Mucocutaneous Leishmaniasis
Case Summary
A middle-aged adult presented with a 10-year history of HIV infection and 3-year history of upper respiratory tract leishmaniasis.
Nasolaryngoscopy demonstrated nasal septal perforation and thick yellow discharge along the larynx, base of the tongue, epiglottis, and pyriform sinuses, preventing evaluation of the glottis and subglottis.
Subsequent tongue biopsy demonstrated leishmania amastigotes. The patient improved with liposomal amphotericin treatment.
Imaging Findings
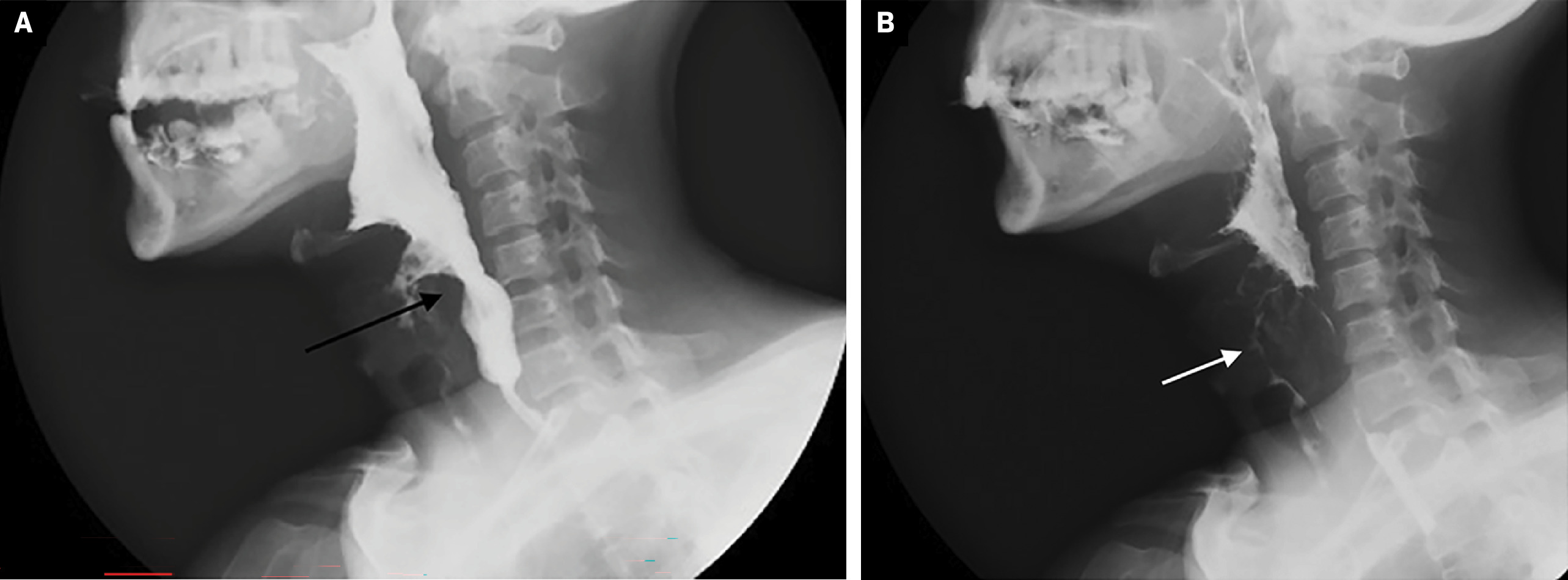
A barium swallow study demonstrated a lesion originating in the larynx and indenting the anterior hypopharyngeal contrast column, with pharyngeal contrast accumulation and delayed emptying, associated with aspiration of contrast into the airway and irregular oropharyngeal mucosa ( Figure 1 ).
Barium swallow (A) study shows a lesion originating in the larynx and indenting the anterior margin of the hypopharynx (arrow). Pharyngeal contrast (B) retention with delayed evacuation and aspiration of contrast into the airway (arrow), Note the irregular oropharyngeal mucosa.
