MRI Contrast Selection: Greater Stability or Higher Relaxivity?
Images
Foreword
Gadolinium-based contrast agents (GBCAs) have been used for decades to enhance the diagnostic performance of magnetic resonance imaging (MRI). Unfortunately, the discovery that gadolinium (Gd) can be retained in the brain and bodily tissues in patients who have been administered GBCAs has led to widespread concern over their safety. Although no clinical sequelae resulting from Gd retention have been reported, GBCA guidelines recommend taking measures to reduce patient Gd exposure during MRI examinations. However, while reducing Gd exposure may decrease the levels of retained Gd, it may also lead to less-than-optimal contrast enhancement and thus sub-optimal diagnostic performance.
GBCA relaxivity and stability are the key properties to consider when selecting a contrast agent for MRI, and achieving the correct balance between high-quality images and low Gd exposure is a primary aim of radiologists. To this end, risk-benefit analyses should be tailored to each patient. For example, more stable macrocyclic GBCAs may be better suited to younger patients or those requiring serial MRI examinations, whereas higher-relaxivity agents may be more appropriate in cases where better diagnostic performance has the potential to impact the diagnosis, prognosis, and/or treatment plan.
Ideally, an MR contrast agent would possess both high relaxivity and high stability, rendering moot the need to choose between these two properties for a given MRI application.1 Such an agent would potentially permit a reduction in contrast dose, resulting in lower Gd exposure, without sacrificing image quality or diagnostic performance.
Recently, a highly stable, high-relaxivity GBCA, gadopiclenol (Vueway™; Bracco Diagnostics Inc.),2 was approved by the U.S. Food and Drug Administration (FDA) for a wide range of MR imaging applications at reduced dose. However, while gadopiclenol may fulfill many, if not all, of the requirements of an ideal MR contrast agent, this agent has not entered clinical practice as of this writing (October 2022), and radiologists must still choose between high-relaxivity and high-stability agents.
This monograph summarizes the presentations, conclusions, and recommendations of a panel of experts who reviewed the safety, efficacy, and use of GBCAs for several MRI applications. Much of the discussion focuses on identifying situations more appropriate for GBCAs with greater stability versus those for which higher relaxivity is of greater importance. The insights and information presented here are intended to help radiologists make informed decisions with respect to selection of a GBCA in clinical practice.
Howard Rowley, MD, FACR
Joseph F. Sackett Endowed Professor of Radiology
Professor of Radiology, Neurology, and Neurosurgery
University of Wisconsin School of Medicine and Public Health
- Lancelot E, Raynaud JS, Desché P. Current and Future MR Contrast Agents: Seeking a Better Chemical Stability and Relaxivity for Optimal Safety and Efficacy. Invest Radiol. 2020;55:578-588.
- Robic C, Port M, Rousseaux O, et al, Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium Chelate With High T1 Relaxivity. Invest Radiol. 2019;54:475-484.
