MRI Screening for Hepatocellular Carcinoma
Images
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Primary liver cancer is the fifth- leading cause of cancer-related death in men and seventh-leading cause of cancer-related death in women in the U.S.1 Worldwide, the most common risk factors for hepatocellular carcinoma (HCC) are chronic hepatitis B infection (44%) and chronic hepatitis C infection (21%).2 In North America, the fraction of cases attributable to major HCC risk factors are alcohol (32%), obesity (24%), and chronic hepatitis C infection (17%).2 All patients with cirrhosis are at risk for HCC. Patients with smaller tumors can undergo potentially curative surgery, including liver transplantation, whereas patients with larger, unresectable tumors or extrahepatic tumor spread have a poor prognosis.
Role of Screening
Surveillance is associated with earlier HCC detection, higher rates of cure, and higher overall survival in patients with cirrhosis.3,4 According to the 2018 guidance document issued by the American Association for the Study of Liver Diseases (AASLD), surveillance ultrasound imaging should be performed every 6 months.5 Surveillance should be offered to cirrhosis patients when risk of HCC is ≥1.5%/year, and to hepatitis B carriers without cirrhosis when HCC risk is ≥0.2%/year.5 Hepatitis B carriers are at risk of developing HCC even without the presence of cirrhosis. Therefore, surveillance is recommended for Asian male hepatitis B carriers over age 40, Asian female carriers over age 50, African and African-Americans with hepatitis B, and hepatitis B carriers with a family history of HCC.5 Although the risk of HCC is reduced in hepatitis C patients who experience a sustained virologic response with newer antiviral therapy agents, those patients with cirrhosis remain at risk for HCC and should continue imaging surveillance.5
According to the AASLD, 6-month imaging surveillance can be performed with or without monitoring of serum alpha-fetoprotein (AFP) levels.5 A serum AFP level > 20 ng/mL is considered positive and has a sensitivity of approximately 60% and a specificity of approximately 90% for HCC.5
Computed tomography (CT) or magnetic resonance (MR) imaging is recommended where a nondiagnostic ultrasound is highly likely, such as in obese patients. In addition, follow-up imaging with multiphase abdominal CT or MRI should be pursued when surveillance ultrasound is positive for a ≥ 10 mm lesion or AFP is > 20 ng/mL.5
Liver Transplant and HCC
Tumor Size Criteria for Transplant
Most HCCs develop in patients with underlying cirrhosis.6 In such patients, tumor resection is typically not possible, as the patient’s remnant liver would be too poorly functioning to be life sustaining. A landmark 1996 publication by Mazzaferro et al of the National Cancer Institute in Milan, Italy, reported 83% recurrence free survival at 4 years for patients with unresectable “small” HCCs, specifically one tumor ≤ 5 cm in diameter or up to three tumors ≤ 3 cm without extrahepatic involvement or macrovascular invasion.7 These thresholds are now known as the Milan criteria. Larger tumors can potentially be downstaged to within Milan criteria using locoregional therapy. Other classification systems also are utilized, such as the University of California San Francisco criteria (one lesion 5 - 6.5 cm in diameter, or up to three lesions, each measuring ≤ 4.5 cm with a total tumor diameter of ≤ 8 cm).8
Transplant Priority
Donor livers are a scare resource. Approximately 8,000 liver transplants were performed in the United States in 2018 while more than 13,000 candidates were on the liver transplant wait list.9 Eligibility for the transplant list relies on the judgement of a multidisciplinary committee, typically consisting of hepatologists, transplant surgeons, coordinators, and social workers who meet regularly to discuss patient candidacy. Factors typically considered include the patient’s overall health status and nonhepatic comorbidities, surgical complexity, recent or active substance use, and psychosocial barriers. Transplant livers are allocated according to the Model of End Stage Liver Disease (MELD) score, which is based upon serum bilirubin, International Normalized Ratio (INR), sodium, and creatinine values.10 Donor livers go to patients with the highest MELD score; these patients are usually gravely ill.