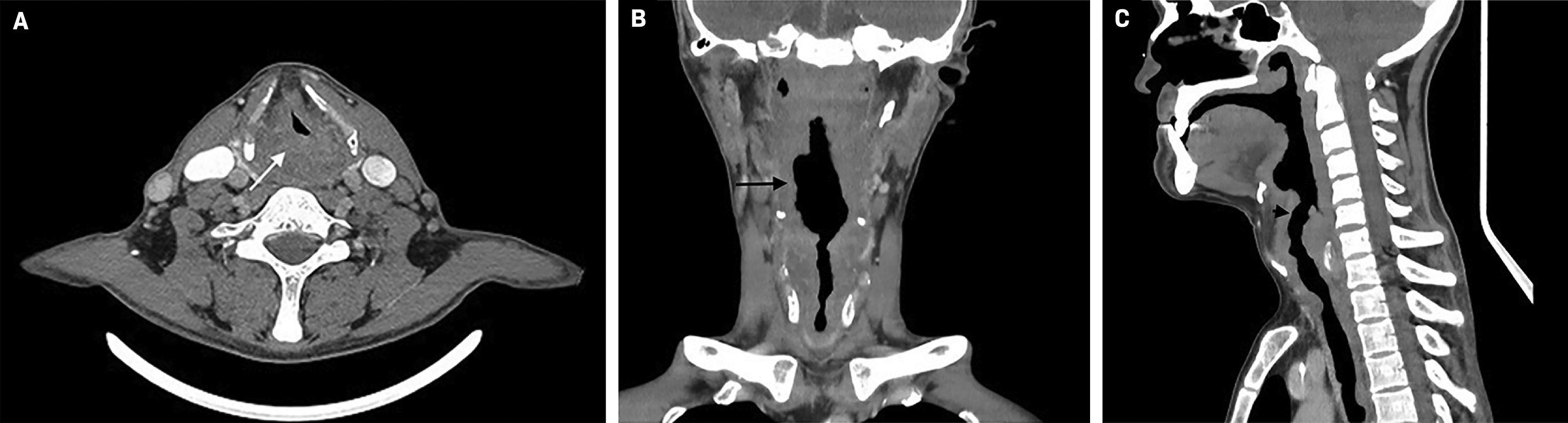
Contrast-enhanced neck CT demonstrated nonspecific thickening of the oropharyngeal, laryngeal, and tracheal mucosa. Left level V and right paratracheal lymphadenopathy were present ( Figure 2 ).
Contrast-enhanced CT of the neck in the axial plane ( A ) shows soft-tissue mass at the hypopharyngeal level (white arrow). The mass extends asymmetrically, to the proximal aspect of the larynx, causing a decrease in its lumen of more than 60%. Coronal ( B ) and sagittal ( C ) plane reformations show nodularity of the mucosa of the oropharynx, larynx, and trachea (black arrow).
Diagnosis
Mucocutaneous leishmaniasis.
The differential diagnosis is tracheal tuberculosis.
Discussion
Mucocutaneous leishmaniasis is a parasitic infectious disease manifest by destruction of the oronasopharyngeal mucosa and cartilage, occasionally involving the larynx.1 It is characterized by destructive lesions of the nasal septum, lips, and palate and as a result of the strong immunopathological response by the host to the parasite.1 The disease is potentially life-threatening and may be highly disfiguring; laryngeal involvement leads to aspiration pneumonia.1 Orofacial disease may include nasal obstruction, dysphagia, mucosal bleeding, and/or hoarseness.2 The disease is thought to be more common in immunocompromised individuals.1
Leishmania protozoans belong to the family Trypanosomatidae and can be present in 2 forms: flagellated, or promastigote, found primarily in the digestive tract of the insect vector; and aflagellated, or amastigote, found in the tissues of vertebrate hosts.3
Observation of amastigotes in a specimen establishes the diagnosis, but combination tissue sampling approaches are recommended.1
It is estimated that approximately 0.7 to 1 million cases of cutaneous leishmaniasis occur worldwide each year.1 In the Americas, multiple Leishmania species are circulating in the same geographical areas, multiple reservoir hosts, and multiple vectors. The pathogenic species in the Americas belongs to Leishmania mexicana or Leishmania braziliensis . About 1% to 10% of patients infected with a strain of the subgenus Viannia develop mucocutaneous leishmaniasis.1
Most cutaneous leishmaniasis lesions heal spontaneously in 2 to 18 months.1 Systemic treatment is usually reserved for immunosuppressed patients, extensive lesions, refractory disease, and mucocutaneous manifestations.1
Manifestations of the disease on CT are variable and nonspecific. In a study by Camargo et al, the most common findings were paranasal sinus thickening, nasal perforation, nasal conchal changes, maxillary sinus retention cysts, and collapse of the nasal pyramid.4
In another case report from Italy, a senior immunocompetent patient presented with a diffuse annular increase in tracheal wall thickness and mild homogeneous enhancement.5 In a multicenter case series of 7 patients with oral leishmaniasis, the most common site affected was the tongue and the most common clinical presentation was an exophytic lesion.6
References
Citation
Brito-Araujo JD, Aluja-Jaramillo F, MHPE. Mucocutaneous Leishmaniasis. Appl Radiol. 2024; (6):42 - 43.
doi:10.37549/AR-D-24-0048
December 1, 2024