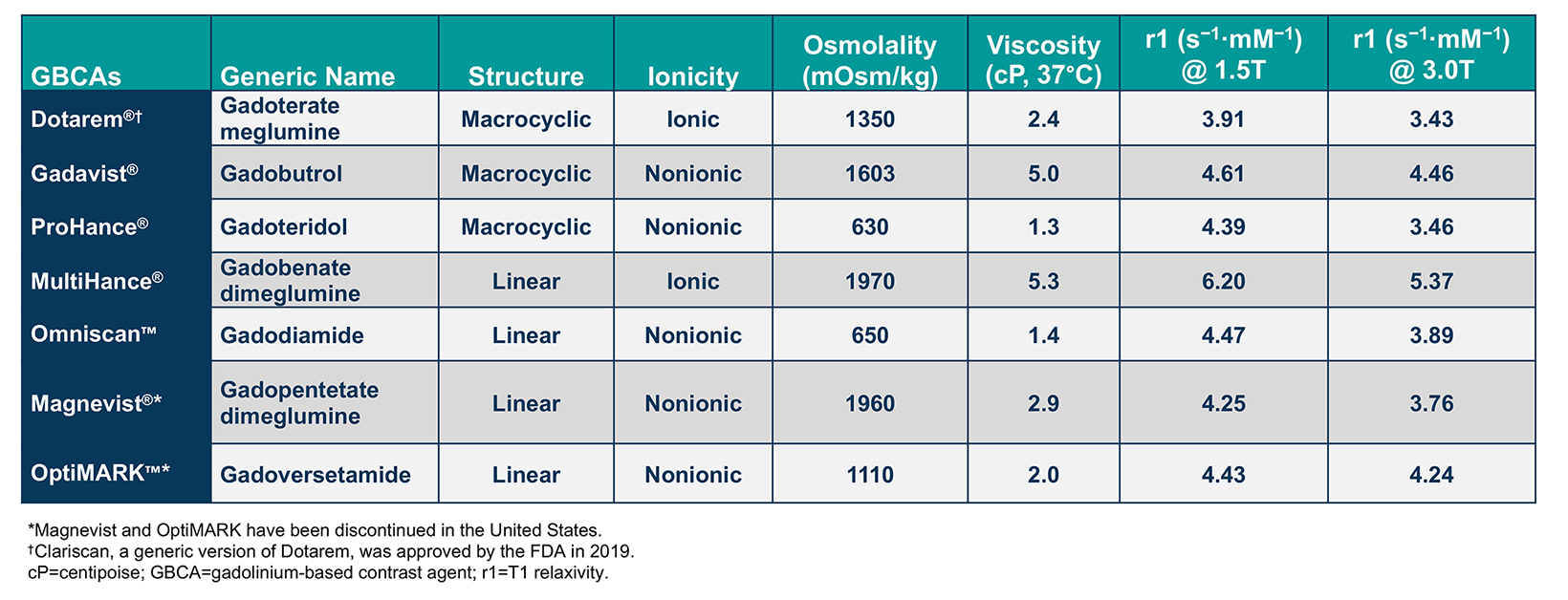
Gadolinium-based contrast agents (GBCAs) are frequently administered to improve the sensitivity and/or specificity of diagnostic magnetic resonance imaging (MRI). With more than 450 million doses administered worldwide over the past 4 decades, GBCAs have a proven record of both safety and efficacy.1 Currently, 6 general-use GBCAs are available and approved for a variety of MRI indications, including brain, body, and breast imaging in adults and children. (Table 1) These agents are the macrocyclic agents Dotarem® (gadoterate meglumine); Gadavist® (gadobutrol); and ProHance® (gadoteridol); the high-relaxivity linear agent MultiHance® (gadobenate dimeglumine); and the standard-relaxivity linear agent Omniscan™ (gadodiamide).2-6 In addition, Clariscan™ (gadoterate meglumine), a generic version of Dotarem, has recently become available.9 The standard-relaxivity linear agents, Magnevist (gadopentetate dimeglumine) and OptiMARK (gadoversetamide), have been discontinued.7-8
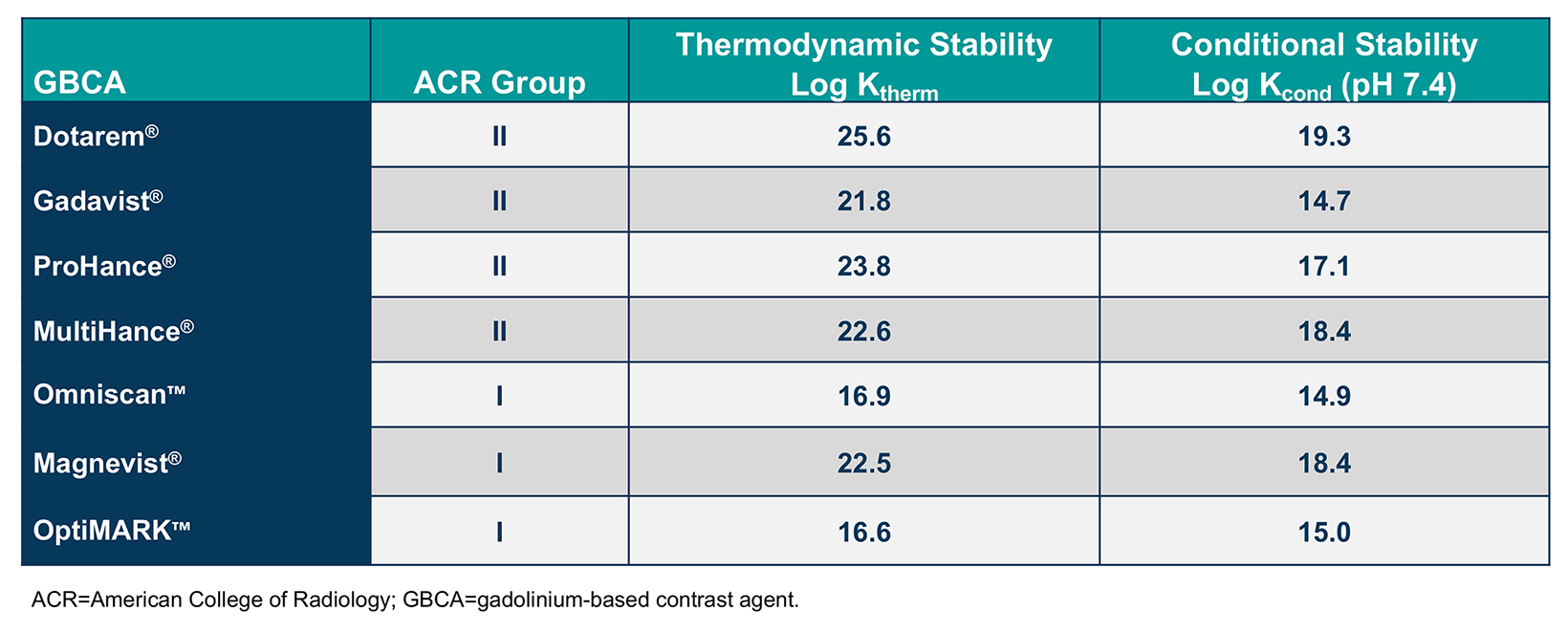
Gadolinium-based contrast agents comprise a gadolinium (Gd) ion bound to an organic ligand to form a chelate. The structure of each chelate imparts unique characteristics to that GBCA. Specifically, whether the chelate is linear or macrocyclic and ionic or nonionic impacts the size and stability of the Gd-chelate complex, as well as its distribution and elimination. In general, macrocyclic agents are more stable than linear agents and, therefore, are less likely to dissociate and release free Gd.13-15 (Tables 1 and 2)
The chelate structure also impacts the relaxivity, or r value, of the GBCA, which dictates the ability to provide contrast relative to background &emdash; the higher the r1, the greater the signal intensity of the enhancing tissue or structure on T1-weighted images.16 (Table 1) This higher relaxivity has been demonstrated in dozens of studies to translate into superior performance for MR applications in the central nervous system, liver, breast, and vascular system (as summarized in the next section).16
GBCA selection should be tailored to the individual patient and clinical setting following a careful risk-benefit analysis, implemented at the individual or protocol level by the radiologist. Familiarity with different safety and efficacy profiles of the various GBCAs can be beneficial in the clinical decision-making involved in GBCA selection. Additional factors may include cost, ease of use, and approved indications, as well as any physician, pharmacy, and/or patient preferences.
Here, we present discussions from a recent Expert Panel Forum focused on the safety and efficacy of GBCAs, as well as specific considerations related to GBCA selection for neuroimaging, pediatric imaging, body imaging, and breast imaging applications.
Effects of Stability and Relaxivity on Safety and Efficacy
Greater Stability → Less Gd Release