Based on MELD scores, many patients with cirrhosis and HCC who fall within the Milan criteria would not qualify for a donor liver; these patients may then become eligible for “MELD exception points” to better reflect their risk of poor outcomes from HCC and their urgency for transplant. MELD exception points permit access to transplantation that would otherwise not be available for HCC patients with preserved liver function. Based on the most current policy created by the United Network for Organ Sharing (UNOS), HCC patients who meet the Milan criteria are initially listed for transplant with their laboratory MELD score. After a 6-month waiting period, they are awarded their MELD exception score, which is 3 points lower than the median patient MELD score at transplant in the local distribution area during the previous 365 days .11
Diagnostic Criteria
Imaging vs Biopsy
Hepatocellular carcinoma is one of the few malignancies that can be diagnosed based on imaging without requiring tissue confirmation. MRI with optimized techniques has high (> 90%) diagnostic sensitivity and specificity for HCCs ≥ 2 cm.12 When imaging is conclusive, treatment (eg, liver transplant for cirrhotic patients within the Milan criteria or locoregional therapy for patients outside the criteria) proceeds without biopsy, owing to the potential risk, albeit low (0 - 0.13%), for track seeding with biopsy.13,14 Cirrhotic patients with liver masses not definitively diagnosed as HCC at imaging should undergo biopsy, as risks associated with inappropriate treatment (eg, liver transplant and lifelong immunosuppresion for lesions other than HCC) outweigh those of biopsy track seeding. It is also important to note that a diagnosis of HCC cannot be made with noninvasive imaging alone in the absence of cirrhosis, except in the setting of hepatitis B. Biopsy is typically required in such cases; benign hyperplastic nodules can have imaging features similar to HCC in non-cirrhotic, non-hepatitis B livers.5
Imaging Characteristics
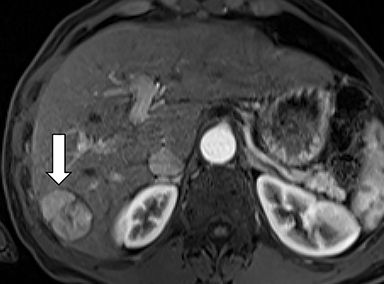
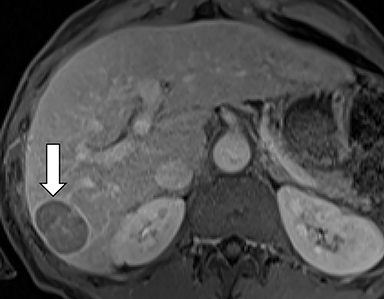
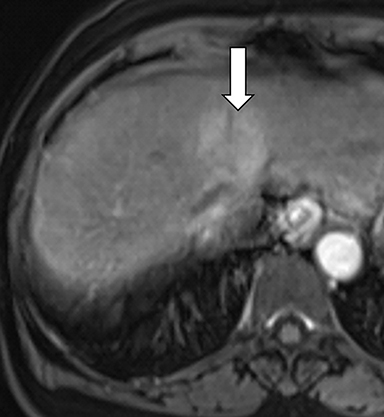
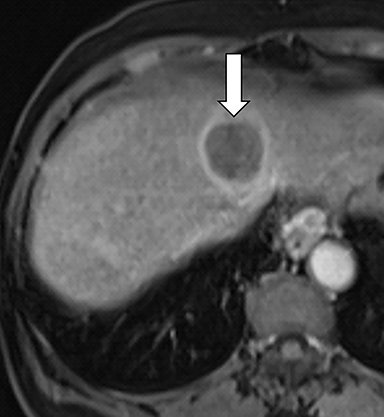
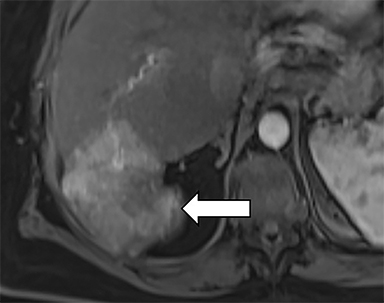
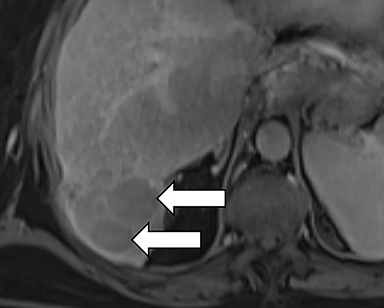
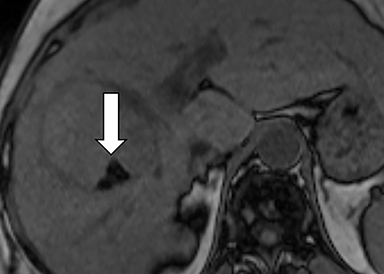
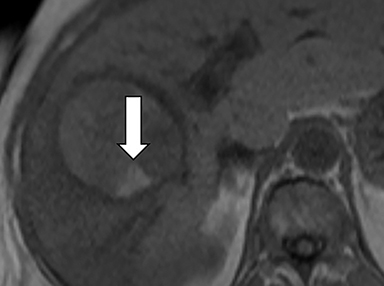
As imaging plays a critical role in diagnosing and managing HCC cases, accurate image interpretation is paramount. Diagnostic criteria have been established by the Organ Procurement and Transplantation Network (OPTN) and by the American College of Radiology-sponsored Liver Imaging Reporting And Data System (LI-RADS) group.15,16 Both sets of criteria consider suspicious features to be arterial phase hyperenhancement, washout in the latter phases of imaging, the presence of a capsule or pseudocapsule around the lesion, and interval growth (Figures 1,2).
