Next Top Model: An Overview of Breast Cancer Risk Assessment Models
Images
CME credits are available for this article here.
In 2023, an estimated 298,000 women in the United States were diagnosed with breast cancer.1 Incidence rates for breast cancer have been increasing by approximately 0.5% per year since the mid-2000s.2 Significant racial disparities in breast cancer mortality exist, with mortality rates in Black women being approximately 40% higher than those for White women, despite similar incidence rates.1 Disparities are also prominent among young women. When comparing Black women age ≤50 years to White women in the same age group, mortality rates were 1.9-2.6 times higher in Black women versus 1.1-1.2 times higher in the groups aged ≥70 years.3
Identifying a diverse population of young women at high risk for breast cancer with dedicated risk assessment models can help to address these existing disparities in mortality. Clinicians perform breast cancer risk assessment by asking a series of questions about such characteristics as family and breast health history and inputting the answers into an electronic tool, which calculates a woman’s risk for breast cancer. Women identified as being at high risk (lifetime risk ≥20%) can be offered guideline-based early screening mammography and supplemental screening with MRI. This early or supplemental screening in high-risk women can identify cancers at earlier stages, prevent delays in diagnosis, and initiate earlier treatment. Ultimately, identification of a diverse population of young, high-risk women has the potential to improve mortality and address existing breast cancer disparities.
While numerous risk assessment models have been developed to identify women at high risk for developing breast cancer, these models remain underutilized in the clinical setting. The purpose of this activity is to
- introduce breast cancer risk assessment models for estimating an individual’s risk of developing breast cancer or risk for carrying a gene mutation that may predispose to developing breast cancer,
- review the strengths and limitations of each model and the model’s applicability to underrepresented populations, and
- provide a case study that demonstrates the use of risk assessment models.
Risk Factors for Breast Cancer
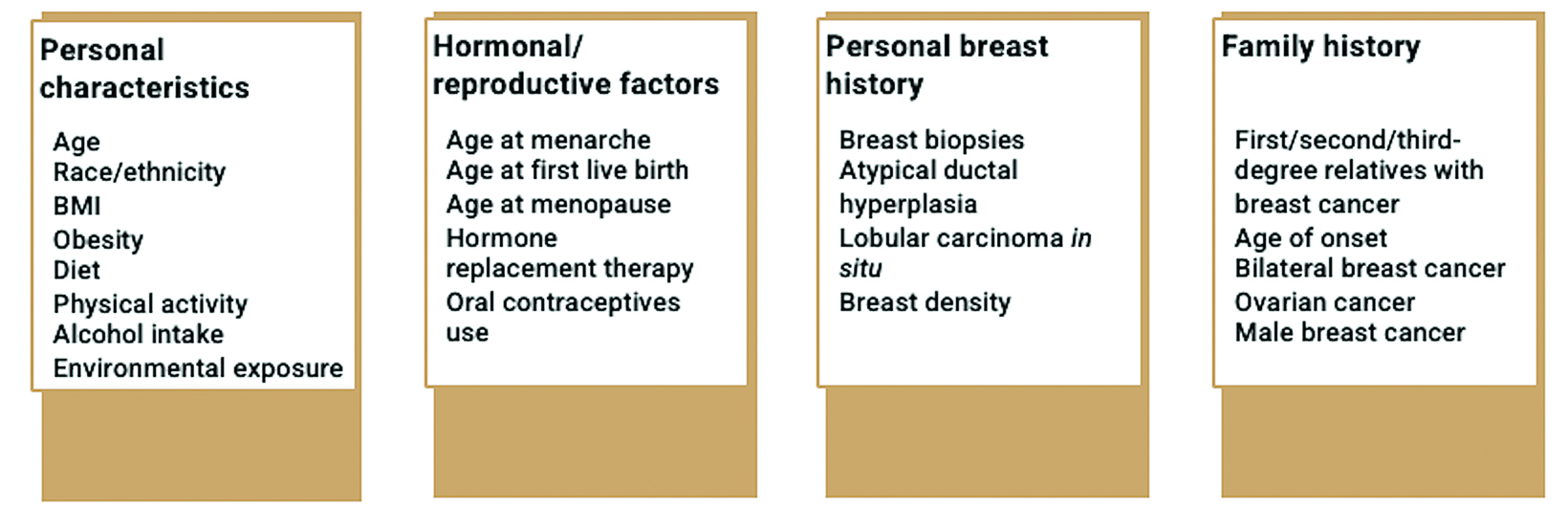
Many risk factors can increase an individual’s likelihood of developing breast cancer (Figure 1).4 Nonmodifiable risk factors include increasing age, being born female, personal or family history of breast cancer, and inherited genetic changes in breast cancer susceptibility genes.4 Hormonal and reproductive risk factors include long menstrual history and having children later in life.4 Breastfeeding for at least one year can serve as a protective factor and decrease risk.4 Potentially modifiable risk factors include excess body weight, menopausal hormone therapy, physical inactivity, and excess alcohol consumption.4 Medical risk factors include high breast tissue density and history of radiation to the chest.4 Additional factors that can increase risk include history of breast biopsies and diagnosis of atypical hyperplasia or lobular carcinoma in situ (LCIS).4
Screening Guidelines Based on Calculated Lifetime Risk
The American College of Radiology (ACR) and Society of Breast Imaging (SBI) recommend risk assessment no later than age 25.5 For those at average risk, annual screening with mammography is recommended starting at age 40. If a patient is found to have a lifetime risk ≥20%, ACR guidelines recommend annual screening beginning at age 30 and annual breast MRI beginning at age 25-30. More specific guidelines are available for women with other factors that may increase personal risk for breast cancer (eg, history of chest radiation therapy; genetics-based increased risk; personal histories of breast cancer and dense breast tissue; family history of breast cancer at a young age; personal history of atypical ductal hyperplasia (ADH), atypical lobular hyperplasia, or LCIS).
National Comprehensive Cancer Network (NCCN) guidelines also recommend screening mammography for high-risk women at younger ages and supplemental screening with contrast-enhanced breast MRI.6,7 Specifically, patients with a lifetime risk ≥20% should receive an annual screening mammogram beginning either at age 40 or 10 years prior to when the youngest family member was diagnosed with the disease, but not prior to age 30 (whichever comes first). Tomosynthesis is recommended. Additionally, patient with a lifetime risk ≥20% should either undergo annual breast MRI beginning at age 40 or 10 years prior to when the youngest family member was diagnosed with breast cancer, but not prior to age 25 (whichever comes first). If a patient cannot undergo MRI, then contrast-enhanced mammography or whole breast ultrasound should be considered. Additional guidelines are available for women with other high-risk factors (eg, those with thoracic radiation between ages 10 and 30, increased 5-year risk of invasive breast cancer, ADH and ≥20% lifetime risk, lobular neoplasia and ≥20% lifetime risk, or pedigree suggestive of genetic predisposition).