Starting in 2014, several groups found that visible areas of T1 shortening could be identified on noncontrast MR images of the brain, specifically in the dentate, red nucleus, and globus pallidus.17 Such enhancement was confirmed to be the result of trace quantities of Gd retained in the brain of patients who had received a contrast-enhanced scan in the past. Early studies investigating this phenomenon showed that Gd retention tends to be higher in patients who had received multiple and/or higher doses of a linear GBCA vs a macrocyclic GBCA.18-21 However, Gd retention has been shown to occur to some degree with all GBCAs, even in patients with normal renal function and an intact blood-brain barrier.22 Among the macrocyclic agents Dotarem, Gadavist, and ProHance, there is some preliminary evidence in animal studies to suggest that lower levels of Gd are retained with ProHance20,23-25 and that ProHance is cleared more rapidly.20,25 However, larger confirmatory studies are warranted, especially in humans. Most importantly, despite MRI-visible and biochemical evidence of trace Gd retention, no related adverse clinical sequelae have been identified.22,26
Based on radiotracer studies conducted decades ago, it was known that some Gd is retained in the body following GBCA administration.13-15 Yet the only known clinical disease has been nephrogenic systemic fibrosis (NSF), a potentially fatal condition shown to occur almost exclusively with administration of higher and/or repeat doses of less stable agents to patients with end-stage renal disease.27-29 Such less-stable agents, which have been deemed by the American College of Radiology (ACR) to be Group I GBCAs, include Omniscan, Magnevist, and OptiMARK.12 (Table 2) Regulatory guidelines and institutional protocols aimed at reducing exposure of at-risk patients to those specific GBCAs have all but eradicated NSF.30
The Group I agents have been contraindicated by the US Food and Drug Administration (FDA) for use in patients with acute kidney injury (AKI) or chronic, severe kidney disease (ie, an estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2).6-8 The remaining agents, ie, ACR Group II agents, include the macrocyclic GBCAs and the high-relaxivity, linear agent MultiHance.12 The European Union (EU) response to Gd retention differed from the FDA response: the EU chose to exclude all linear agents, including MultiHance, except for use in the liver, leaving no high-relaxivity option in the EU.
So, what is being done to investigate Gd retention? The NIH/ACR/RSNA held a workshop to identify known information and prioritize research needed on Gd chelates.22 A chief priority is to collect more data to ascertain where Gd is retained in the body and for how long. For example, in a recent evaluation of CSF taken from patients administered the macrocyclic agent Gadavist, Gd could be detected in the spinal fluid for weeks after administration.31 In the meantime, guidelines suggest that radiologists: (1) consider noncontrast scans whenever possible; (2) consider non-Gd-based contrast agents (eg, ferumoxytol), if appropriate; and (3) use lower-dose Gd imaging, either by using a lower dose of a high-relaxivity GBCA and/or coupling low-dose imaging with new technology to help simulate full-dose images.32
Higher Relaxivity → Greater Efficacy