Neoangiogenesis is thought to be responsible for HCC hyperenhancement in the arterial phase.17 This type of hyperenhancement is defined as non-rim enhancement of the lesion (or “observation,” if using LI-RADS terminology) that exceeds the signal intensity of the surrounding liver parenchyma (Figures 1,2).17 Arterial phase hyperenhancement has a sensitivity of 65-98%, specificity of 62-97%, positive predictive value of 67-99%, and negative predictive value of 54-89% for HCC.17
Washout is defined as decreased enhancement of a liver lesion between the earlier and later phases and lower signal intensity in the lesion than in the background liver parenchyma (Figures 1,2).17 The later phases of contrast enhancement should be the portal venous and delayed phases when using extracellular contrast agents, and the portal venous, transitional, and hepatobiliary phases when administering gadoxetate disodium.17 Washout has 50-79% sensitivity, 62-100% specificity, 55-100% positive predictive value, and 49-83% negative predictive value for HCC.17
A capsule appearance is defined as an enhancing rim that surrounds a lesion, owing either to a true capsule or compressed liver parenchyma (ie, pseudocapsule) (Figures 1,2).17 A capsule has 42-64% sensitivity, 86-96% specificity, 89-96% positive predictive value, and 47-87% negative predictive value for HCC.17
More than one suspicious feature is required to diagnose HCC noninvasively with imaging; the number required declines as lesion size increases. To be diagnosed as HCC based on OPTN criteria, for example, lesions ≥ 1 cm and < 2 cm must demonstrate late hepatic arterial phase hyperenhancement, washout, and a capsule or pseudocapsule; or demonstrate late hepatic arterial phase hyperenhancement and growth by ≥ 50% documented with serial MR images ≤ 6 months apart.15 Also under OPTN criteria, a lesion with a maximum diameter ≥ 2 cm and ≤ 5 cm can be diagnosed as HCC if it demonstrates late hepatic arterial phase hyperenhancement and washout or a capsule or pseudocapsule or growth by ≥ 50% documented on serial MR images ≤ 6 months apart.15 Lesions with a maximum diameter > 5 cm, late arterial phase hyperenhancement and washout or a capsule or pseudocapsule are diagnostic of HCC also under OPTN criteria.15 The assignment of LI-RADS categories is similarly based on lesion size, presence or absence of non-rim arterial phase hyperenhancement, number of major features (eg, enhancing capsule, nonperipheral washout, threshold growth), and presence or absence of ancillary features.
