Nested Stromal Epithelial Tumor
Images
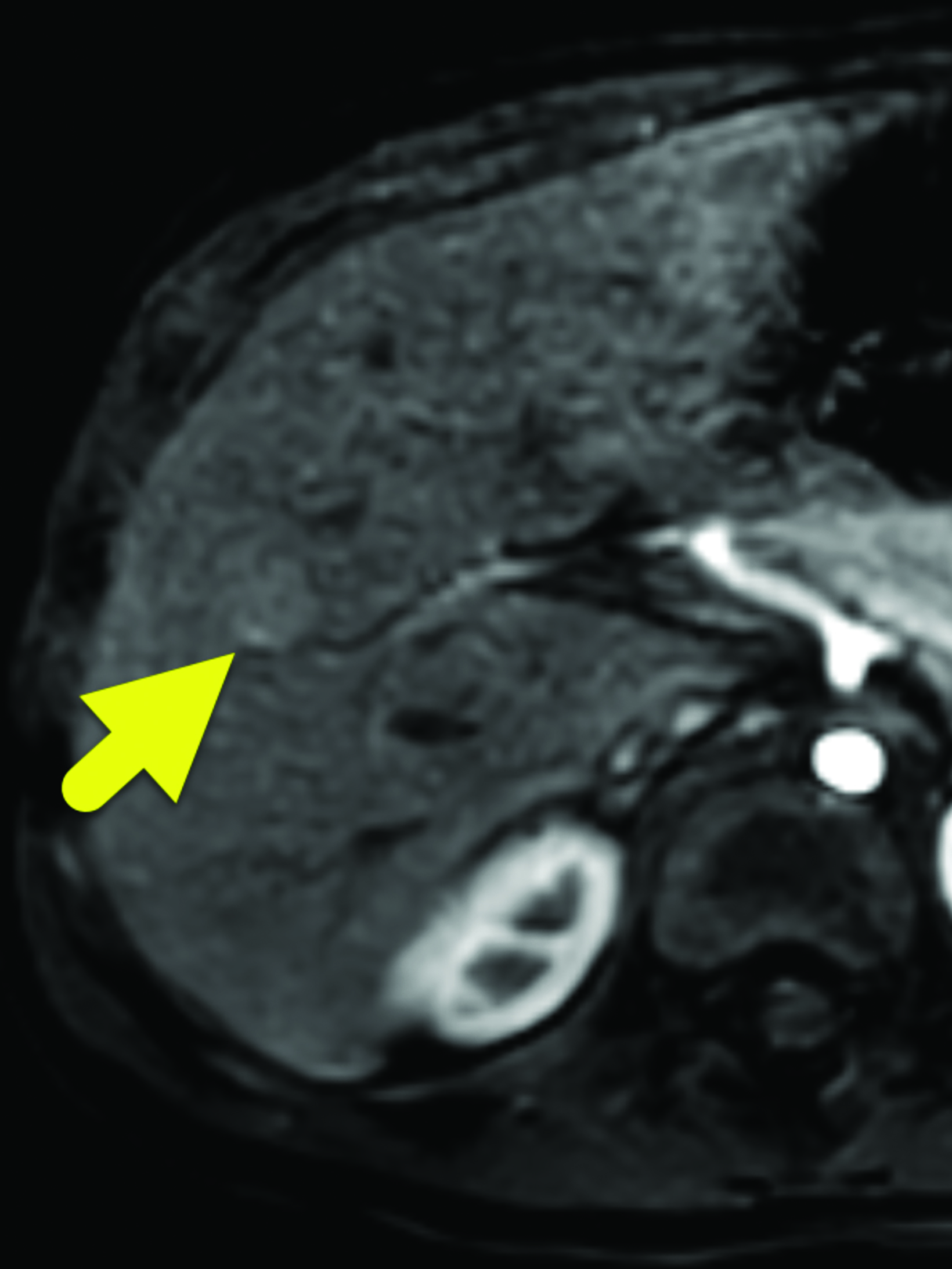
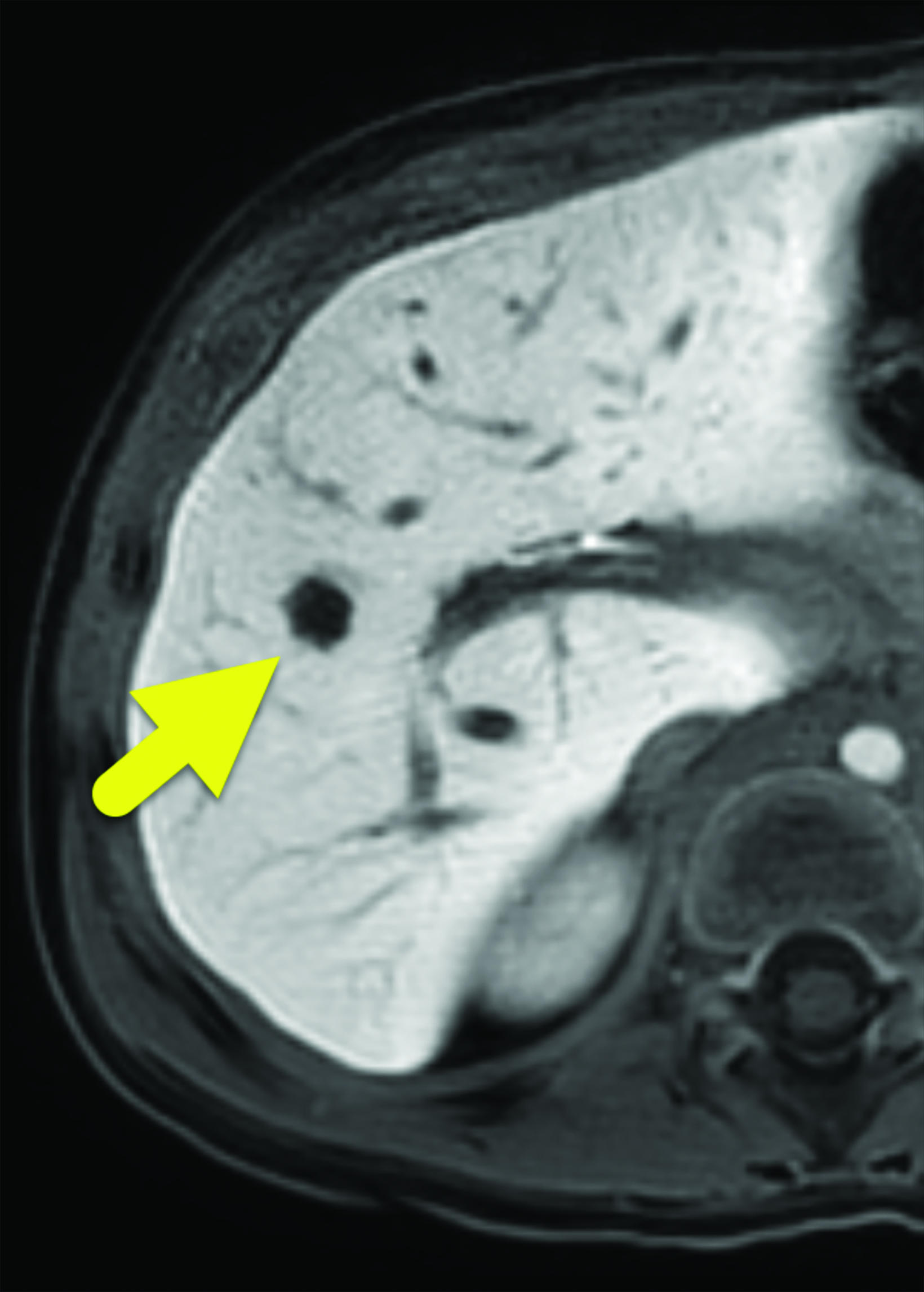
Case Summary
An infant with Beckwith-Wiedemann syndrome (BWS) was found to have an incidental hepatic mass on screening ultrasound. Alpha fetal protein (AFP) levels were monitored serially and remained normal.