Overview of Risk Assessment Models
Gail Model
The Gail model is one of the earliest models of breast cancer risk assessment, first published in 1989.8 The data was derived from 243,221 White women in the Breast Cancer Detection Demonstration Project (BCDDP), a screening program conducted between 1973 and 1980 in the United States.9 This model was modified in 1992 by the National Surgical Adjuvant Breast and Bowel Project (NSABP) to estimate the absolute risk of developing only invasive breast cancer.10 Researchers have made additional updates to the model to pro- vide more accurate estimates for Black women, Asian and Pacific Islander women, and Hispanic women. The modified model is used in the National Cancer Institute’s (NCI) Breast Cancer Risk Assessment Tool. It estimates a patient’s 5-year and lifetime risk of developing invasive breast cancer and is available at https://bcrisktool.cancer.gov/.11
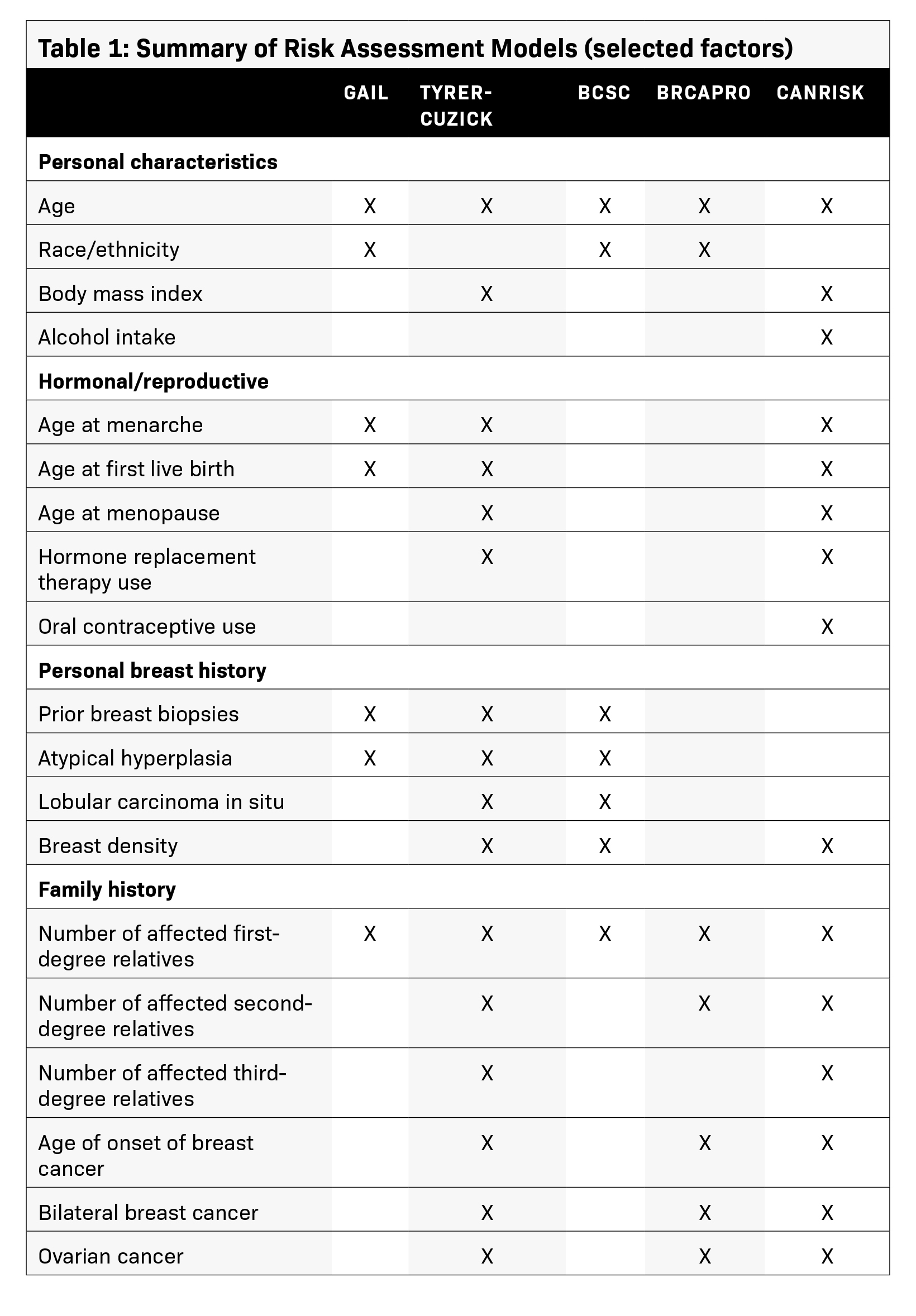
Factors included in the Gail model are age, race/ethnicity, age at menarche, age at first live birth, number of previous breast biopsies, presence of atypical hyperplasia on biopsy, and number of affected first-degree relatives.
While easily accessible, well-calibrated to provide moderate discriminatory accuracy in studies of predominantly White women, and updated to provide more accurate estimates among different populations, the Gail model may underestimate risk in certain populations, such as Black women with previous biopsies and Hispanic women born outside the United States.12-17 Additionally, owing to a lack of data, the model may be inaccurate among Native American and Alaskan Native women.
The Gail model has additional weaknesses that should be considered. For example, it does not account for family history of breast cancer beyond affected first-degree relatives, thereby excluding paternal family history. For this reason, the NCCN guidelines do not list it as a model that should be used to identify candidates for supplemental screening with breast MRI. Instead, a patient found to have a 5-year risk of invasive breast cancer >1.7% in individuals age >35 per the Gail model should receive an annual screening mammogram (to begin when identified as being at increased risk), with tomosynthesis if available.
Furthermore, the Gail model does not include the age at onset of breast cancer among relatives or family history of other cancers. The model also does not consider variables such as mammographic density and should not be used in women under the age of 35. Additionally, the Gail model should not be used in women with known BRCA1/BRCA2 mutations, those with a previous history of breast cancer, those with prior treatment of Hodgkin lymphoma with radiation to the chest, or those with breast cancer-causing syndromes, including Li-Fraumeni syndrome.
International Breast Cancer Intervention Study [IBIS]/Tyrer-Cuzick (version 8)
The Tyrer-Cuzick model, also known as the IBIS model, was developed by scientists at the Wolfson Institute of Preventive Medicine, Queen Mary University of London.18,19 The Tyrer-Cuzick model was developed using data on first breast cancer diagnoses among women in the United Kingdom (Thames Cancer Registry) between 2005-2009. Familial risk is based on data from a Swedish population-based study.19 The latest version of the model (version 8) incorporates additional risk factors such as breast density and single nucleotide polymorphisms (SNPs). The model estimates an individual’s 10-year and lifetime risk for developing breast cancer, as well as the likelihood of carrying a BRCA1 or BRCA2 gene. It is available at https://ibis.ikonopedia.com/ and https://ems-trials.org/ riskevaluator/.20,21
Factors included in the Tyrer-Cuzick model are age, body mass index, reproductive history (age at menarche, age at first live birth, age at menopause), exogenous hormone exposure (hormone replacement therapy duration), results of previous breast biopsy (hyperplasia, presence of LCIS or atypical hyperplasia), breast density, family history (number and age of onset of first, second, or third-degree relatives with breast cancer, ovarian cancer diagnoses, male breast cancer diagnoses, unaffected relatives), Ashkenazi Jewish origin, and previous genetic test results (BRCA1/2).
