New N-13 Ammonia Manufacturing Site Approved in South Florida
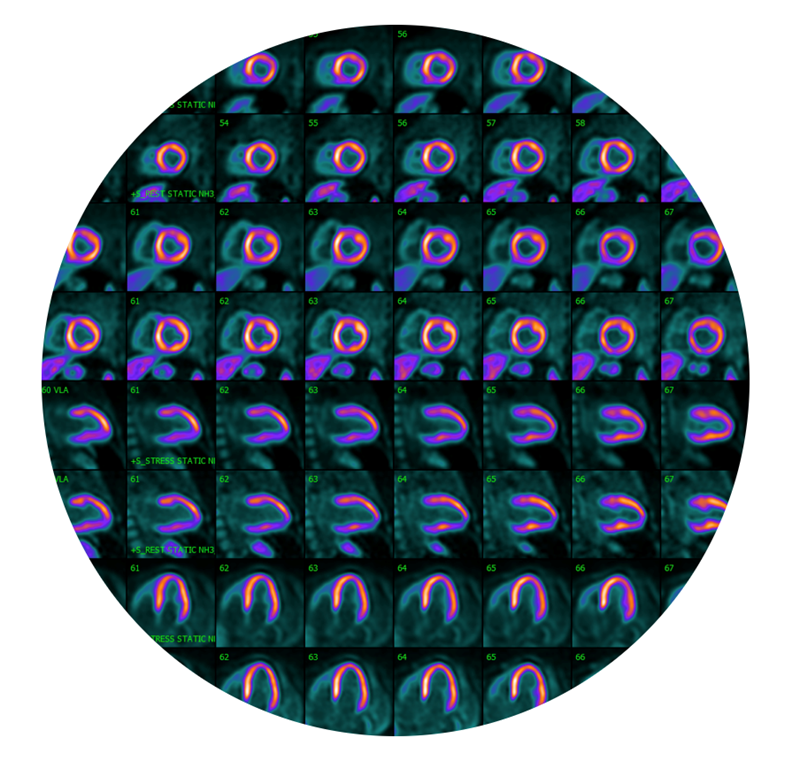 IONETIX has received US FDA approval for N-13 Ammonia manufacturing at their Miami, FL location to support Center for Imaging and Research of America (CIRA). CIRA will be using the N-13 Ammonia manufactured by IONETIX to perform Cardiac PET imaging, which is used to diagnose, and risk stratify patients with coronary artery disease. Its superior image quality and ability to perform quantitative analysis is why it is considered the "gold standard" of non-invasive cardiac imaging for the detection and evaluation of CAD.
IONETIX has received US FDA approval for N-13 Ammonia manufacturing at their Miami, FL location to support Center for Imaging and Research of America (CIRA). CIRA will be using the N-13 Ammonia manufactured by IONETIX to perform Cardiac PET imaging, which is used to diagnose, and risk stratify patients with coronary artery disease. Its superior image quality and ability to perform quantitative analysis is why it is considered the "gold standard" of non-invasive cardiac imaging for the detection and evaluation of CAD.
"The top 16 universities in the US provide Cardiac PET/CT service using N-13 Ammonia, and we are proud to be the first to provide this capability to patients in South Florida." stated Rick Rippin, CIRA CEO. "In my opinion, N-13 is the gold standard in Cardiac PET and provides significant advantages over other tracers by improving image quality, improving quantification of myocardial blood flow, and enabling treadmill stress capability."
Kevin Cameron, CEO of IONETIX added, "CIRA is our 7th site approved by the FDA, and several more sites are underway. We are passionate at IONETIX about bringing our unique solution for N-13 Ammonia as a service to hospitals and outpatient office settings across the United States."
Kevin Cameron, CEO of IONETIX added, "CIRA is our 7th site approved by the FDA, and several more sites are underway. We are passionate at IONETIX about bringing our unique solution for N-13 Ammonia as a service to hospitals and outpatient office settings across the United States."