Neonatal Adrenal Hemorrhage
Images

Case Summary
A newborn with tracheoesophageal fistula, imperforate anus, and tethered cord underwent abdominal ultrasound to evaluate the solid organs for further anomalies.
Imaging Findings
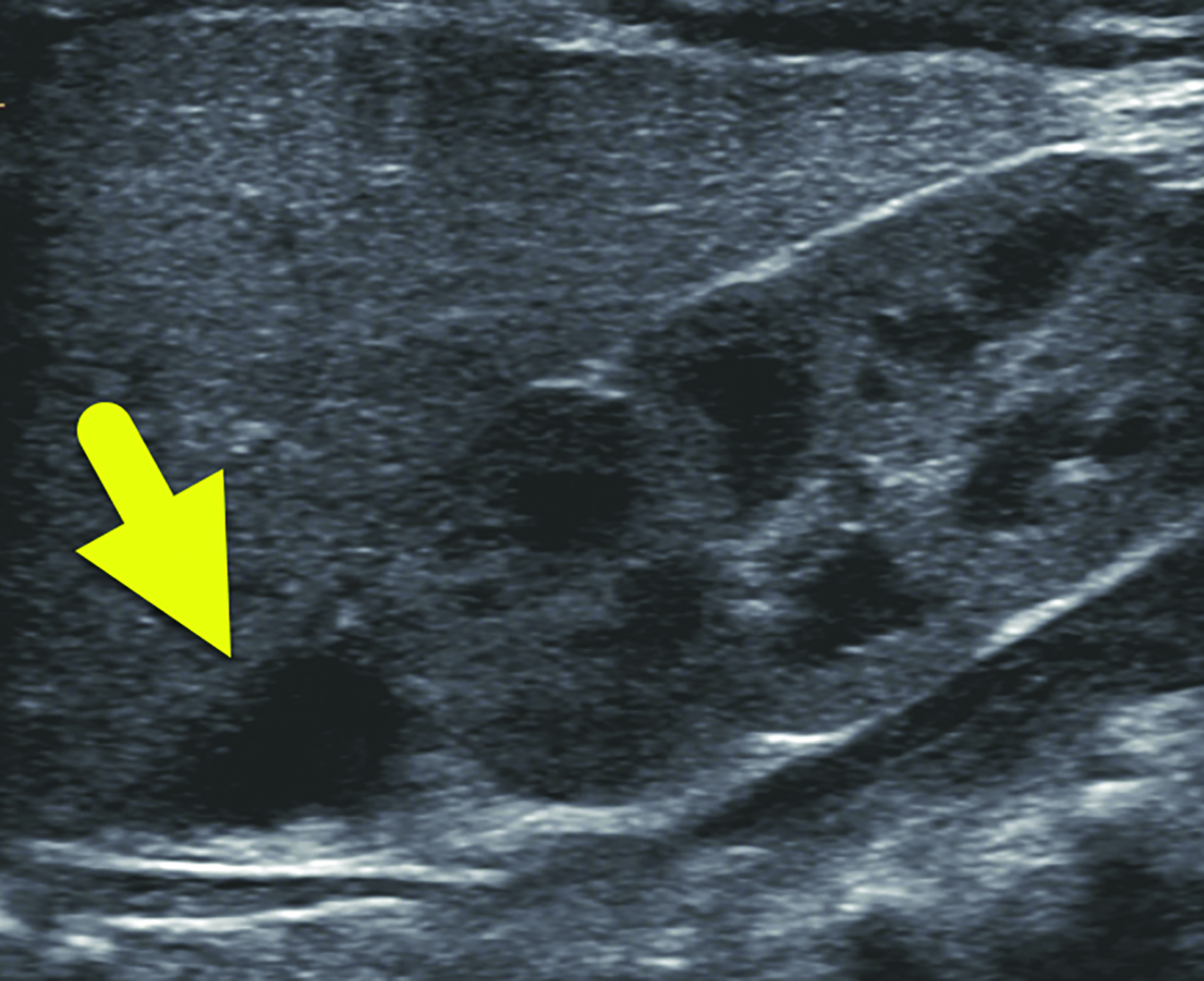
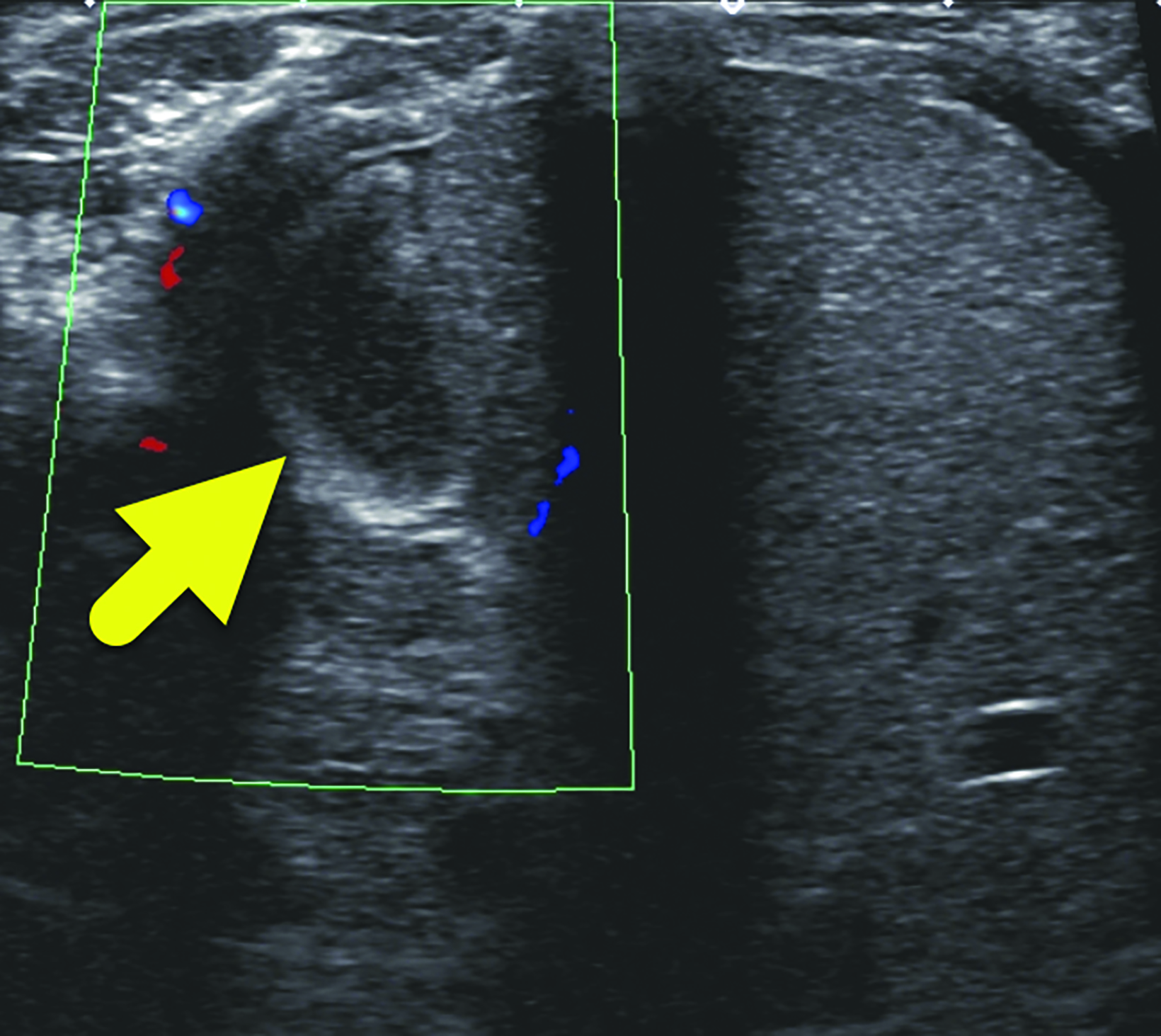
Ultrasound (Figure 1) showed a 1.2 cm asymptomatic hypoechoic mass within the right adrenal gland. The lesion had no internal color Doppler flow, and it was not present on earlier ultrasound. It decreased in size on subsequent ultrasound (Figure 2) before later resolving. Abdominal ultrasounds were repeated at two weeks, one month, and four months. Throughout this time, the mass decreased in size, remained adreniform in configuration, and appeared hypoechoic to anechoic, consistent with the suspected diagnosis of adrenal hemorrhage.
Diagnosis
Adrenal hemorrhage.
The differential diagnosis for a suprarenal mass in a neonate includes neuroblastoma, mesoblastic nephroma, and subdiaphragmatic extralobar pulmonary sequestration1 risk factors and clinical presentations of neonatal adrenal haemorrhage (NAH
Discussion
Adrenal hemorrhage is a relatively uncommon condition in neonates, occurring in just 0.2-0.55% of live births.1 The vascular architecture of the adrenal gland is unique and particularly vulnerable to arteriolar rupture and bleeding. Three arteries supply the gland, dividing into fifty to sixty small branches, forming a subcapsular plexus. A relatively small number of venules drain the subcapsular plexus. This intrinsically vulnerable network, termed a vascular dam, is sensitive to vasoconstriction from catecholamines released by the adrenal medulla resulting in increased intravascular pressure.2,3
Adrenal hemorrhage is more common on the right side (70%) of cases, with bilateral involvement occurring in 10% of cases. The greater incidence of right adrenal hemorrhage is thought to occur because the veins drain directly into the inferior vena cava.