The relative efficacy of GBCAs is generally determined using a crossover study design, in which patients undergo MRI with one GBCA, followed by a wash-out period, and then undergo a second MRI with the other GBCA. Other than the contrast, all parameters are kept constant. A side-by-side comparison of the two sets of images, therefore, shows only the relative effect of the GBCAs. Two contrast agents with different properties are typically compared in this way, at either similar or different doses. For example, more than a dozen MRI crossover studies have been published comparing GBCAs specifically in diagnostic neuroimaging,33-47 but also in imaging of the body,48-50 breast,51-53 and vasculature.54-58
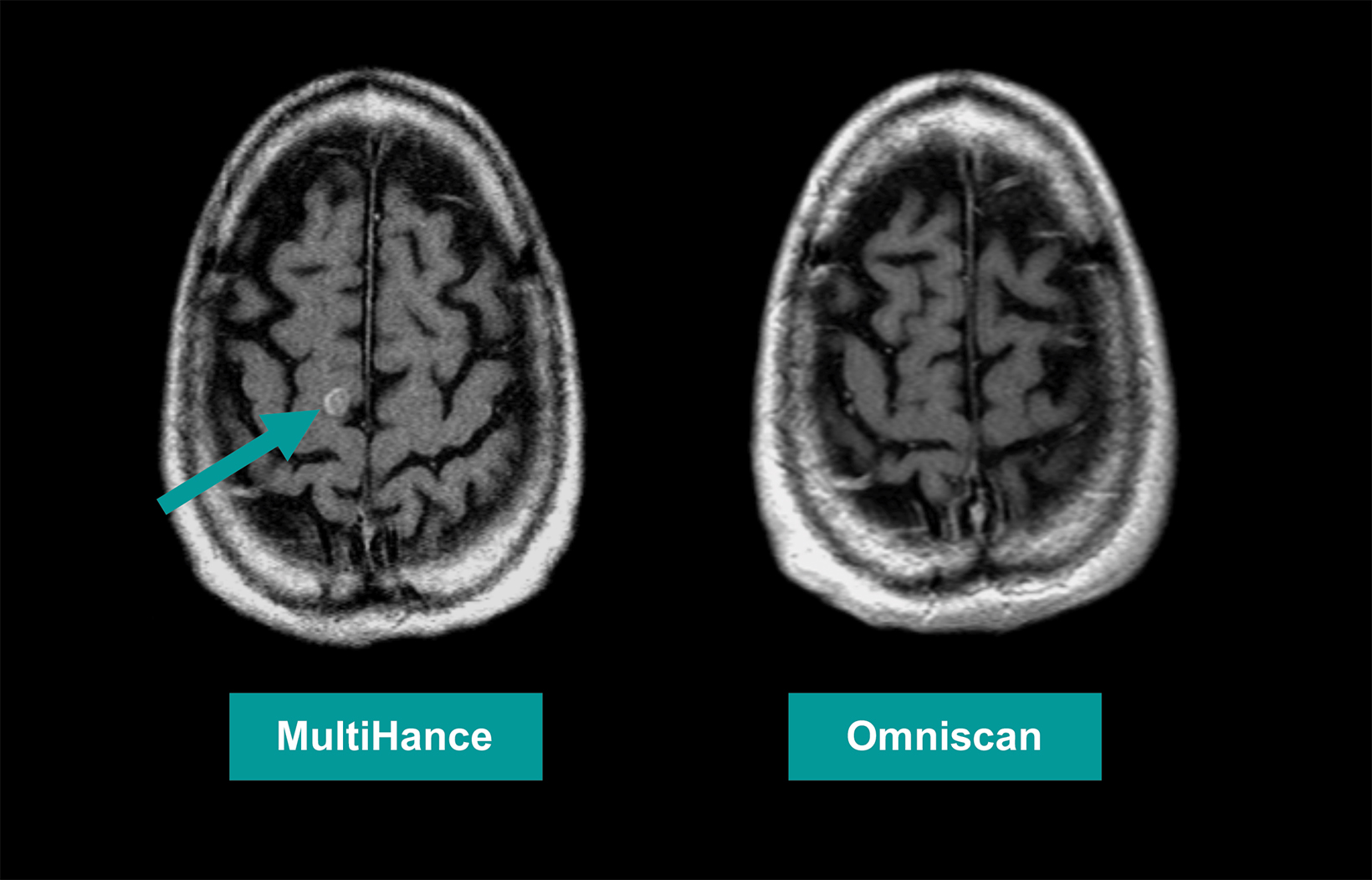
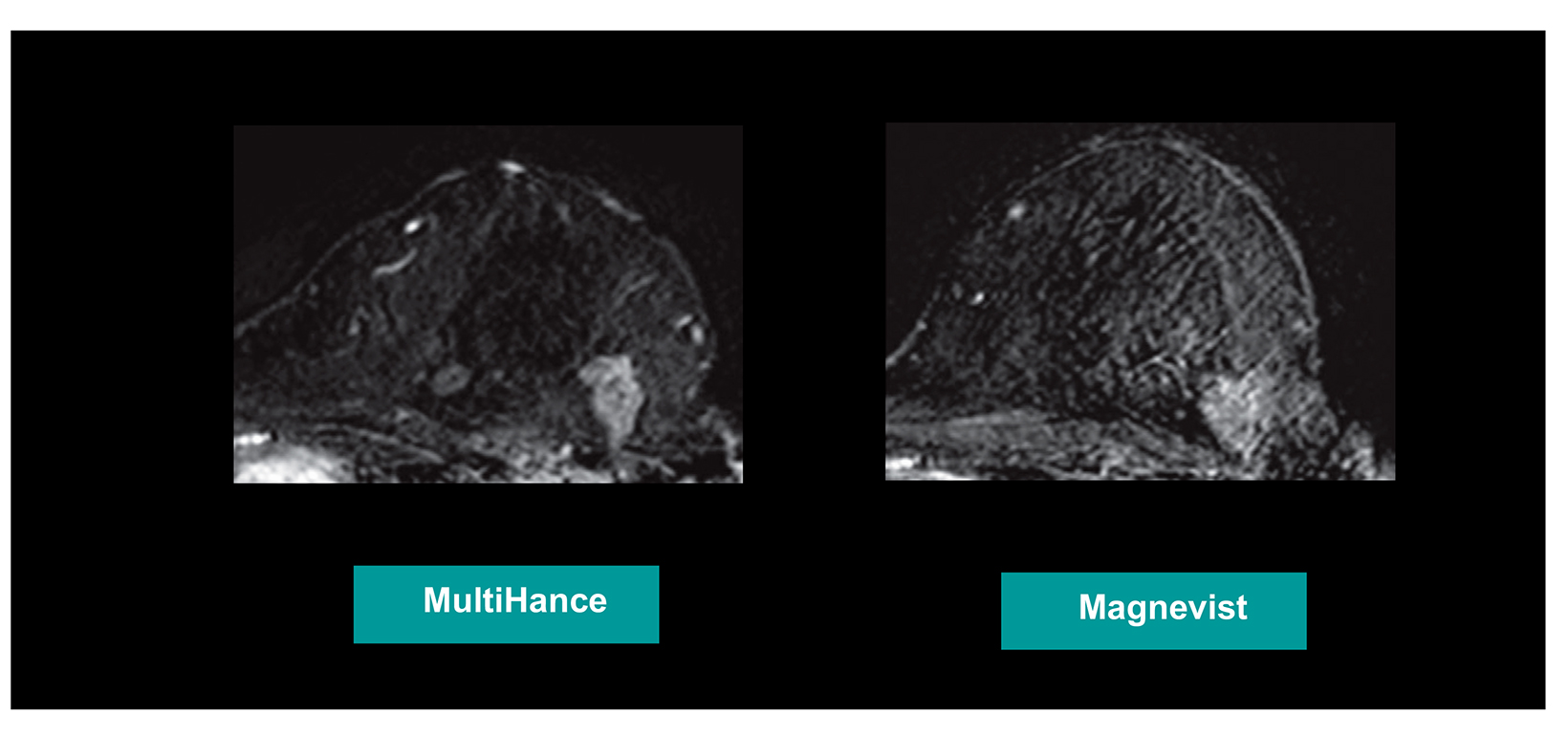
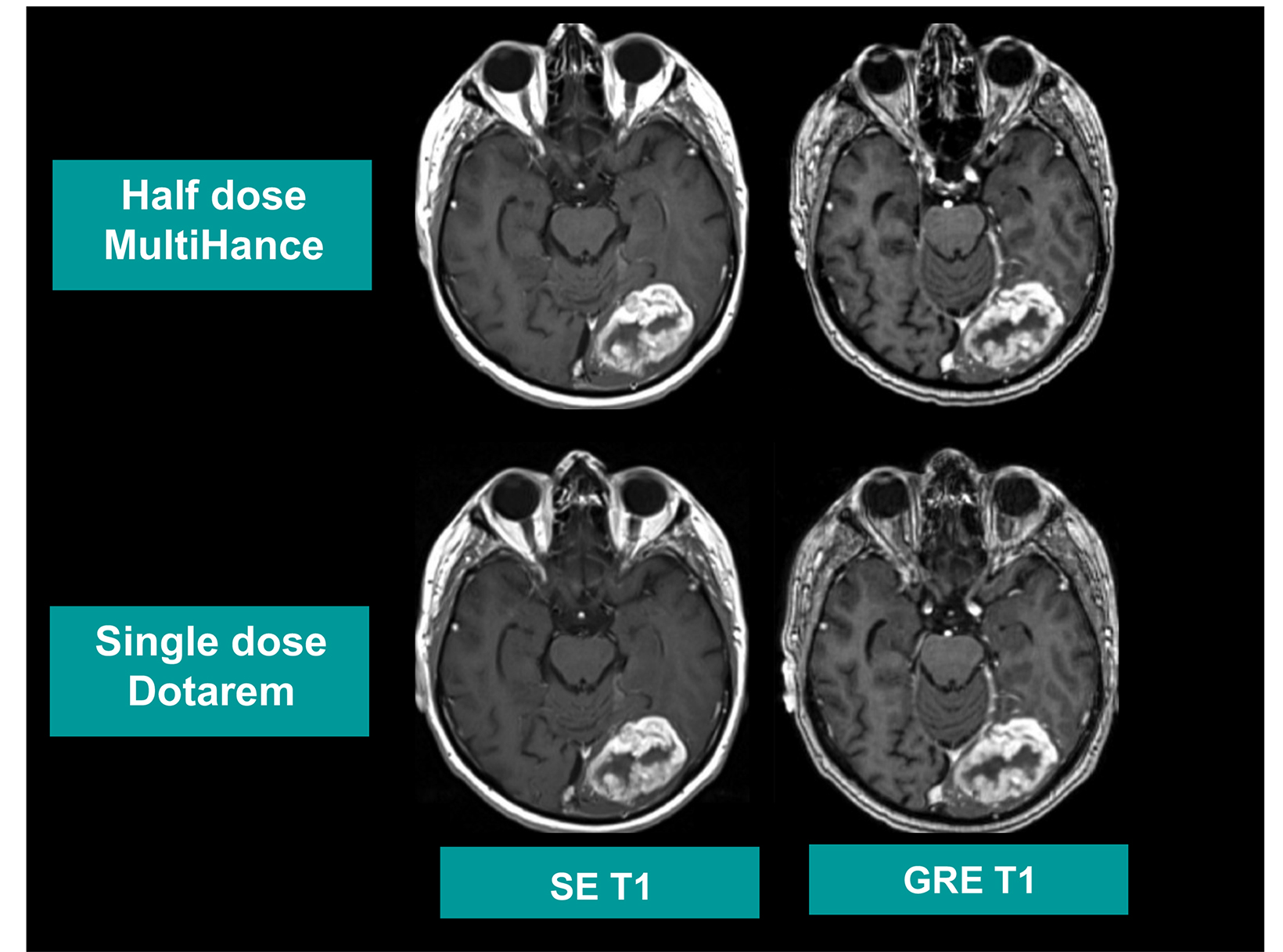
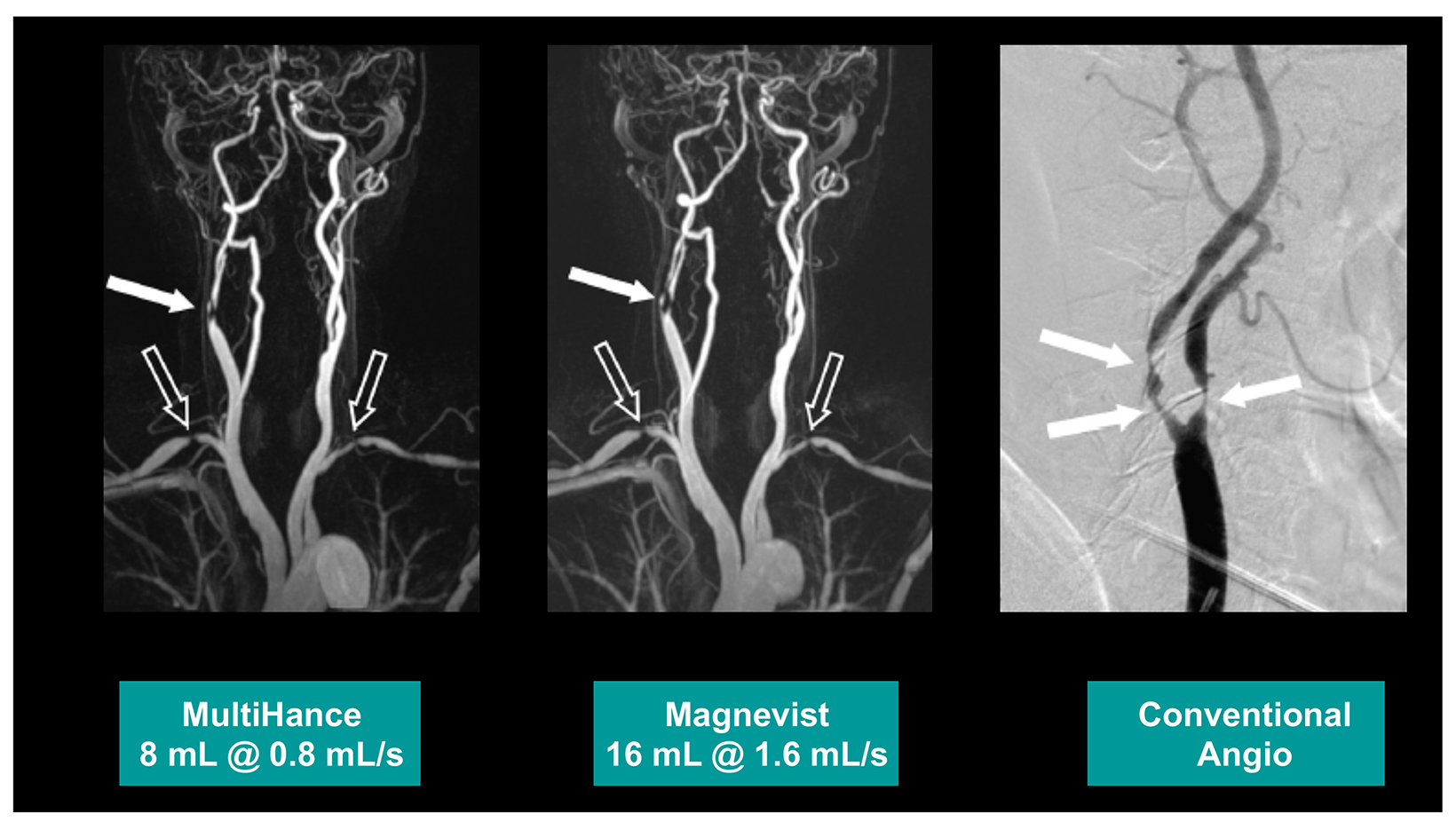
Many of these studies have been performed to assess whether a single dose of the high-relaxivity linear contrast agent MultiHance is superior to an equivalent dose of a standard-relaxivity agent, and also whether a half-dose of MultiHance can be used to obtain results similar to a full, single dose of a standard-relaxivity agent. Such studies have consistently shown that at the same dose, the high-relaxivity linear GBCA MultiHance performs better in lesion detection and characterization compared to standard-relaxivity GBCAs.35-41,47,52,53,55 (See Figures 1 and 2 for examples) Additionally, a half dose of MultiHance performs similarly to a full dose of a standard-relaxivity GBCA, potentially allowing for a reduction in Gd exposure in at-risk patients.47-51,56-58 (See Figures 3 and 4 for examples)
Moreover, this improved performance has been demonstrated to be true even when the comparator to MultiHance is the more concentrated 1M Gadavist: given equivalent amounts of Gd, the higher concentration of Gadavist has not been shown to provide greater lesion conspicuity compared to MultiHance.43 Also, Gadavist has been shown in crossover studies to perform similarly to ProHance, a standard-relaxivity, 0.5M macrocyclic agent.3,46
Contrast Selection for Specific MRI Applications
When is GBCA stability a greater consideration than relaxivity? In general terms, greater stability is more important in younger patients and those requiring serial imaging. Higher relaxivity, on the other hand, can be advantageous in cases where a more accurate depiction of lesion size and number may contribute to clinical decision-making, as well as in cases where immediate clinical needs take precedence over potential long-term concerns related to asymptomatic Gd retention.
In addition, there exists the overall goal of reducing GBCA exposure to a minimum while still obtaining a diagnostic scan. Methods to reduce GBCA exposure include the use not only of noncontrast sequences, but also of contrast-enhanced sequences that may be abbreviated or adaptive, and of lower GBCA doses, which may be more feasible using a higher-relaxivity agent. New technologies are also available that may be relevant to reducing GBCA exposure; eg, artificial intelligence (AI) to “enhance” scans and thereby permit lower doses of Gd.
The presentation and group discussions from the Expert Panel Forum are summarized below for each MRI application (neuro-imaging, pediatric imaging, body imaging, and breast imaging). Each group member focused on the contrast agents they use and why, as well as the tools and techniques they use to minimize GBCA exposure while still obtaining diagnostic images.
