NeoNavia Pulse Biopsy System Receives FDA Clearance
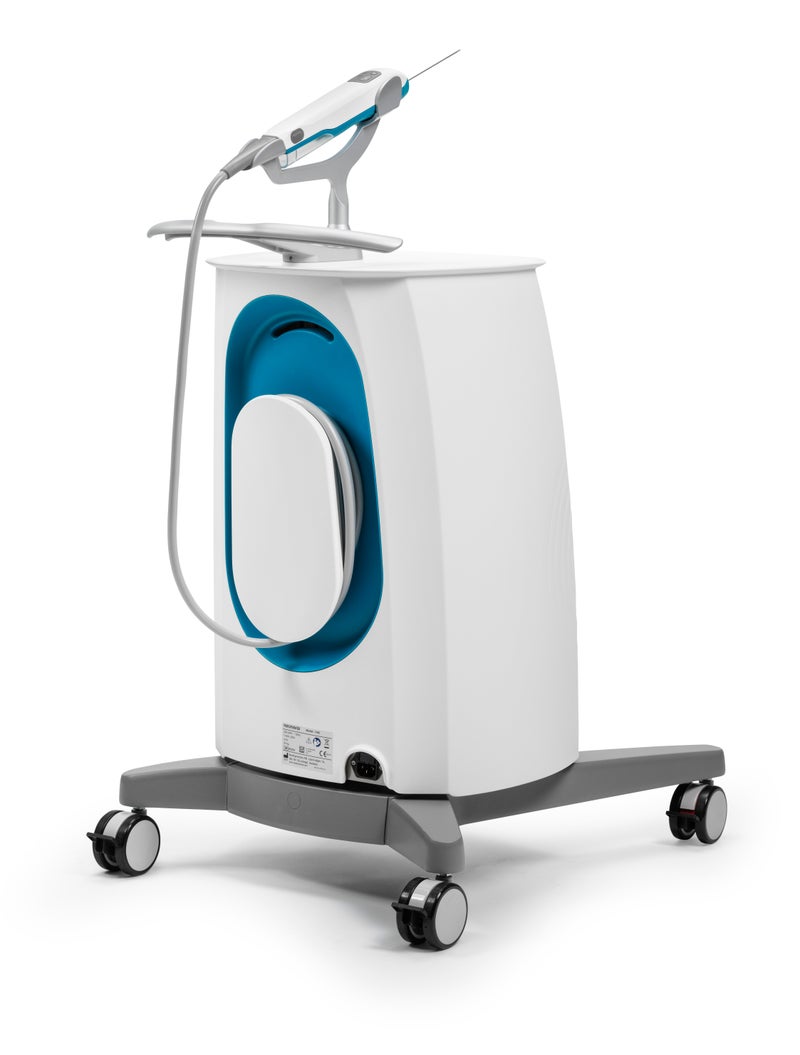 NeoDynamics has received US FDA 510(k) clearance for its pulse biopsy system NeoNavia.
NeoDynamics has received US FDA 510(k) clearance for its pulse biopsy system NeoNavia.
NeoNavia is a breast biopsy system that uses a patented pulse technology for controlled and accurate needle insertion, which is based on research at Karolinska Institutet. It consists of a base unit, a handheld driver and three different types of biopsy needles. Each needle type is driven by the pulse technology providing a more controlled needle insertion and precise placement of the needle in the tumour whilst enabling high-quality tissue samples from both breasts and lymph nodes. The pulse biopsy system NeoNavia is designed to offer clinicians and patients accurate lesion targeting and high tissue yield for correct diagnosis and individualized treatment.
“The FDA approval for NeoNavia is a major milestone for NeoDynamics and a stamp of quality for both the product and NeoDynamics as a whole," said Anna Eriksrud, CEO of NeoDynamics. "The US represents the potentially largest market for the product, where we now also can build on the experience gained with the system in Europe. Preparation for the launch is ongoing and we expect to begin introducing the system to clinicians and potential partners within the next few months."
NeoDynamics intends to work with US clinics to further document the product in clinical practice to support its marketing and sales strategy. A similar strategy is successfully being executed on in Europe, demonstrating the benefits of the product while forging relationships with important clinics to facilitate a broad acceptance and uptake of the system.
The product also has CE approval in Europe, where it is in clinical use.