This configuration makes the gland more susceptible to pressure changes, which ultimately lead to arteriolar rupture. Neonates are at risk for adrenal hemorrhage owing to the relatively large size of their adrenal glands and their resultant increased vascularity.4 Other factors that increase the risk of hemorrhage in neonates include the hormone release during the antenatal period and changing pressures as the neonate passes through the vaginal canal.5
Though some risk factors for adrenal hemorrhage have been reported, the etiology of bleeding in many cases remains unknown. As previously mentioned, physiologic stress predisposes neonates to adrenal hemorrhage. Thus, acidemia, asphyxia, septicemia, prolonged labor, hypotension, and difficult delivery all increase the risk of adrenal hemorrhage.5 Additionally, male gender and macrosomia have been identified as risk factors, likely owing to increased size of the newborn and potential for stress during delivery.6
The presentation of neonatal adrenal hemorrhage is variable and nonspecific. Many neonates are asymptomatic. The most common presenting symptom in neonatal adrenal hemorrhage is indirect hyperbilirubinemia, resulting in jaundice. Other common features include anemia, pallor, flank mass, and lethargy/hypotonia.1 Scrotal discoloration has been reported, with an incidence of about 0.2%, and occurs either by dissection along the tissue planes of the retroperitoneum outside the processus vaginalis or by rupture of the posterior peritoneum, resulting in intraperitoneal hemorrhage and descending within the patent processes vaginalis.5 As the adrenal gland has significant regenerative capacity, hemorrhage is typically not associated with adrenal insufficiency.4
Abdominal ultrasound generally serves as the initial diagnostic modality. Findings vary with the age of the hemorrhage. Initially the adrenal hemorrhage appears solid and hyperechoic. As the clot lyses, the echogenicity becomes mixed with a central echogenic region that becomes cystic in appearance. Calcification can develop as early as 1-2 weeks after onset of the bleeding. The hemorrhage usually resolves by about 2 months of age. Calcifications remain visible on radiograph and CT. The conspicuity of the calcification on radiographs likely decreases as the child grows into adulthood.
In the neonatal period, distinguishing neuroblastoma from adrenal hemorrhage is important. Imaging is not always conclusive, since neuroblastoma can also present as a solid, cystic, or mixed lesion exerting mass effect on the upper pole of the kidney. Doppler ultrasound can help distinguish the lesions. Neonatal adrenal hemorrhage is often hypovascular or has no blood flow, while neuroblastoma may have increased blood flow. The shape of the lesions may also help to distinguish neuroblastoma from hemorrhage. Neuroblastoma often appears mass-like, while the adrenal gland may maintain its triangular shape with hemorrhage.
There is no treatment for neonatal adrenal hemorrhage, as most cases spontaneously resolve. Standard practice is watchful waiting with serial sonography performed at follow-up to check for regression of the lesion. If the lesion does not regress as expected or increases in size, it may be biopsied to exclude malignancy.8 Alternatively, neuroblastoma could be confirmed with MIBG scan or the presence of urine or serum catecholamine levels.
Conclusion
Ultrasound remains the primary modality for assessing and following neonates with suspected adrenal hemorrhage. Regression of the adrenal mass and absence of vascular flow on color Doppler are the main differentiating features of neonatal adrenal hemorrhage from neuroblastoma. Though the symptoms of neonatal adrenal hemorrhage are variable and non-specific, the index of suspicion should increase when a neonate presents with unexplained jaundice, anemia, or hypotonia.
References
- Mutlu M, Karagüzel G, Aslan Y, Cansu A, Ökten A. Adrenal hemorrhage in newborns: A retrospective study. World J Pediatr. 2011;7(4):355-357. doi:10.1007/ s12519-011-0259-7
- Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. Published online 1999. doi:10.1148/ radiographics.19.4.g99jl13949
- Jordan E, Poder L, Courtier J, Sai V, Jung A, Coakley F V. Imaging of nontraumatic adrenal hemorrhage. Am J Roentgenol. Published online 2012. doi:10.2214/AJR.11.7973
- Toti MS, Ghirri P, Bartoli A, et al. Adrenal hemorrhage in newborn: How, when and why- from case report to literature review. Ital J Pediatr. 2019;45(1):1-8. doi:10.1186/s13052-019-0651-9
- Roupakias S, Papoutsakis M, Mitsakou P. Blunt adrenal gland trauma in the pediatric population. Asian J Surg. Published online 2011. doi:10.1016/j.as-jsur.2011.08.003
- Gyurkovits Z, Maróti Á, Rénes L, Németh G, Pál A, Orvos H. Adrenal haemorrhage in term neonates: A retrospective study from the period 2001-2013. J Matern Neonatal Med. Published online 2015. doi:10.3109/14767058.2014.976550
- Dorai CRT, Smith AJ, Dewan PA. Adrenal haemorrhage: Presenting as acute scrotal swelling in a neonate. J Paediatr Child Health. Published online 1994. doi:10.1111/j.1440-1754.1994.tb00571.x
- Wang CH, Chen SJ, Yang LY, Tang R Bin. Neonatal adrenal hemorrhage presenting as a multiloculated cystic mass. J Chinese Med Assoc. Published online 2008. doi:10.1016/S1726-4901(08)70153-9
Citation
H T, RB T, CM S, AJ T.Neonatal Adrenal Hemorrhage. Appl Radiol. 2024; (1):48a-48c.
January 24, 2024