External validation studies for the current version of the Tyrer-Cuzick model are ongoing. The Tyrer-Cuzick model has been found to be well-calibrated overall in non-Hispanic White women and Black women, with good calibration for Asian/Pacific Islanders and Native Americans, although sample sizes were small.22 The model may overestimate risk for Hispanic women.22 The addition of mammographic density was found to increase the discriminatory accuracy of the model.23 One study suggests the Tyrer-Cuzick model may overestimate risk for women at the highest-risk decile.24
A major strength of the Tyrer-Cuzick model is that it includes a diverse range of risk factors, including breast density and a comprehensive family history. Like the Gail model, it is easily accessible online and has undergone periodic updates to incorporate additional data on breast cancer incidence. Unlike the Gail model, the Tyrer-Cuzick model can be used in women ages <35 years and can calculate the risk for BRCA1 or BRCA2 mutations.
The Tyrer-Cuzick model should not be used to assess risk in women who have already been diagnosed with breast cancer and may overestimate risk in women with atypical hyperplasia and LCIS.25,26
The Breast Cancer Surveillance Consortium (BCSC) Risk Calculator version 2.0
The BCSC Risk Calculator model was developed in 2008 using data from 1,095,484 women in seven mammography registries participating in the NCI-funded BCSC in the United States.27 The study included women ages >35 with at least 1 mammogram with breast density measured using the Breast Imaging Reporting and Data System (BI-RADS) classification system. The model was updated in 2015 (version 2) to include benign breast diagnoses.28 The BCSC Risk Calculator estimates a patient’s 5- and 10-year risk of developing invasive breast cancer and is available at: https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm.29
Factors included in the BCSC Risk Calculator are age, race/ethnicity, history of first-degree relatives with breast cancer (yeso), history of a breast biopsy with benign breast disease diagnoses if known, and BI-RADS breast density.
The original model was externally validated among patients in the Mayo Mammography Health Study (MMHS) cohort.30 Version 2 of the model was validated in a cohort of women in Chicago and was well-calibrated but found to underestimate risk in younger women, Hispanic and non-Hispanic Black women, and those with almost entirely fat breast density.28
The major strengths of the BCSC Risk Calculator are that it incorporates BI-RADS breast density and is easily accessible. This model cannot be used in women with a previous diagnosis of breast cancer or DCIS, prior breast augmentation, prior mastectomy, or those aged <35 or >74. Additionally, it does not account for a family history of breast cancer beyond affected first-degree relatives, thereby excluding paternal family history.
BRCAPRO
The BRCAPRO model was developed in 1997 based on estimates of BRCA1 mutation frequencies in the general population and age-specific incidence rates of breast and ovarian cancers in carriers and noncarriers of mutations.31 It was expanded in 1998 to include BRCA2.32 The model uses Mendelian genetics and Bayes’ theorem to calculate a patient’s likelihood of carrying a germline mutation in the BRCA1 or BRCA2 genes, developing invasive breast cancer, or developing contralateral breast cancer. Access to the model can be requested at https://projects.iq.harvard.edu/bayesmendel/bayesmendel-r-package.33
Factors included in the BRCAPRO model are age, race/ethnicity, number/age at onset of first or second-degree relatives with breast cancer, family history of bilateral breast cancer or male breast cancer, personal or family history of ovarian cancer, and Ashkenazi Jewish origin.
Validation studies demonstrate variation in the performance of the BRCAPRO model with some studies demonstrating appropriate performance and other studies finding the model to underpredict risk.34,35
Like the Tyrer-Cuzick and the CanRisk models, one of the strengths of the BRCAPRO model is its ability to assess the likelihood of carrying a BRCA1 and/or BRCA2 gene mutation. The model also considers information about unaffected relatives and is routinely updated.
The BRCAPRO model does not include non-hereditary risk factors, such as age at menarche, age at first live birth, age at menopause, or specific results of prior breast biopsies. It also excludes family history of third-degree relatives.
Additionally, this model may underestimate risk in patients without BRCA gene mutations, as well as in families with prostate or
ovarian cancer.36 The model is not immediately accessible; however, it can be requested through an online form.
CanRisk (BOADICEA v5)
The Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) model calculates the probabilities of carrying rare loss-of-function variants in several breast or ovarian cancer susceptibility genes in addition to estimating the risk of developing breast and ovarian cancer.37-39 It has undergone numerous updates since its development in 2002 and incorporates the effects of common genetic variants (summarized as polygenic risk scores, PRS), pathogenic variants in other genes, mammographic density, and additional risk factors. The latest version of the model (v6) is available to use via a web tool called CanRisk (https://www.canrisk.org/).37,38,40-44
Factors included in the CanRisk Tool for breast cancer risk estimation are age, body mass index, height, daily alcohol intake, age at menarche, age at first live birth, use of menopause hormone therapy, use or oral contraception, parity, mammographic density, family and personal-proband history of breast, ovarian, and pancreatic cancer, rare pathogenic variants in moderate and high-risk susceptibility genes, age information on unaffected family members, information on year of birth to capture birth cohort, Ashkenazi Jewish origin, and common cancer genetic susceptibility variants (Polygenic Risk Scores).45
The model has been validated in several studies, largely consisting of women of European ancestry, and has been found to be well-calibrated.24,46-48 However, it may not be as reliable in populations at lower risk for breast cancer or those of non-European ancestry.49
Like the Tyrer-Cuzick model, the CanRisk Tool includes a diverse range of risk factors, including comprehensive family history. Additionally, this is the only model that includes lifestyle risk factors such as alcohol consumption. The model is easily accessible online but requires the user to create an account for access. Unlike other models, it can be used in patients with a previous diagnosis of breast cancer.
The CanRisk tool should not be used in patients with personal history ductal carcinoma in situ (DCIS). Additionally, it will underestimate risk in those with Ataxia-Telangiectasia or homozygous carriers of pathogenic CHEK2 pathogenic truncating variants and should not be used in these patients. The CanRisk tool does not incorporate information on prior breast biopsies (number or result).
Table 1 provides a summary of the factors included in several risk assessment models.