Neuroimaging
Max Wintermark, MD, summarized trends in the use of GBCAs in neuroradiology. Dr. Wintermark stated that his group relies on the ACR-ASNR Position Statement on the Use of Gadolinium Contrast Agents,59 which states:
“GBCAs provide crucial, life-saving medical information. Each time a gadolinium-enhanced MRI study is considered, it would be prudent to consider the clinical benefit of the diagnostic information or treatment result that MRI or MRA may provide against the unknown potential risk of gadolinium deposition in the brain for each individual patient. Particular attention should be paid to pediatric and other patients who may receive many GBCA-enhanced MRI studies over the course of their lifetimes. If the decision for an individual patient is made to use a GBCA for an MRI study, multiple factors need to be considered when selecting a GBCA, including diagnostic efficacy, relaxivity, rate of adverse reactions, dosing/concentration, and propensity to deposit in more sensitive organs such as the brain.”
Many studies have demonstrated the benefit of the high-relaxivity linear agent MultiHance in neuroimaging applications. Compared to standard-relaxivity agents, MultiHance provides more contrast enhancement (better lesion-to-brain contrast and better conspicuity of CNS lesions), leading to increased diagnostic information and/or dosing flexibility, and better performance, with a similar acute reaction safety profile.35,39,40,43,47 Dr. Wintermark, therefore, prefers MultiHance for most neuro applications, save for patient insistence on a different agent.
The panel discussed concerns regarding Gd retention but noted the need to balance those concerns with the potential benefits to patients. Dr. Wintermark acknowledged that such calls can be controversial, even among neuroradiologists. An example he cited from his institution is Gd contrast use in patients with multiple sclerosis (MS), who typically require multiple MRIs over their lifetime. Even among neurologists caring for these patients, consensus is difficult to achieve: some request Gd for every exam, while others are willing to alternate with noncontrast exams.
Low-dose or noncontrast scans in neuroradiology can be associated with clinical risks. If Gd is deemed unlikely to provide direct clinical benefit, then contrast can be avoided; however, Gd is required for many applications in neuroradiology. For example, Dr. Wintermark said that he and his colleagues at Stanford Medicine tried unsuccessfully to use half-dose MR perfusion to image stroke patients; while results were obtained, the quantitative assessment of the penumbra was found to be suboptimal.60
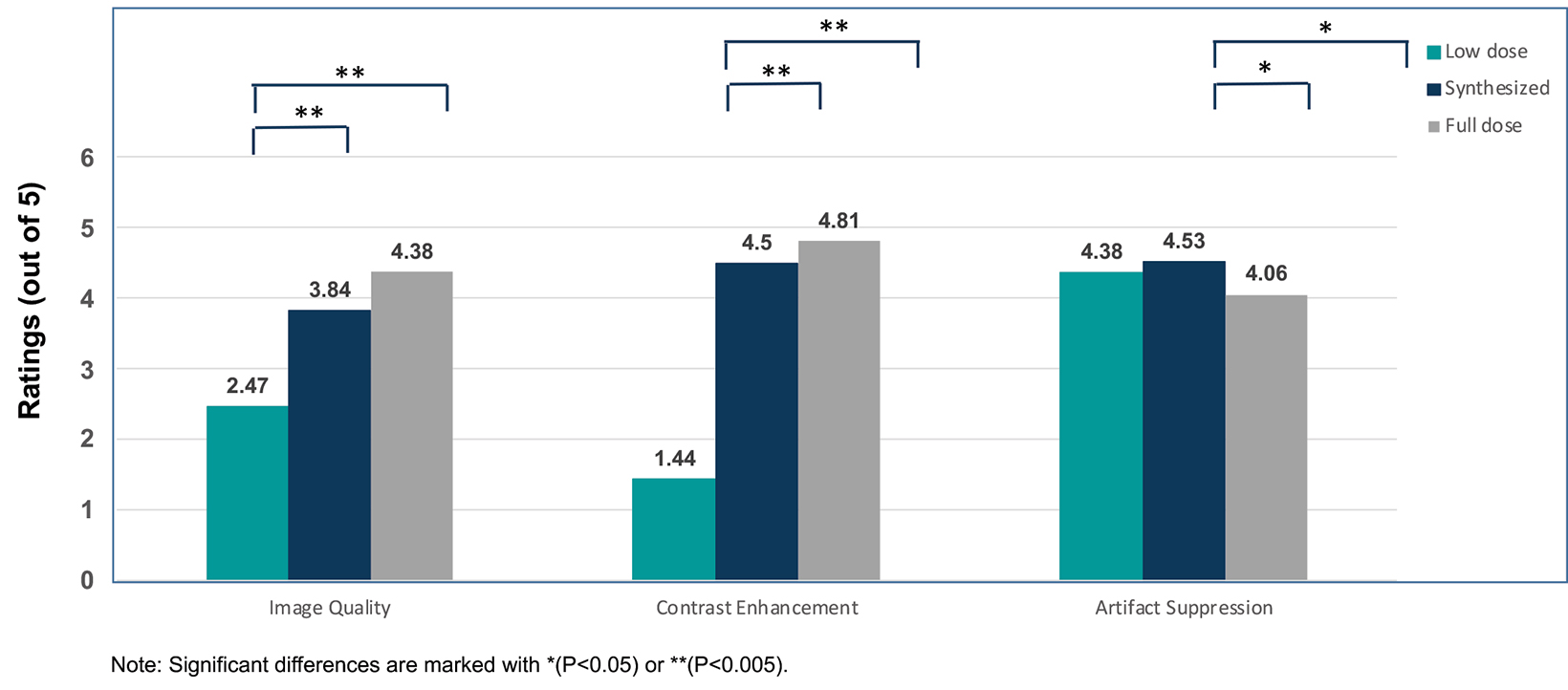
New techniques are being evaluated to reduce contrast use in neuro MRI. Dr. Wintermark discussed AI in some detail, noting that AI is being used primarily for research, but its clinical use is likely to increase in the future. For example, it was recently demonstrated that by combining AI with other technological advancements for brain MR imaging, it is possible to reduce the Gd dose by tenfold; such “synthesized” images appear more similar to full-dose than low-dose exams.32 (Figure 5)
Other ways to reduce Gd exposure include adaptive or tailored imaging, where the scan is checked and the protocol potentially modified based on early findings or prior scans. For some indications, such as acute emergent stroke, obtaining all possible information immediately is critical; thus, a fast but comprehensive exam with no stopping points is preferred. However, for other applications, such as monitoring the size of a meningioma over time, a much more focused/adaptive protocol is possible. Often, some combination of standardized and adaptive imaging is optimal, and striking a balance is important: the need to stop for a “rad check” can be valuable, but it can also negatively impact workflow. Note that AI may help alleviate workflow issues by automatically checking for patient positioning, FOV, and other factors that concern technologists.
Pediatric Imaging
Lorna P Browne, MD, summarized the state of current thinking on Gd contrast use in children. A trend toward using macrocyclics in pediatric patients has been documented: a 2017 survey of children’s hospitals found that 80% had just switched to a macrocyclic GBCA, and 57% had switched in the previous year.61 However, this trend toward preference of macrocyclic agents among pediatric radiologists is not due primarily to concerns for NSF, which is rare in children.62 Rather, it is the potential for unknown clinical sequalae later in life due to Gd retention.
Dr. Browne stated that in general, but especially for pediatric imaging, the best strategy is to reserve contrast administration for when it is deemed clinically necessary and then to use an agent most likely to provide a diagnostic scan without risking repeat scans and, potentially, resedation. Her pediatric imaging practice, she said, employs MultiHance for general use based on its higher relaxivity and overall excellent safety profile. She also noted that MultiHance is approved for use in children over 2 years of age at a dose of 0.2 mL/kg (0.1 mmol/kg) and in pediatric patients below 2 years at 0.1 to 0.2 mL/kg.5
Dr. Browne also reviewed the literature on Gd retention following administration of linear and macrocyclic GBCAs, pointing out that there are confounders to studying Gd retention in children. For example, many patients in the existing studies may be exposed to whole-brain radiation, and whether that might increase predisposition to Gd retention remains unknown. Also, there are inconsistencies in methodology across pediatric studies, making interpretation problematic. But, while there is less literature on Gd retention in children than in adults, dose-dependent T1 signal intensity has been demonstrated in children in deep-brain nuclei on noncontrast scans after administration of a linear GBCA.63-65 Initial studies suggested there was no discernable retention of GBCA in the deep brain nuclei following macrocyclic GBCA administration.66-68 However, two recent studies suggest possible deep brain nuclei deposition following serial macrocyclic GBCA administration in linear-GBCA&emdash;naïve patients. Rossi Espagnet, et al, demonstrated an increase in signal intensity in the dentate nuclei after serial administrations of Dotarem in 50 children;69 and Topcuoglu, et al, found increased T1 signal intensity in the dentate nuclei in 45 children after at least 3 doses of Dotarem.70 Additionally, Stanescu, et al, found Gd deposits in the brain (white matter and deep brain nuclei) of 5 autopsy specimens exposed only to macrocyclic agents (both ionic and nonionic), albeit at a significantly lower level than linear agents.71
Finally, Murata, et al, found that macrocyclic GBCA retention in cortical bone can be 23 times higher than in brain tissue, which may be mobilized during rapidly growing phases of childhood development and in children with altered calcium metabolism.72 Hence, it is reasonable to hypothesize that even a single dose of a macrocyclic agent may result in detectable deposition. However, the clinical significance of this deposition in childhood, and whether there is long-term retention in children who may have yearslong intervals between doses, have not been determined.