Imaging Findings
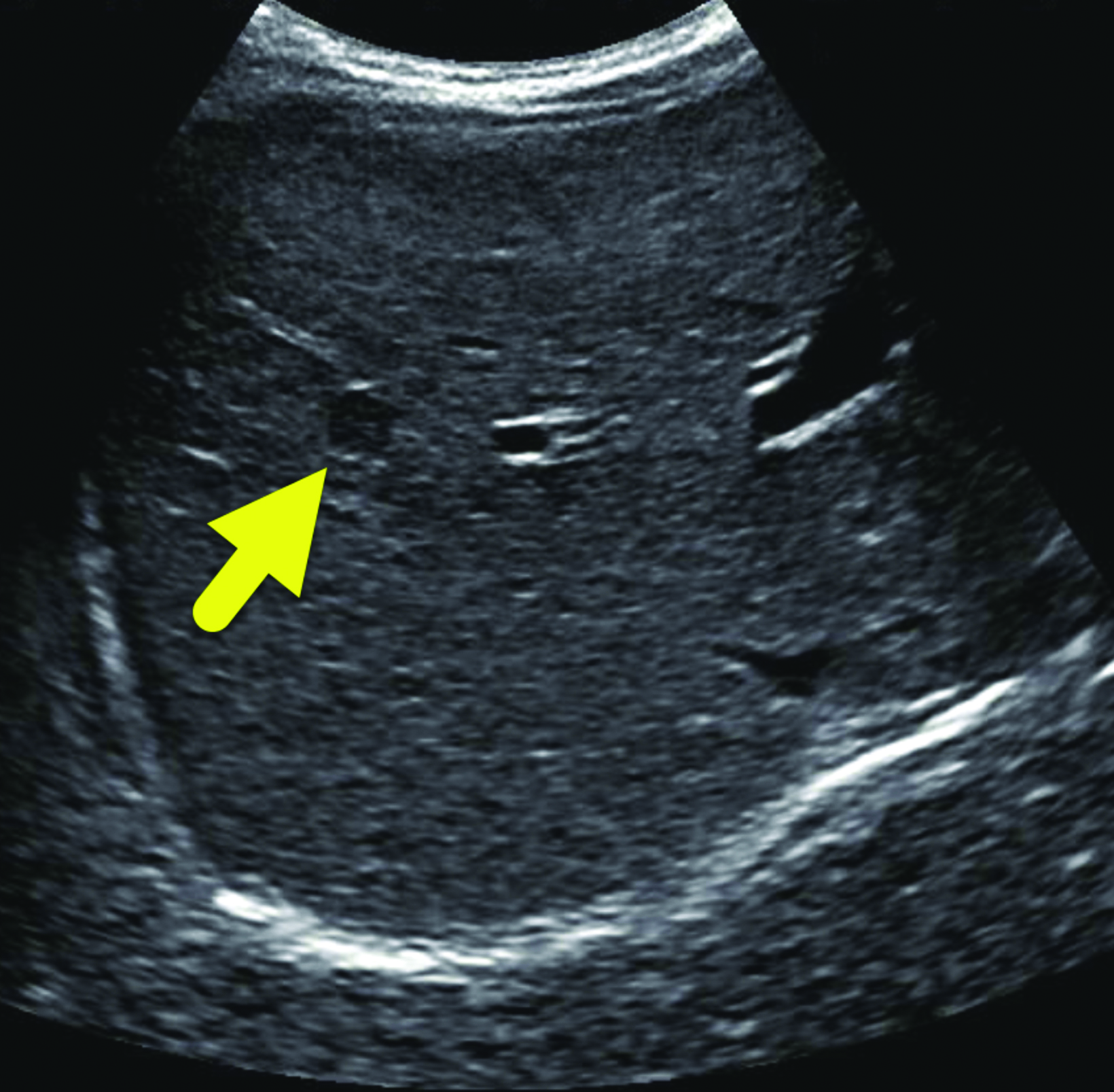
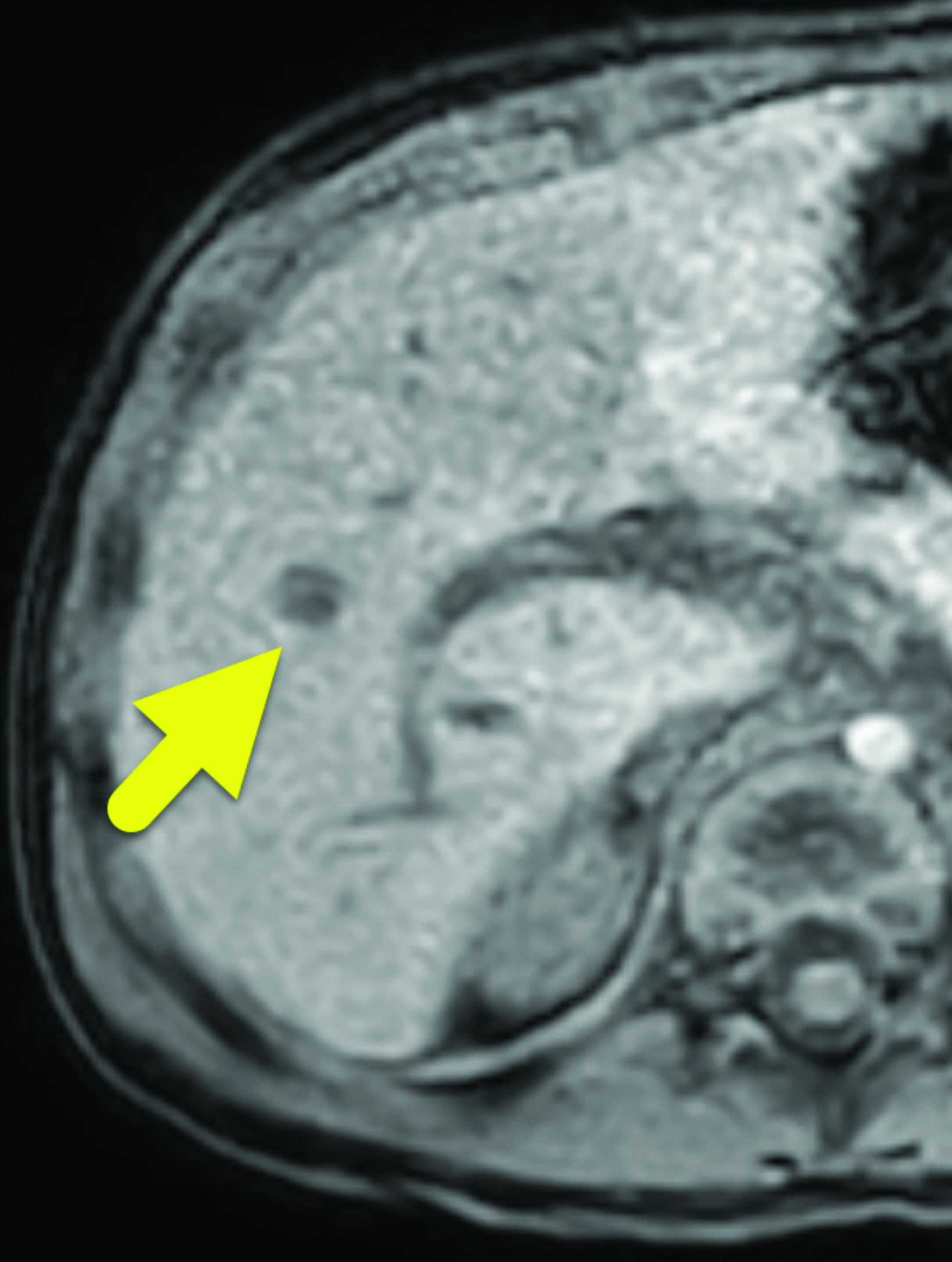
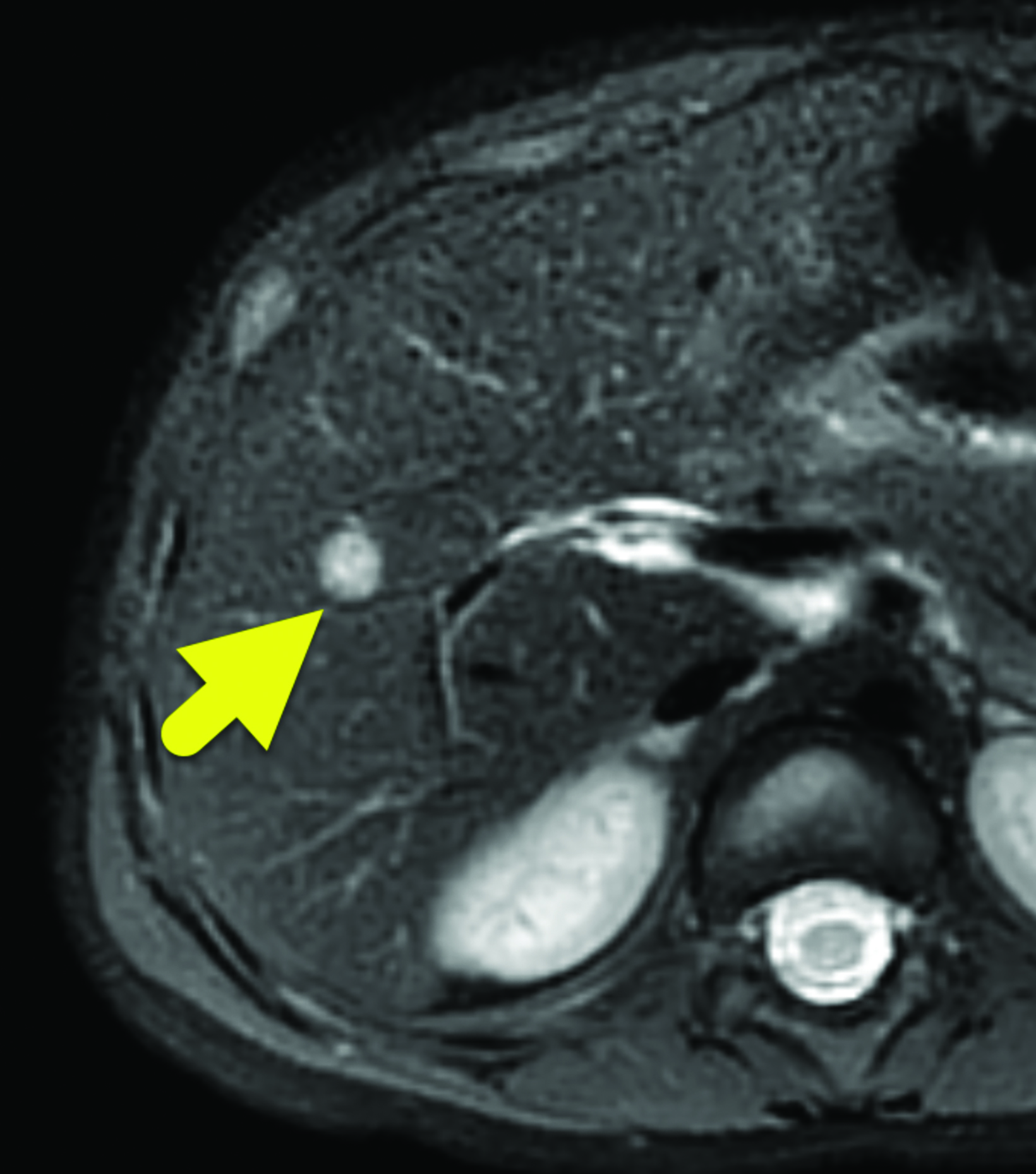
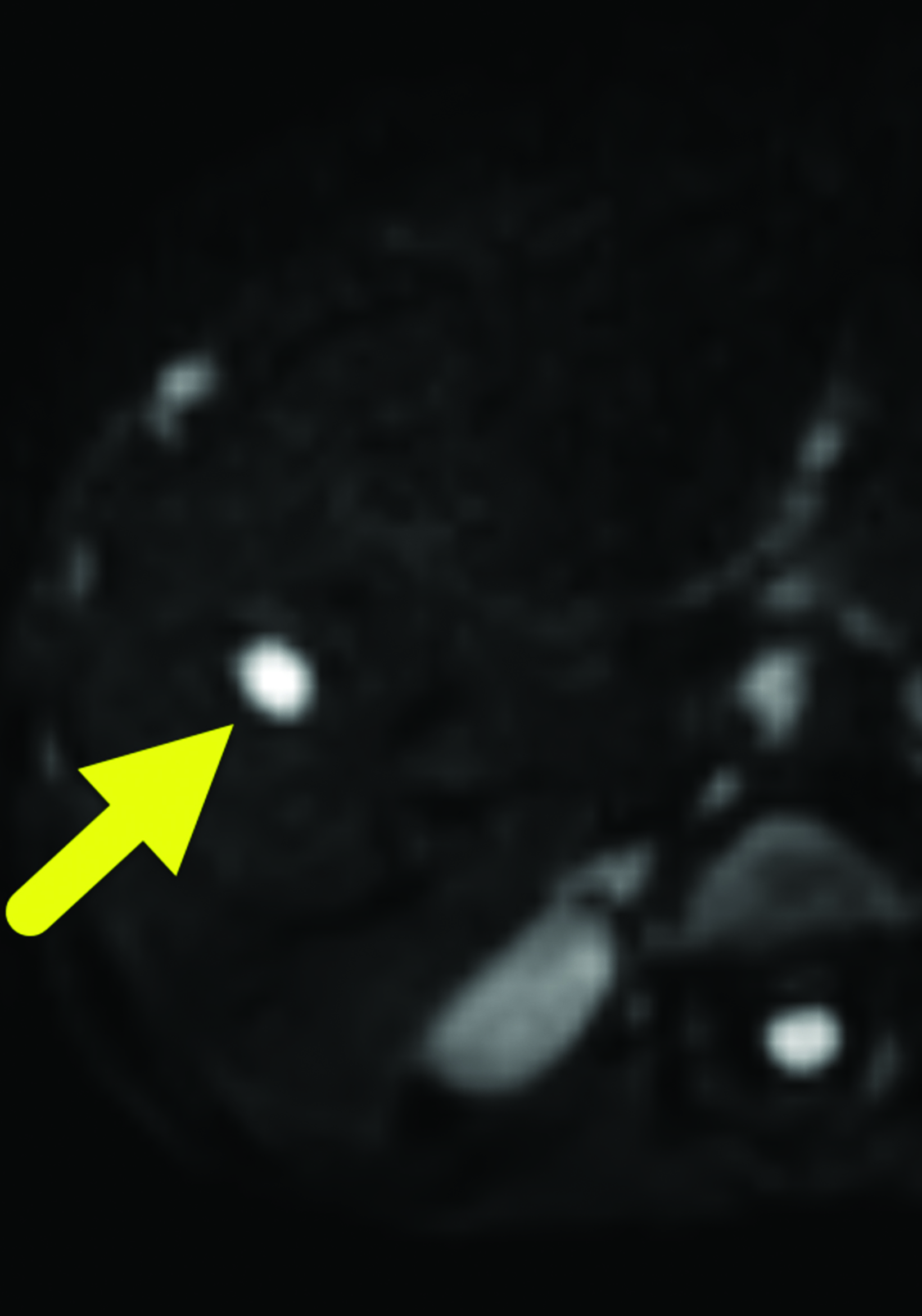
A 7 mm, oval-shaped, hypoechoic lesion was identified within the right lobe of the liver on screening ultrasound (Figure 1). Six weeks later, the lesion had slightly increased in size, measuring 1 cm in maximum diameter. MRI performed one week later showed the lesion to be hypointense on T1 images, hyperintense on T2 images, and with restricted diffusion. On arterial phase imaging, the lesion enhanced slightly more than the background liver. It was isointense to liver parenchyma on the portal venous and systemic venous spaces. On the delayed hepatobiliary phase of imaging, the lesion was hypointense to the remainder of the liver parenchyma (Figure 2).