Case Study
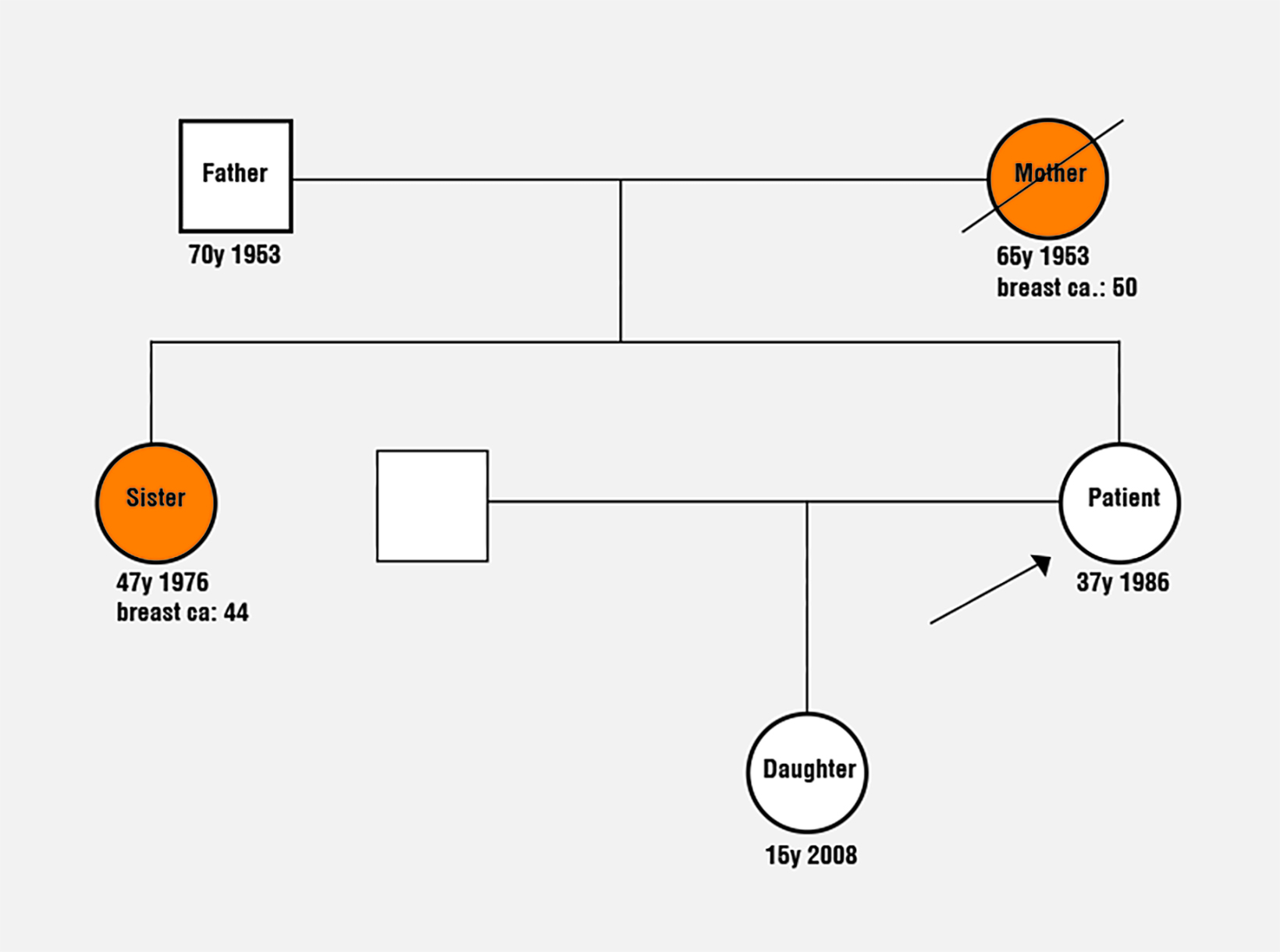
A 37-year-old White female wishes to know her lifetime risk for developing breast cancer. She has no significant medical history and is not Ashkenazi Jewish. She has never been tested for genetic mutations, had a mammogram, or had a breast biopsy. Menarche was at age 14, and she is premenopausal. She had a daughter at the age of 22, who is currently 15 and healthy. The patient’s mother (diagnosed at age 50, deceased age 65) and sister (diagnosed at age 44, alive (currently 47) had unilateral breast cancer (Figure 2). Her father is living, age 70, and healthy. There is no family history of ovarian cancer. Genetic testing for the patient’s relatives has never been performed. She is 5 foot 4 inches, weighs 150 pounds, and does not drink alcohol. She has never used hormone replacement therapy or oral contraceptives. She has never had an SNP array/PRS calculated.
What is the patient’s lifetime risk for developing breast cancer? What are the appropriate breast cancer screening recommendations?
Risk Model Assessment
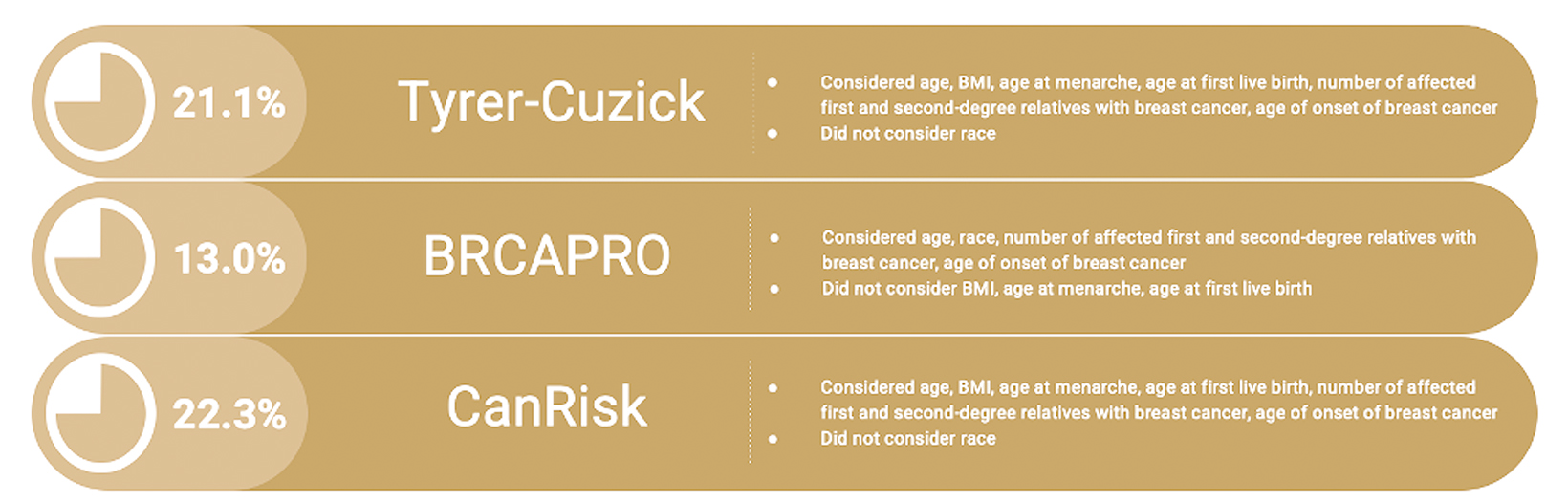
The Tyrer-Cuzick and CanRisk models calculate the patient’s lifetime risk for breast cancer as ≥20%. The BRCAPRO model calculates the patient’s lifetime risk for breast cancer as <20%. Figure 3 depicts the risk assessment values for each model and the factors included in each model.