With so many unknowns regarding potential GBCA retention in children, there is a trend in neonatal and pediatric imaging toward noncontrast MRI examinations. This has been facilitated by the fact that many diagnoses in pediatric patients are not based purely on contrast kinetics, but on location and patient age, allowing for accurate diagnoses based upon noncontrast sequences.
Also, other imaging modalities, such as ultrasonography, are frequently employed first to evaluate patients prior to MRI and, therefore, the diagnostic question can be more focused and the MRI exam more tailored, potentially permitting noncontrast scanning. Also, when attempting to image young children without sedation or anesthesia, it can be preferable to proceed without contrast, as painful pokes for an IV can upset the child significantly and eliminate the opportunity to avoid anesthesia. For all of these reasons, noncontrast pediatric MRI is becoming more common.
Body Imaging
Kevin Chang, MD, pointed out that many body MRI applications use contrast, particularly when solid organs—eg, the liver, spleen, pancreas, kidneys, and heart—are being assessed for suspected masses. Many of these scans are dynamic contrast-enhanced, multi-phase exams that call for specific timing intervals for postcontrast acquisitions.
With respect to GBCA preference, Dr. Chang indicated that his practice no longer uses any Group I agents, instead defaulting to the macrocyclic agent ProHance. However, their formulary also contains MultiHance and Eovist. Although not as high in relaxivity as MultiHance, ProHance is approved for up to a triple-dose administration and, in preclinical studies, the agent appears to be associated with the least amount of Gd retention of any GBCA.20,23,24 There is no robust evidence of efficacy differences among the macrocyclics; thus, if Gd retention is of concern, using the agent with the lowest Gd retention seems prudent.
Contrast considerations as they pertain to liver and cardiac MRI, and MRA, were discussed in some detail by the panel. In liver imaging, MultiHance or Eovist may be used when a hepatobiliary agent is warranted. MultiHance excretion is 5% biliary and 95% renal, requiring a wait of 2 hours before acquiring the hepatobiliary phase, whereas Eovist is 50% biliary and 50% renal, requiring a waiting time of only 15-20 minutes. These decisions can impact workflow and throughput. Dr. Chang noted that in cases of suspected hepatocellular carcinoma (HCC), using an agent such as MultiHance with the highest relaxivity can help to achieve maximal arterial enhancement. Moreover, high relaxivity is particularly beneficial in cirrhotic livers.73
Dr. Chang indicated that cardiac imaging has been performed with a double (0.2 mmol/kg) dose of a GBCA since the 1990s. While some radiologists have experience using a 1.5x dose of MultiHance, achieving single-dose imaging is difficult. However, there are some cardiac applications for which too bright a signal is not good; specifically, a high dose of a high-relaxivity agent can produce
a signal so bright that it masks endocardial lesions. Therefore, a standard-relaxivity macrocyclic agent with low Gd retention would be preferred in these cases.
In magnetic resonance angiography (MRA), a high-relaxivity agent provides some additional signal with a slightly longer tail; therefore, MultiHance is the agent of choice for contrast-enhanced MRA. Dr. Chang provided some technical tips and tricks for MRA to ensure adequate visualization of small, distal branches. The panel also discussed the advantages and caveats regarding safe and efficacious administration of ferumoxytol for MRA.
Breast Imaging
Christopher Comstock, MD, FACR, reviewed contrast-enhanced breast MRI, focusing specifically on breast cancer screening in both normal- and high-risk women. The goal of breast cancer screening is to find tumors at their smallest, which can translate to earlier-stage cancer detection and treatment.
Several studies show that lesion size at detection is clearly linked to outcomes and relative risk of mortality; annual screening reduces the risk of mortality from breast cancer on the order of 20-40%.74,75 Finding cancers earlier also lowers morbidity, owing to fewer mastectomies and the potential to use less aggressive therapeutic regimens.
Dr. Comstock discussed some of the differences between morphologic screening (eg, mammography, ultrasound) and vascular-based screening (eg, breast MRI, and the newer contrast mammography). First, they detect cancer in different ways: vascular-based imaging shows vascular changes, which can be seen with smaller lesions at earlier stages, compared to morphologic features. Specifically, MRI detects cancers at an average size of 0.7-0.8 centimeters, whereas morphology-based screening tests typically detect cancers at an average size of 1.4-1.5 centimeters.76 In addition, in a study designed to determine the “biological detection profile” of each type of modality, vascular-based screening found more aggressive, higher-grade entities, while mammography tended to find lower grade tumors.77 Therefore, not only is vascular-based screening finding smaller cancers, it is also finding the more biologically-concerning cancers.
Based on American Cancer Society guidelines, patients who should get annual breast MRI screening in addition to mammography include:78
- BRCA1 or BRCA2 gene mutation carriers;
- Untested first-degree relatives of BRCA gene mutation carriers; and,
- Those with a ≥20-fold lifetime risk, defined by BRCAPRO or other models, depending on family history.
The panel discussed the value of abbreviated protocols for breast MRI screening. The goal of the abbreviated MR (AB-MR) protocol is to increase access to the most sensitive test in order to detect more cancers. Regardless of advances in breast cancer treatment, early detection of smaller cancers will always be beneficial. Kuhl, et al, demonstrated that AB-MR protocols stripped of some sequences retained high sensitivity while significantly lowering costs.79 This shortened protocol consists of one T2-weighted, precontrast sequence with or without fat saturation, one precontrast T1-weighted sequence, and one postcontrast series.
A recent multicenter study compared the performance of AB-MR (performed in less than 10 minutes) and digital breast tomosynthesis (DBT) for breast cancer screening in 1,444 average-risk women with dense breasts.80 Abbreviated breast MRI with MultiHance at 0.1 mL/kg detected all invasive cancers in all 17 women with the disease, and ductal carcinomas in situ (DCIS) in 5 of 6 women with the disease, while DBT detected invasive cancers in 7 of 17 women, and DCIS in 2 of 6 women. Sensitivity for both types of cancer was significantly higher with AB-MR than with DBT (95.7% vs 39.1% [P=0.001]).
Dr. Comstock summarized the results of this study, noting that AB-MR is well tolerated by patients, can be performed in less than 10 minutes, and is significantly more sensitive than DBT. In addition, AB-MR appears to detect more biologically significant breast cancers. By shortening the time to perform breast MRI, AB-MR will increase access to the most powerful breast cancer screening tool. However, he stressed that the study was performed in average-risk patients; the full protocol is currently still recommended in high-risk patients.
Dr. Comstock cautioned that the same results attained in the trial may not be achieved if centers perform AB-MR differently than the way it was performed in the trial. For example, the high sensitivities seen in the study were based on a specific protocol, the use of high-relaxivity MultiHance, and AB-MR reader training.
In addition, using a macrocyclic GBCA to minimize Gd retention may be prudent, as women undergoing screening are typically relatively young and require annual screening with MRI. But a multi-institution assessment of moderate- to high-risk women undergoing screening breast MRI showed that overall, women prioritized imaging sensitivity over the potential for immediate adverse events, Gd retention, and procedure cost. Low-income patients were an exception, prioritizing out-of-pocket cost over the other factors.81 The results of this study highlight the importance of patient preferences when selecting a GBCA.