OPTN vs LI-RADS
There are differences in the OPTN and LI-RADS criteria. For example, the OPTN criteria are generally binary (eg, HCC yes or no) and specifically characterize HCC for liver transplant priority assignment. By comparison, the LI-RADS system classifies observations on a sliding scale consisting of LR-5 (definitely HCC), LR-4 (probably HCC), LR-3 (intermediate probability of malignancy), LR-2 (probably benign), LR-1 (definitely benign), LR-M (probably or definitely malignant but not HCC specific), and LR-TIV (tumor in vein).14 This classification is based on the presence or absence of major features and ancillary features and the use of a table to assign a final LI-RADS category.
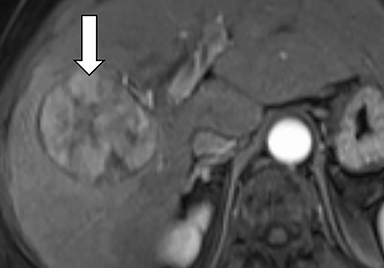
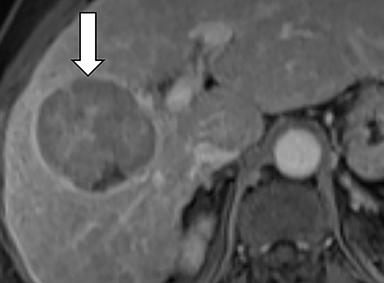
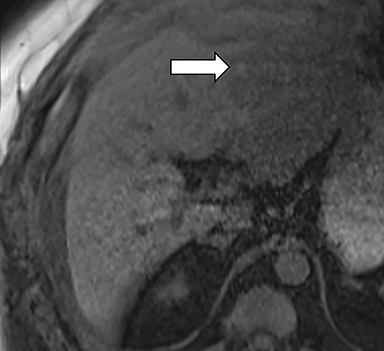
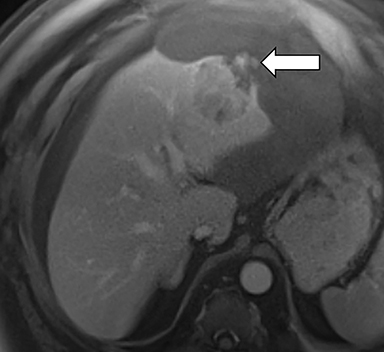
Ancillary features are divided into three categories: those favoring HCC, including nodule-in-nodule architecture (Figure 3), fat located within the mass more than in adjacent liver (Figure 4), and blood in mass (Figure 5); those favoring malignancy not specific for HCC, including restricted diffusion; and features favoring benignity, including size stability ≥2 years, size reduction, greater iron level in the lesion than in background liver, and marked T2-hyperintensity.16
Physician practices using OPTN or LI-RADS criteria are encouraged to remain up-to-date on the most recent version of each diagnostic criteria. The LI-RADS criteria, for example, are updated approximately every 3-4 years. Those practices contemplating a transition to LI-RADS are encouraged to read the current version of the LI-RADS core document, which is freely available on the American College of Radiology website.