Based on the results of the Tyrer-Cuzick and CanRisk models, the patient is considered high risk. Per NCCN guidelines, they should consider screening mammography and screening MRI 10 years prior to the age of diagnosis of the youngest first-degree relative, but not before age 30. Because the patient’s sister was diagnosed with breast cancer at age 44, screening mammography and screening MRI could have been considered as early as age 34.
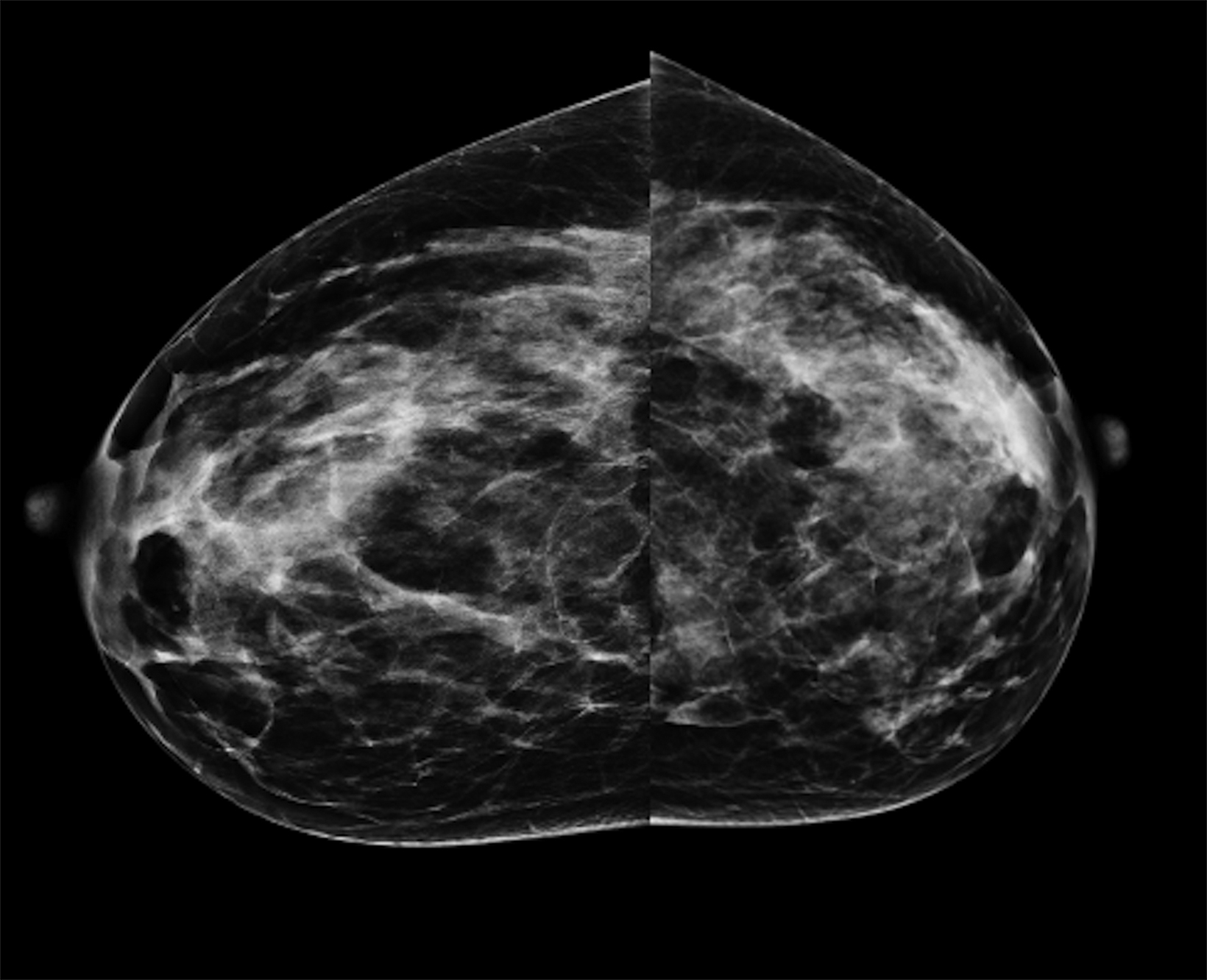
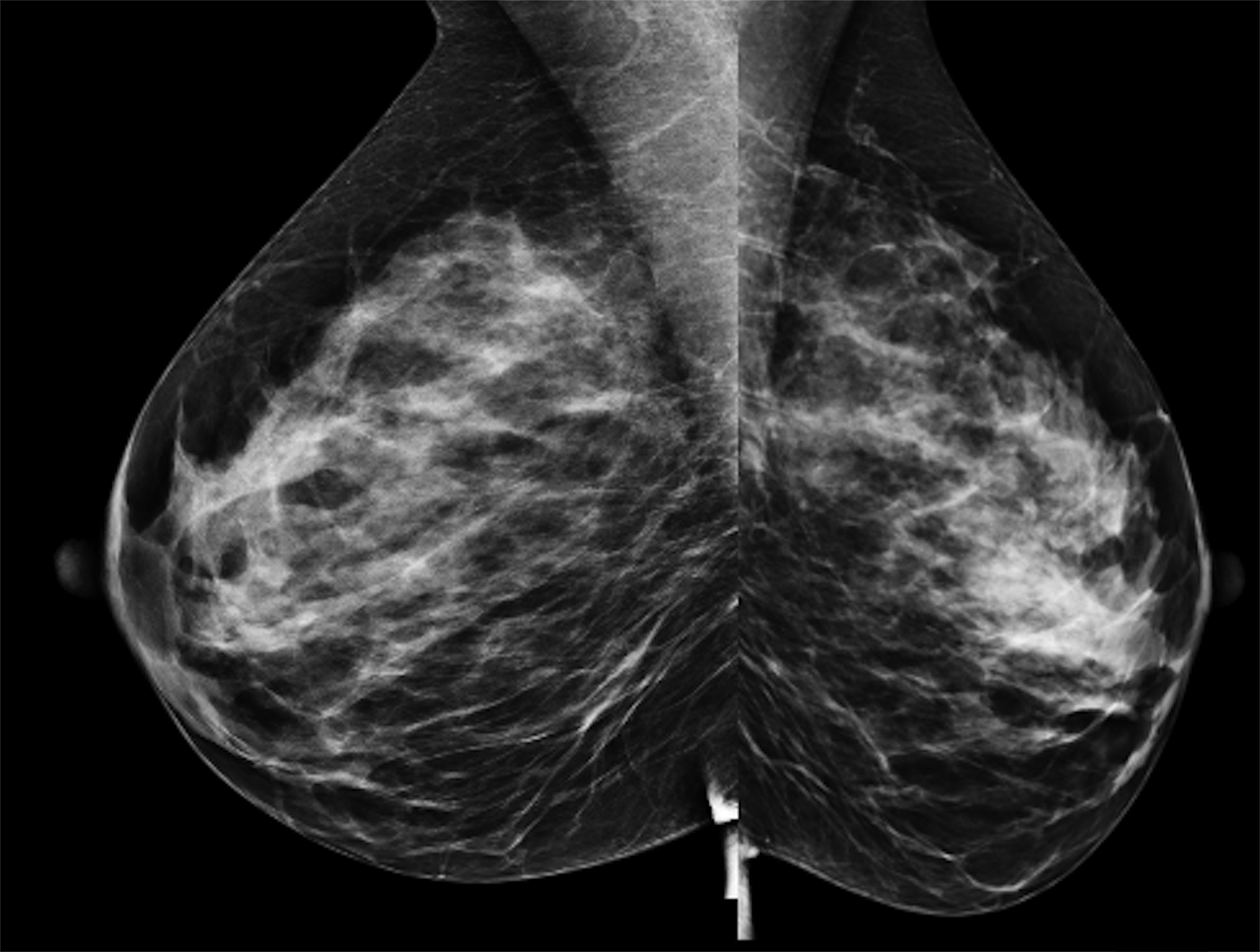
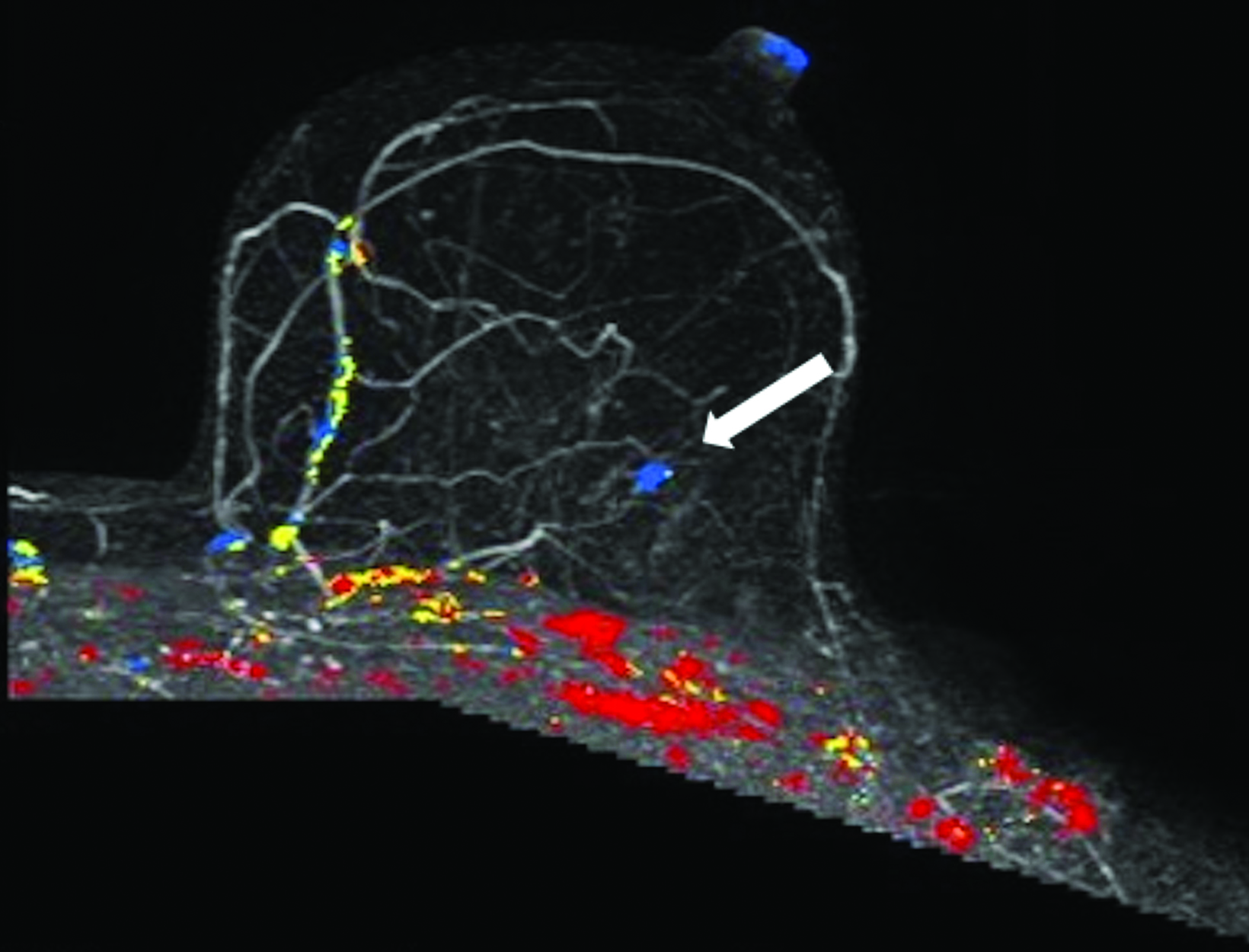
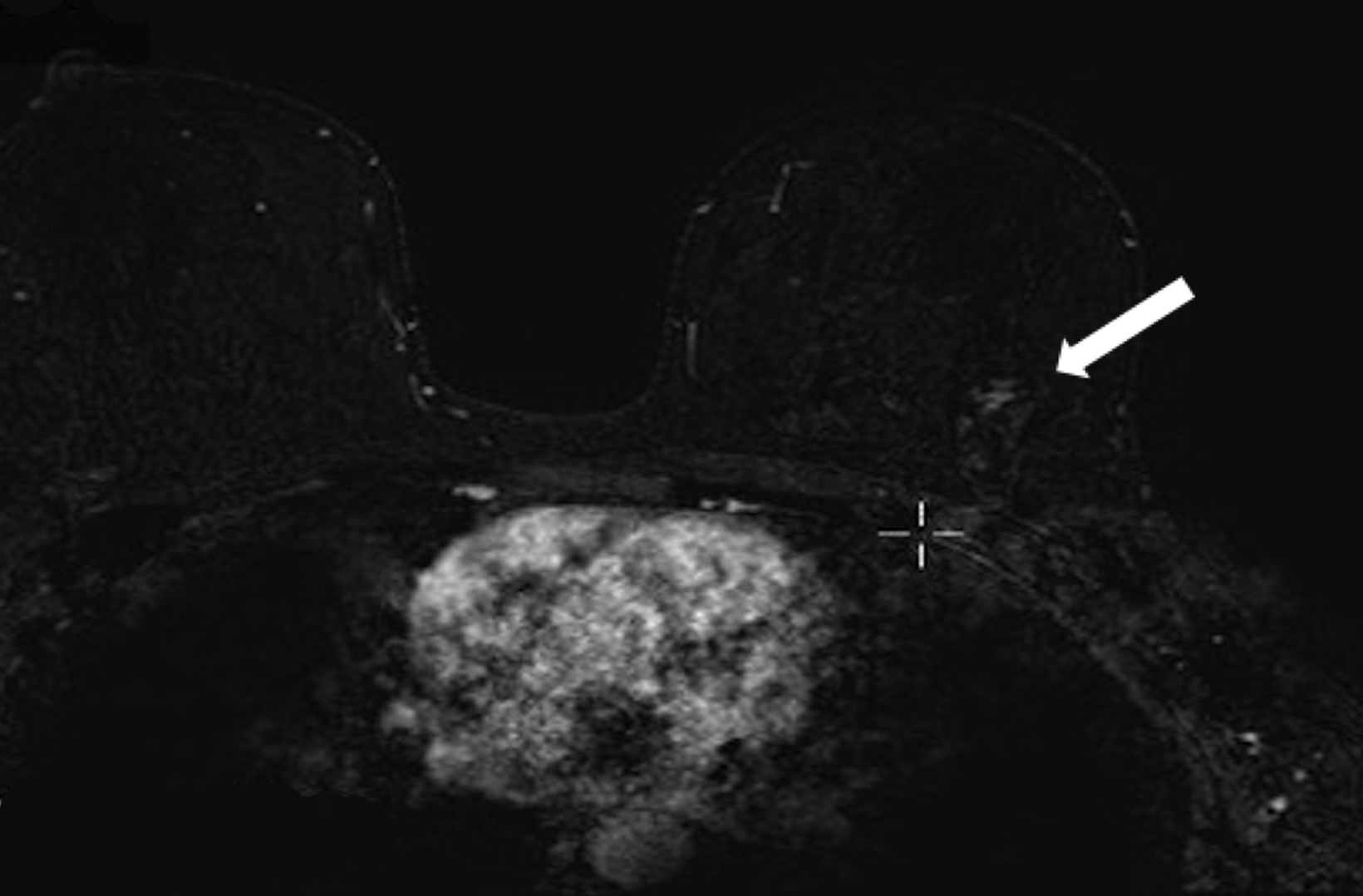