Noncontrast sequences/protocols for breast MRI remain in the development stages.82 Currently, they do not appear to have detection rates equal to those of contrast-enhanced breast MRI.83
GBCA Summary and Future Considerations
When deciding whether to prioritize stability or relaxivity in a given contrast-enhanced MRI setting, radiologists should employ risk-benefit analyses that include patient age and the need for repeat exams: younger patients requiring a lifetime of annual MRI may warrant the use of more stable agents associated with lower Gd retention. On the other hand, high-relaxivity agents may be preferred to maximize the signal-to-noise ratio in cases where sensitivity of MRI will directly impact the diagnosis, prognosis, and/ or treatment plan, or in patients where the diagnosis or prognosis precludes long-term safety concerns.
Imaging guidelines tend to focus on GBCA risk; however, the potential benefit cannot be understated &emdash; in many clinical scenarios, missing or mischaracterizing a lesion can have a devastating effect on patient outcome. Table 3 summarizes the expert panel’s recommendations with respect to contrast-enhanced imaging. Ultimately, a patient-centered approach should be taken with the goal of obtaining accurate and clinically relevant diagnostic information with maximum patient safety.
References
- McDonald JS, McDonald RJ. MR imaging safety considerations of gadolinium-based contrast agents: gadolinium retention and nephrogenic systemic fibrosis. Magn Reson Imaging Clin N Am. 2020;28:497-507.
- Dotarem®(gadoteric acid) [prescribing information]. Bloomington, IN: Guerbet LLC; September, 2017.
- Gadavist® (gadobutrol) injection [prescribing information]. Wayne, NJ; Bayer HealthCare Pharmaceuticals; April 2016.
- ProHance® (Gadoteridol) Injection, [prescribing information]. Princeton, NJ: Bracco Diagnostics Inc.; November 2013.
- MultiHance®(gadobenate dimeglumine) injection [prescribing information]. Princeton, NJ; Bracco Diagnostics Inc.; July 2013.
- Omniscan™ (gadodiamide) injection [prescribing information]. Princeton, NJ: GE Healthcare; August 2013.
- Magnevist®(gadopentetate dimeglumine) injection [prescribing information]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; June 2014.
- OptiMARK™ (gadoversetamide) injection [prescribing information]. Bloomington, IN: Guerbet LLC; August 2016.
- Clariscan™ (gadoterate meglumine) [prescribing information]. Marlborough, MA: GE Healthcare Inc.; November, 2019.
- Shen Y, Goerner FL, Snyder C, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330-338.
- Tweedle MF. The ProHance story: the making of a novel MRI contrast agent. Eur Radiol. 1997;(7 Suppl) 5:225-230.
- American College of Radiology (ACR) Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, 2021. Available at: https://www.acr.org/ Clinical-Resources/Contrast-Manual. Accessed December 31, 2021.
- Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol. 2004;39:138-142.
- Tweedle MF, Wedeking P, Kumar K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol. 1995;30:372-380.
- White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41:272-278.
- Pintaske J, Martirosian P, Graf H, et al. Relaxivity of gadopentetate dimeglumine (Magnevist), gadobutrol (Gadovist), and gadobenate dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Invest Radiol. 2006;41: 213-221 [erratum in Invest Radiol. 2006;41:859].
- Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834-841.
- McDonald RJ, McDonald JS, Dai D, et al. Comparison of Gadolinium Concentrations within Multiple Rat Organs after Intravenous Administration of Linear versus Macrocyclic Gadolinium Chelates. Radiology. 2017;285:536-545.
- Robert P, Violas X, Grand S, et al. Linear Gadolinium-Based Contrast Agents Are Associated With Brain Gadolinium Retention in Healthy Rats. Invest Radiol. 2016;51:73-82.
- Jost G, Frenzel T, Boyken J, Lohrke J, Nischwitz V, Pietsch H. Long-term excretion of gadolinium-based contrast agents: linear versus macrocyclic agents in an experimental rat model. Radiology. 2019;290:340-348.
- Kobayashi M, Levendovszky SR, Hippe DS, et al. Comparison of Human Tissue Gadolinium Retention and Elimination between Gadoteridol and Gadobenate. Radiology. 2021;300:559-569.
- McDonald RJ, Levine D, Weinreb J, et al. Gadolinium Retention: A research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018;289:517-534.
- Bussi S, Coppo A, Botteron C, et al. Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging. 2018;47:746-752.
- Bussi S, Coppo A, Celeste R, et al. Macrocyclic MR contrast agents: evaluation of multiple-organ gadolinium retention in healthy rats. Insights Imaging. 2020;11:11.
- Bussi S, Coppo A, Bonafè R, et al. Gadolinium Clearance in the First 5 Weeks After Repeated Intravenous Administration of Gadoteridol, Gadoterate Meglumine, and Gadobutrol to rats. J Magn Reson Imaging. 2021;54:1636-1644.
- Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564-570.
- Thomsen HS, Morcos SK, Dawson P. Is there a causal relation between the administration of gadolinium based contrast media and the development of nephrogenic systemic fibrosis (NSF)? Clin Radiol. 2006;61:905-906.
- Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148-157.
- Weinreb JC, Rodby RA, Yee J, et al. Use of Intravenous Gadolinium-based Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2021;298:28-35.
- Davenport MS. Virtual elimination of nephrogenic systemic fibrosis: A medical success story with a small asterisk. Radiology. 2019;292:387-389.
- Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288:416-423.
- Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48:330-340.
- Greco A, Parker JR, Ratcliffe CG, Kirchin MA, McNamara MT. Phase III, randomized, double-blind, cross-over comparison of gadoteridol and gadopentetate dimeglumine in magnetic resonance imaging of patients with intracranial lesions. Australas Radiol. 2001;45:457-463.
- Anzalone N, Gerevini S, Scotti R, Vezzulli P, Picozzi P. Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol. 2009;50:933-940.
- Essig M, Tartaro A, Tartaglione T, Pirovano G, Kirchin MA, Spinazzi A. Enhancing lesions of the brain: intraindividual crossover comparison of contrast enhancement after gadobenate dimeglumine versus established gadolinium comparators. Acad Radiol. 2006;13:744-751.
- Colosimo C, Ruscalleda J, Korves M, et al. Detection of intracranial metastases: a multicenter, intrapatient comparison of gadobenate dimeglumine-enhanced MRI with routinely used contrast agents at equal dosage. Invest Radiol. 2001;36:72-81.
- Colosimo C, Knopp MV, Barreau X, et al. A comparison of Gd-BOPTA and Gd-DOTA for contrast-enhanced MRI of intracranial tumours. Neuroradiol. 2004;46:655-665.
- Knopp MV, Runge VM, Essig M, et al. Primary and secondary brain tumors at MR imaging: bicentric intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine. Radiology. 2004;230:55-64.
- Maravilla KR, Maldjian JA, Schmalfuss IM, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology. 2006;240:389-400.
- Rowley HA, Scialfa G, Gao PY, et al. Contrast-enhanced MR imaging of brain lesions: a large scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol. 2008;29:1684-1691.
- Rumboldt Z, Rowley HA, Steinberg F, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine in MRI of brain tumors at 3 Tesla. J Magn Reson Imaging. 2009;29:760-767.
- Katakami N, Inaba Y, Sugata S, et al. Magnetic resonance evaluation of brain metastases from systemic malignances with two doses of gadobutrol 1.0 m compared with gadoteridol: a multicenter, phase ii/iii study in patients with known or suspected brain metastases. Invest Radiol. 2011;46:411-418.
- Seidl Z, Vymazal J, Mechl M, et al. Does higher gadolinium concentration play a role in the morphologic assessment of brain tumors? Results of a multicenter intraindividual crossover comparison of gadobutrol versus gadobenate dimeglumine (the MERIT Study). AJNR Am J Neuroradiol. 2012;33:1050-1058.
- Anzalone N, Scarabino T, Venturi C, et al. Cerebral neoplastic enhancing lesions: multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0M) and gadoterate meglumine (0.5M) at 0.1 mmol Gd/kg body weight in a clinical setting. Eur J Radiol. 2013;82:139-145.