Diagnosis
Nested stromal-epithelial tumor (NSET).
The differential diagnosis includes fibrolamellar hepatocellular carcinoma, calcified hemangioma, hemangioendothelioma, and hamartoma.
Discussion
NSETs are exceedingly rare neoplasms of the liver. There have been approximately 40 reported cases since its first description as an ossifying stromal-epithelial tumor by Ishak et al in 2001.1 The name refers to the typical cellular arrangement of the tumors with nests of epithelial cells separated by stromal cells. NSETs are thought to derive from hepatic mesenchymal precursor cells.2
BWS is an imprinting disorder of chromosome 11p15 affecting approximately 1 in 10,000 live births. It has a wide phenotypic presentation with a classic triad of macroglossia, hemihypertrophy, and abdominal wall defects. In addition to its phenotypic features, BWS is a cancer predisposition syndrome. Children affected by BWS are at increased risk of developing embryonal tumors – most commonly Wilms tumor (52% of all tumors), hepatoblastoma (14% of all tumors), and neuroblastoma (10% of all tumors).3 Because patients with BWS are at an increased risk of developing a tumor, surveillance imaging is recommended.3 While the recommendations differ based on an individual’s specific mutation, most patients receive an ultrasound of the abdomen every 3 months until 7-years-of-age to evaluate for the common abdominal tumors.3
There is a known correlation between BWS and NSETs, with three reported cases of NSETs in patients with molecularly confirmed BWS and one in a patient with suspected BWS.4-6 Whether there is a causal relationship is unclear, but there are reasons to suspect such a link. NSETs in the setting of BWS have, in multiple cases, stained positively for Wilm tumor 1 (WT-1) protein.7 WT-1 is a multifunctional transcription factor that is thought to be involved in NSET pathogenesis, as it is a regulator of mesenchymal to epithelial transition.2 As WT-1 is also located on 11p it can be hypothesized that the epigenetic changes that induce BWS can also affect the gene locus. However, this remains supposition as the cellular origin of NSETs and pathophysiology remain unknown.
NSETs are most frequently diagnosed as incidental findings from abdominal imaging. Patients typically describe nonspecific abdominal pain and symptoms related to Cushing syndrome.8,9 When present, the Cushing symptoms are caused by the ectopic production of adrenocorticotropic hormone’ by the tumor.8 The neoplasm has most frequently followed an indolent course and does not tend to recur after excision. Metastatic potential is difficult to assess due to the rarity of NSETs, but there is one documented case of an NSET metastasizing to the lungs.9
NSETs appear as well-circumscribed, lobulated masses on gross examination. They tend to calcify and are referred to as calcifying NSETs (CNSETs) by multiple sources. Magnetic resonance imaging shows a well-circumscribed lesion that is hypointense on T1 imaging and hyperintense on T2 imaging. Surgical resection has been utilized as the preferred method for treatment; for larger tumors partial hepatectomies and liver transplants have been documented.9 Our patient underwent a successful microwave ablation of the NSET and follow-ups with oncology for surveillance as necessary.
Conclusion
NSETs are rare tumors of unknown histologic origin that occur in the liver. There is an association with BWS, but the degree of causality is unknown.
References
Citation
DD P, RB T, AJ T.Nested Stromal Epithelial Tumor. Appl Radiol. 2023; (2):36-38.
March 7, 2023