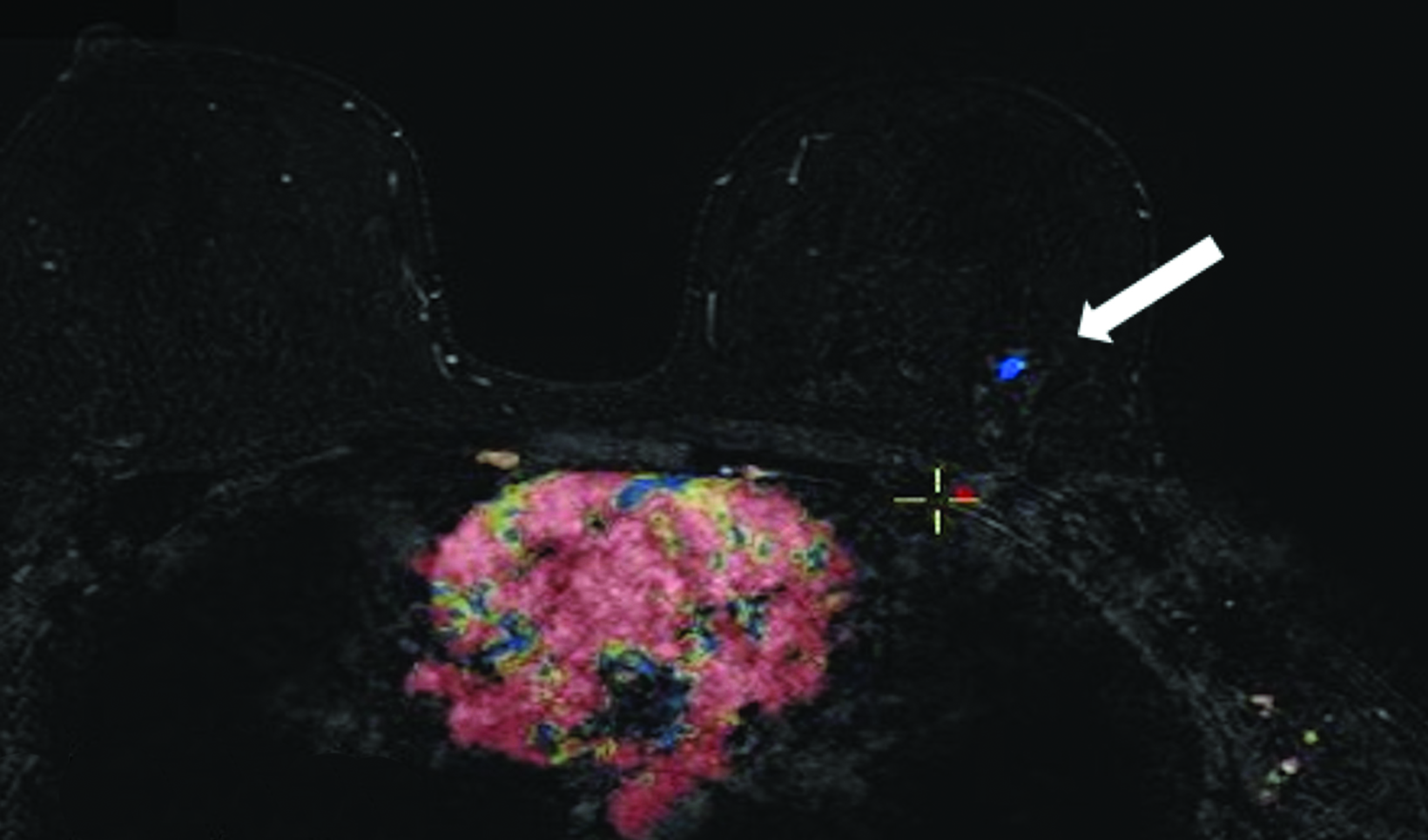
The patient pursued screening mammography and MRI (Figure 4). The mammogram was normal, with dense breast tissue. Screening MRI demonstrated focal clumped non-mass enhancement in the left outer breast. MRI-guided biopsy was performed and revealed DCIS with a microinvasive component.
Conclusion
Numerous risk assessment models are available to calculate a woman’s lifetime risk of developing and/or carrying a gene mutation that may predispose her to developing breast cancer. Knowledge of risk may help to inform individual screening practices.
Risk assessment models have different strengths and weaknesses that may increase or limit use in certain populations. Further understanding and evaluation of risk assessment models are needed to increase their utilization. Increased breast cancer risk assessment among diverse populations can identify women who may be at high risk for breast cancer. Guideline-based breast cancer screening in these populations serves as an opportunity to address known breast cancer mortality disparities.
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524-541.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438-451.