- Koenig M, Schulte-Altedorneburg G, Piontek M, et al. Intra-individual, randomised comparison of the MRI contrast agents gadobutrol versus gadoteridol in patients with primary and secondary brain tumours, evaluated in a blinded read. Eur Radiol. 2013;23:3287-3295.
- Maravilla KR, Smith MP, Vymazal J, et al. Are there differences between macrocyclic gadolinium contrast agents for brain tumor imaging? Results of a multicenter intraindividual crossover comparison of gadobutrol with gadoteridol (the TRUTH study). AJNR Am J Neuroradiol. 2015;36:14-23.
- Vaneckova M, Herman M, Smith MP, et al. The benefits of high relaxivity for brain tumor imaging: results of a multicenter intraindividual crossover comparison of gadobenate dimeglumine with gadoterate meglumine (the BENEFIT study). AJNR Am J Neuroradiol. 2015;36:1589-1598.
- Schneider G, Maas R, Schultze Kool L, et al. Low-dose gadobenate dimeglumine versus standard dose gadopentetate dimeglumine for contrast-enhanced magnetic resonance imaging of the liver: an intra-individual crossover comparison. Invest Radiol. 2003;38:85-94.
- Homayoon B, Diwakar H, Strovski E, et al. Half-dose gadobenate dimeglumine versus standard-dose gadodiamide in dynamic magnetic resonance imaging of non-cirrhotic livers: a retrospective intra-individual crossover comparison. Abdom Imaging. 2014;39:955-962.
- Cheong BY, Duran C, Preventza OA, Muthupillai R. Comparison of low-dose higher-relaxivity and standard-dose lower-relaxivity contrast media for delayed-enhancement MRI: a blinded randomized crossover study. AJR Am J Roentgenol. 2015;205:533-539.
- Clauser P, Helbich TH, Kapetas P, et al. Breast lesion detection and characterization with contrast-enhanced magnetic resonance imaging: Prospective randomized intraindividual comparison of gadoterate meglumine (0.15 mmol/kg) and gadobenate dimeglumine (0.075 mmol/kg) at 3T. J Magn Reson Imaging. 2019;49:1157-1165.
- Martincich L, Faivre-Pierret M, Zechmann CM, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for Breast MR imaging (DETECT Trial). Radiology. 2011;258:396-408.
- Pediconi F, Catalano C, Padula S, et al. Contrast-enhanced MR mammography: improved lesion detection and differentiation with gadobenate dimeglumine. AJR Am J Roentgenol. 2008;191:1339-1346.
- Gerretsen SC, le Maire TF, Miller S, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for MR angiography of peripheral arteries. Radiology. 2010;255:988-1000.
- Spampinato MV, Nguyen SA, Rumboldt Z. Comparison of gadobenate dimeglumine and gadodiamide in the evaluation of spinal vascular anatomy with MR angiography. AJNR Am J Neuroradiol. 2010;31:1151-1156.
- Wang J, Yan F, Liu J, et al. Multicenter, intra-individual comparison of single dose gadobenate dimeglumine and double dose gadopentetate dimeglumine for MR angiography of the peripheral arteries (the Peripheral VALUE Study). J Magn Reson Imaging. 2013;38:926-937.
- Xing X, Zeng X, Li X, et al. Contrast-enhanced MR angiography: does a higher relaxivity MR contrast agent permit a reduction of the dose administered for routine vascular imaging applications? Radiol Med. 2015;120:239-250.
- Li Y, Li X, Li D, et al. Multicenter, intraindividual comparison of single-dose gadobenate dimeglumine and double-dose gadopentetate dimeglumine for MR angiography of the supra-aortic arteries (the Supra-Aortic VALUE study). AJNR Am J Neuroradiol. 2013;34:847-854.
- ASNR Website. ACR&emdash;ASNR Position Statement on the Use of Gadolinium Contrast Agents. May 2016. Available at: https://www.asnr.org/wp-content/uploads/2017/03/ACR_ASNR_Position_Statement_on_the_Use_of_Gadolinium_Contrast_Agents.pdf. Accessed February 3, 2022.
- Heit JJ, Christensen S, Mlynash M, et al. MR perfusion imaging: Half-dose gadolinium is half the quality. J Neuroimaging. 2021;31:1014-1019.
- Mithal LB, Patel PS, Mithal D, Palac HL, Rozenfeld MN. Use of gadolinium-based magnetic resonance imaging contrast agents and awareness of brain gadolinium deposition among pediatric providers in North America. Pediatr Radiol. 2017;47:657-664.
- >Nardone B, Saddleton E, Laumann AE, et al. Pediatric nephrogenic systemic fibrosis is rarely reported: a RADAR report. Pediatr Radiol. 2014;44:173-180.
- Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev. 2016;38:331-336.
- Flood TF, Bhatt PR, Jensen A, Maloney JA, Stence NV, Mirsky DM. Age-dependent signal intensity changes in the structurally normal pediatric brain on unenhanced T1-weighted MR imaging. AJNR Am J Neuroradiol. 2019;40:1824-1828.
- Miller JH, Hu HH, Pokorney A, Cornejo P, Towbin R. MRI Brain Signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics. 2015;136:e1637-40.
- Ozturk K, Nascene D. Dentate nucleus signal intensity changes on T1-weighted MRI after repeated administrations of linear and macrocyclic gadolinium-based contrast agents: a pediatric intraindividual case-control study. Acta Radiol. 2021:2841851211018809.
- Renz DM, Kümpel S, Böttcher J, et al. Comparison of unenhanced T1-weighted signal intensities within the dentate nucleus and the globus pallidus after serial applications of gadopentetate dimeglumine versus gadobutrol in a pediatric population. Invest Radiol. 2018;53:119-127.
- Tibussek D, Rademacher C, Caspers J, et al. Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology. 2017;285:223-230.
- Rossi Espagnet MC, Bernardi B, Pasquini L, Figà-Talamanca L, Tomà P, Napolitano A. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol. 2017;47:1345-1352.
- Topcuoglu ED, Topcuoglu OM, Semiz Oysu A, Bukte Y. Does Gadoterate meglumine cause gadolinium retention in the brain of children? a case-control study. J Magn Reson Imaging. 2020;51:1471-1477.
- Stanescu AL, Shaw DW, Murata N, et al. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr Radiol. 2020;50:388-396.
- Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51:447-453.
- Marin D, Di Martino M, Guerrisi A, et al. Hepatocellular carcinoma in patients with cirrhosis: qualitative comparison of gadobenate dimeglumine-enhanced MR imaging and multiphasic 64-section CT. Radiology. 2009;251:85-95.
- Michaelson JS, Silverstein M, Wyatt J, et al. Predicting the survival of patients with breast carcinoma using tumor size. Cancer. 2002;95:713-723.
- Smith RA, Duffy SW, Tabár L. Breast cancer screening: the evolving evidence. Oncology. 2012;26. Available at: https://www.cancernetwork.com/view/breast-cancer-screening-evolving-evidence. Accessed February 3, 2022.
- Bae MS, Sung JS, Bernard-Davila B, Sutton EJ, Comstock CE, Morris EA. Survival outcomes of screening with breast MRI in women at elevated risk of breast cancer. J Breast Imaging. 2020;2:29-35.
- Sung JS, Stamler S, Brooks J, et al. Breast cancers detected at screening MR imaging and mammography in patients at high risk: method of detection reflects tumor histopathologic results. Radiology. 2016;280:716-722.
- Saslow D, Boetes C, Burke W, et al.; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Kuhl CK. Abbreviated magnetic resonance imaging (MRI) for breast cancer screening: rationale, concept, and transfer to clinical practice. Annu Rev Med. 2019;70:501-519.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756.
- Woolen SA, Troost JP, Khalatbari S, et al. Prospective multicenter assessment of patient preferences for properties of gadolinium-based contrast media and their potential socioeconomic impact in a screening breast MRI setting. Eur Radiol. 2021;31:9139-9149.
- Bu Y, Xia J, Joseph B, et al. Non-contrast MRI for breast screening: preliminary study on detectability of benign and malignant lesions in women with dense breasts. Breast Cancer Res Treat. 2019;177:629-639.
- Baltzer PAT, Bickel H, Spick C, et al. Potential of noncontrast magnetic resonance imaging with diffusion-weighted imaging in characterization of breast lesions: intraindividual comparison with dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol. 2018;53:229-235.