The next steps in patient management vary based on LI-RADS category. For example, an LR-5 lesion is assumed to be an HCC without requiring a biopsy. By comparison, an LR-4 lesion should undergo multidisciplinary discussion, and potentially a biopsy, to establish a diagnosis.14 In a retrospective review of 181 patients with 146 HCCs, 94.4% of observations categorized as LR-5 were found to be HCC at final pathology; 47.6% of observations categorized as LR-4 were HCC; 25% of observations categorized as LR-3 were HCC; and no observations categorized as LR-1 or 2 were HCC.18
Care must be taken not to confuse OPTN and LI-RADS in the setting of possible liver transplant because OPTN is exclusively used for determining candidacy for HCC MELD exceptions. It is also possible for LR-5 lesions not to meet OPTN criteria (eg, 10-19 mm observations with non-rim arterial phase hyperenhancement and non-rim washout, but without enhancing capsule or threshold growth), and effectively treating a lesion prior to demonstration of OPTN criteria surrenders candidacy for HCC MELD exceptions.16
MRI Technique
Imaging is the primary screening tool for HCC, and accurate description of tumor size in a cirrhotic liver determines a patient’s eligibility for liver transplant. Therefore, high-quality MR images are paramount. Images should be acquired with ≥ 1.5 T field strength using a surface coil.15,16 Required MR sequences according to both the 2011 OPTN/UNOS policy for liver transplant allocation and LI-RADS version 2018 consist of pre- and postcontrast T1 images in the late arterial, portal venous, and delayed phases, as well as T2 images, and T1 in- and opposed-phase images.15,16 LI-RADS version 2018 also suggests subtraction and diffusion weighted imaging.16 Section thickness should be ≤ 5 mm for dynamic imaging and ≤ 8 mm for other sequences.15
The development of abbreviated MR protocols for HCC screening is an area of active research, as these could result in cost-savings, although the ideal abbreviated protocol is a source of debate in the imaging community; some groups advocate coronal T2 images and axial dynamic contrast-enhanced T1 fat-suppressed sequences,19 while others prefer T1pre-and postcontrast imaging alone or diffusion weighted images combined with T1images obtained during the hepatobiliary phase of contrast enhancement.20,21
Summary
MR screening is an established technique for early detection of hepatocellular carcinoma, a unique tumor that can be diagnosed non-invasively based on MR features using established diagnostic criteria. Early detection with MRI, in turn, improves HCC patient outcomes by identifying potential candidates for curative liver transplant.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7-34.
- Baecker A, Liu X, La Vecchia C, Zhang Z-F. Worldwide incident hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev 2018;27(3):205-212.
- Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4): e1001624
- Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099-1106.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases Hepatology. 2018;68(2):723-750.
- Greten TF, Papendorf F, Bleck JS, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92(10):1862-1868.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
- Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8(10):1982-1989.
- National Data Organ Procurement and Transplantation Network. U.S. Department of Health & Human Services. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ Accessed August 19, 2019.
- Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33(2):464-470.
- Organ Procurement and Transplantation Network. Policies. U.S. Department of Health & Human Services. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf Accessed September 7, 2019.
- Becker-Weidman DJS, Kalb B, Sharma P, et al. Hepatocellular carcinoma lesion characterization: single-institution clinical performance review of multiphase gadolinium-enhanced MR imaging-comparison to prior same-center results after MR systems improvements. Radiology. 2011;261(3):824-833.
- Szpakowski J-L, Drasin TE, Lyon LL. Rate of seeding with biopsies and ablations of hepatocellular carcinoma: A retrospective cohort study. Hepatol Commun. 2017;1(9):841-851.
- Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA, Francis IR. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006;187(5):1184-1187.
- Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266(2):376-382.
- LI-RADS Steering Committee. CT/MRI LI-RADS®v2018 CORE. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?la=en Accessed August 28, 2019.
- Tang A, Bashir MR, Corwin MT, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology. 2018;286(1):29-48.
- Ren A-H, Zhao P-F, Yang D-W, et al. Diagnostic performance of MR for hepatocellular carcinoma based on LI-RADS v2018, compared with v2017. J Magn Reson Imaging 2019;50(3):746-755.
- Khatri G, Pedrosa I, Ananthakrishnan L, et al. Abbreviated-protocol screening MRI vs. complete protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS 2018 J Magn Reson Imaging. 2019 June 18 doi: 10.1002/jmri.26835. [Epub ahead of print]
- Lee JY, Huo EJ, Weinstein S, et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol. 2018;43(7):1627-1633.
- Besa C, Lewis S, Pandharipande PV, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol. 2017;42(1):179-190.
Citation
CC M, TP H, JP W,. MRI Screening for Hepatocellular Carcinoma. Appl Radiol. 2020;(4):9-15.
June 30, 2020