- Ban KA, Godellas CV. Epidemiology of breast cancer. Surg Oncol Clin N Am. 2014;23(3):409-422.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18(9):1280-1288.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2023. © National Comprehensive Cancer Network I. All rights reserved. Accessed April 14, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer Risk Reduction V.1.2022. © National Comprehensive Cancer Network I. All rights reserved. Accessed [April 14, 2023]. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886.
- Baker LH. Breast cancer detection demonstration project: five-year summary report. CA Cancer J Clin. 1982;32(4):194.
- >Anderson S, Ahnn S, Duff K. NSABP Breast Cancer Prevention Trial risk assessment program, version 2. NSABP Biostatistical Center Technical Report. 1992;12
- Institute NC. The Breast Cancer Risk Assessment Tool. Accessed April 14, 2023, https://bcrisktool.cancer.gov/
- Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358-366.
- Gail MH. Choosing breast cancer risk models: importance of independent validation. Oxford University Press; 2020. p. 433-435.
- Wang X, Huang Y, Li L, Dai H, Song F, Chen K. Assessment of performance of the Gail model for predicting breast cancer risk: a systematic review and meta-analysis with trial sequential analysis. Breast Cancer Res. 2018;20(1):1-19.
- Matsuno RK, Costantino JP, Ziegler RG, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J Natl Cancer Inst. 2011;103(12):951-961.
- Banegas MP, John EM, Slattery ML, et al. Projecting individualized absolute invasive breast cancer risk in US Hispanic women. J Natl Cancer Inst. 2017;109(2):djw215.
- Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99(23):1782-1792.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111-1130.
- Anderson H, Bladström A, Olsson H, Möller TR. Familial breast and ovarian cancer: a Swedish population-based register study. Am J Epidemiol. 2000;152(12):1154-1163.
- Cuzick J. IBIS Breast Cancer Risk Evaluation Tool.
- IBIS Risk Assessment Tool.
- Kurian AW, Hughes E, Simmons T, et al. Performance of the IBIS/Tyrer-Cuzick model of breast cancer risk by race and ethnicity in the Women’s Health Initiative. Cancer. 2021;127(20):3742-3750.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res. 2015;17:1-10.
- Pal Choudhury P, Brook MN, Hurson AN, et al. Comparative validation of the BOADICEA and Tyrer-Cuzick breast cancer risk models incorporating classical risk factors and polygenic risk in a population-based prospective cohort of women of European ancestry. Breast Cancer Res. 2021;23:1-5.
- Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28(22):3591.
- Lo LL, Milne RL, Liao Y, Cuzick J, Terry MB, Phillips K-A. Validation of the IBIS breast cancer risk evaluator for women with lobular carcinoma in-situ. Br J Cancer. 2018;119(1):36-39.
- Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337-347.
- Tice JA, Miglioretti DL, Li C-S, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137.
- Consortium BCS. Breast Cancer Surveillance Consortium Risk Calculator V2. Updated July 17, 2015. Accessed April 14, 2023. https://tools.bcsc-scc.org/BC5year-Risk/calculator.htm
- Vachon CM, Pankratz VS, Scott CG, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015;107(5):dju397.
- Berry DA, Parmigiani G, Sanchez J, Schildkraut J, Winer E. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J Natl Cancer Inst. 1997;89(3):227-237.
- Parmigiani G, Berry DA, Aguilar O. Determining carrier probabilities for breast cancer–susceptibility genes BRCA1 and BRCA2. Am J Med Genet. 1998;62(1):145-158.
- BayesMendel R package.
- Terry MB, Liao Y, Whittemore AS, et al. 10-year performance of four models of breast cancer risk: a validation study. Lancet Oncol. 2019;20(4):504-517.
- Antoniou AC, Hardy R, Walker L, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet. 2008;45(7):425-431.
- Fernández LO, Márquez-Aragonés M, Romero-Laorden N, et al. Limited value of currently used germline brca mutations predictive tools in prostate cancer. Ann Oncol. 2017;28:v286.
- Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21(8):1708-1718.
- Carver T, Hartley S, Lee A, et al. CanRisk Tool—A web interface for the prediction of breast and ovarian cancer risk and the likelihood of carrying genetic pathogenic variants. Cancer Epidemiol Biomarkers Prev. 2021;30(3):469-473.
- Archer S, Babb de Villiers C, Scheibl F, et al. Evaluating clinician acceptability of the prototype CanRisk tool for predicting risk of breast and ovarian cancer: A multi-methods study. PLoS One. 2020;15(3):e0229999.
- Antoniou AC, Pharoah PD, McMullan G, et al. A comprehensive model for familial breast cancer incorporating BRCA1, BRCA2 and other genes. Br J Cancer. 2002;86(1):76-83.
- Antoniou AC, Cunningham A, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98(8):1457-1466.
- Lee AJ, Cunningham AP, Kuchenbaecker K, Mavaddat N, Easton DF, Antoniou AC. BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer. 2014;110(2):535-545.
- Lee AJ, Cunningham AP, Tischkowitz M, et al. Incorporating truncating variants in PALB2, CHEK2, and ATM into the BOADICEA breast cancer risk model. Genet Med. 2016;18(12):1190-1198.
- Lee A, Mavaddat N, Cunningham A, et al. Enhancing the BOADICEA cancer risk prediction model to incorporate new data on RAD51C, RAD51D, BARD1 updates to tumour pathology and cancer incidence. J Med Genet. 2022;59(12):1206-1218.
- Carver T. What information do the breast and ovarian cancer models use to determine risks? Updated December 5, 2022. Accessed April 13, 2023, https://canrisk. atlassian.net/wiki/spaces/FAQS/pages/3211464/What+information+do+the+breast+and+ovarian+cancer+models+use+to+determine+risks
- Lakeman IM, Rodríguez-Girondo M, Lee A, et al. Validation of the BOADICEA model and a 313-variant polygenic risk score for breast cancer risk prediction in a Dutch prospective cohort. Genet Med. 2020;22(11):1803-1811.
- Lee A, Yang X, Tyrer J, et al. Comprehensive epithelial tubo-ovarian cancer risk prediction model incorporating genetic and epidemiological risk factors. J Med Genet. 2022;59(7):632-643.
- Yang X, Eriksson M, Czene K, et al. Prospective validation of the BOADICEA multifactorial breast cancer risk prediction model in a large prospective cohort study. J Med Genet. 2022;59(12):1196-1205.
- Carver T. Can I use the CanRisk Tool to estimate risks for populations outside Europe? Updated July 22, 2021. Accessed April 13, 2023, 2023. https://canrisk.atlassian. net/wiki/spaces/FAQS/pages/28180481/Can+I+use+the+CanRisk+Tool+to+estimate+risks+for+populations+outside+Europe.
Citation
P A, CM A, LL E, L L, SA R, KM D, R S, M S, LB S. Next Top Model: An Overview of Breast Cancer Risk Assessment Models. Appl Radiol. 2024;(1):8-15.
January 24, 2024