Middle Aortic Syndrome
Images
Case Summary
An adolescent presented with chronic, severe hypertension (systolic >200 mmHg) not controlled by antihypertensive medications. A cardiology workup detected left ventricular hypertrophy.
Imaging Findings
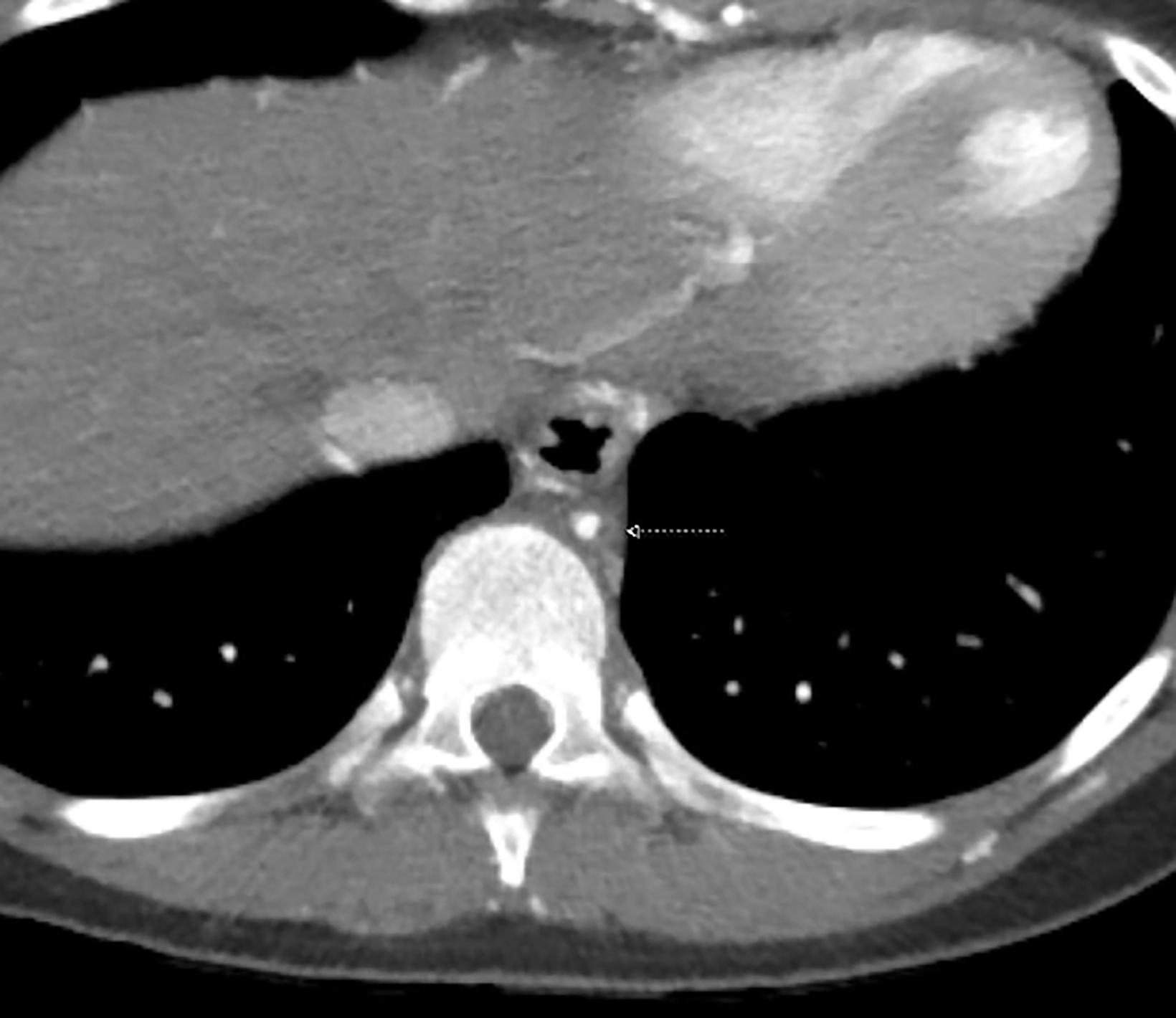
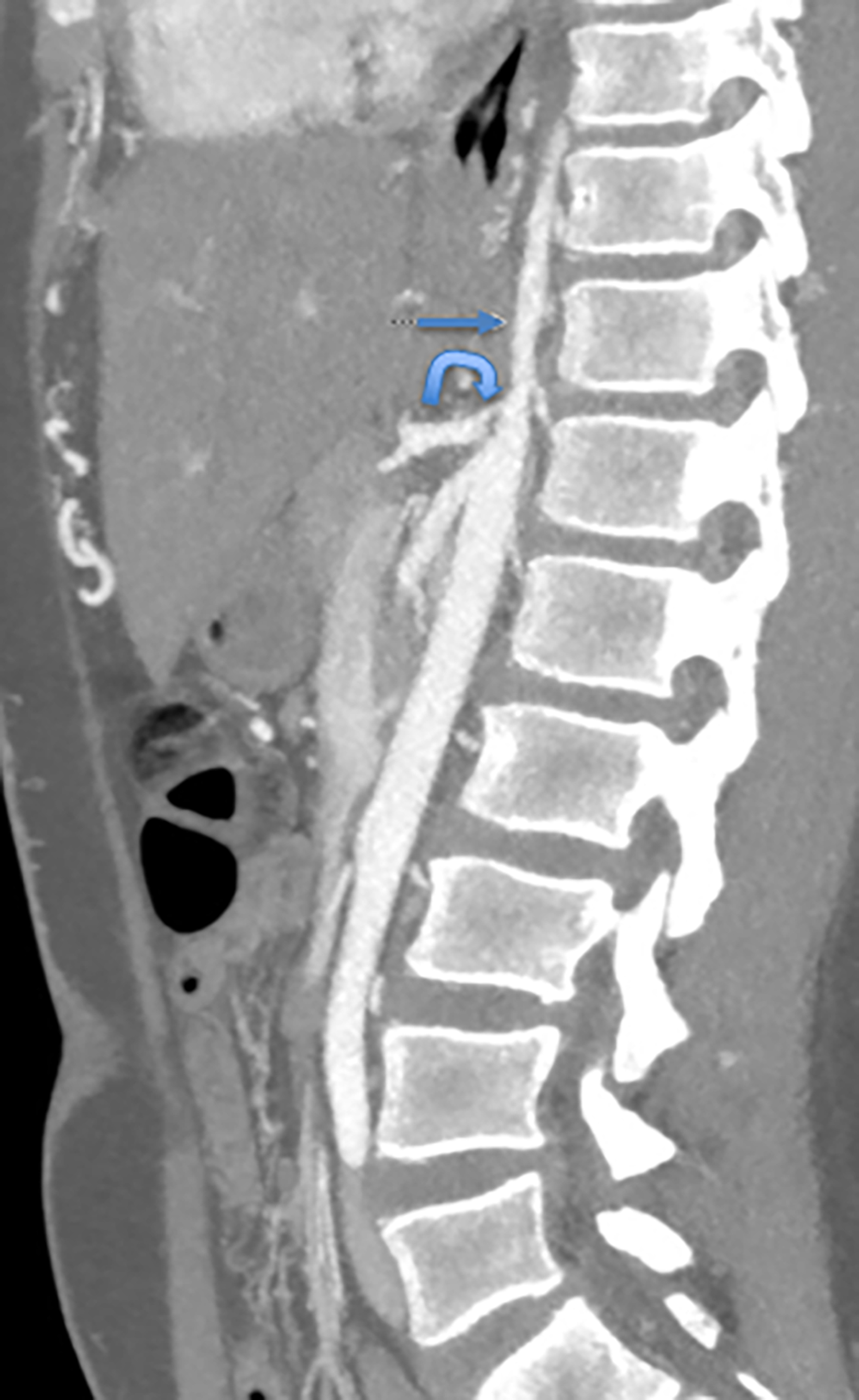
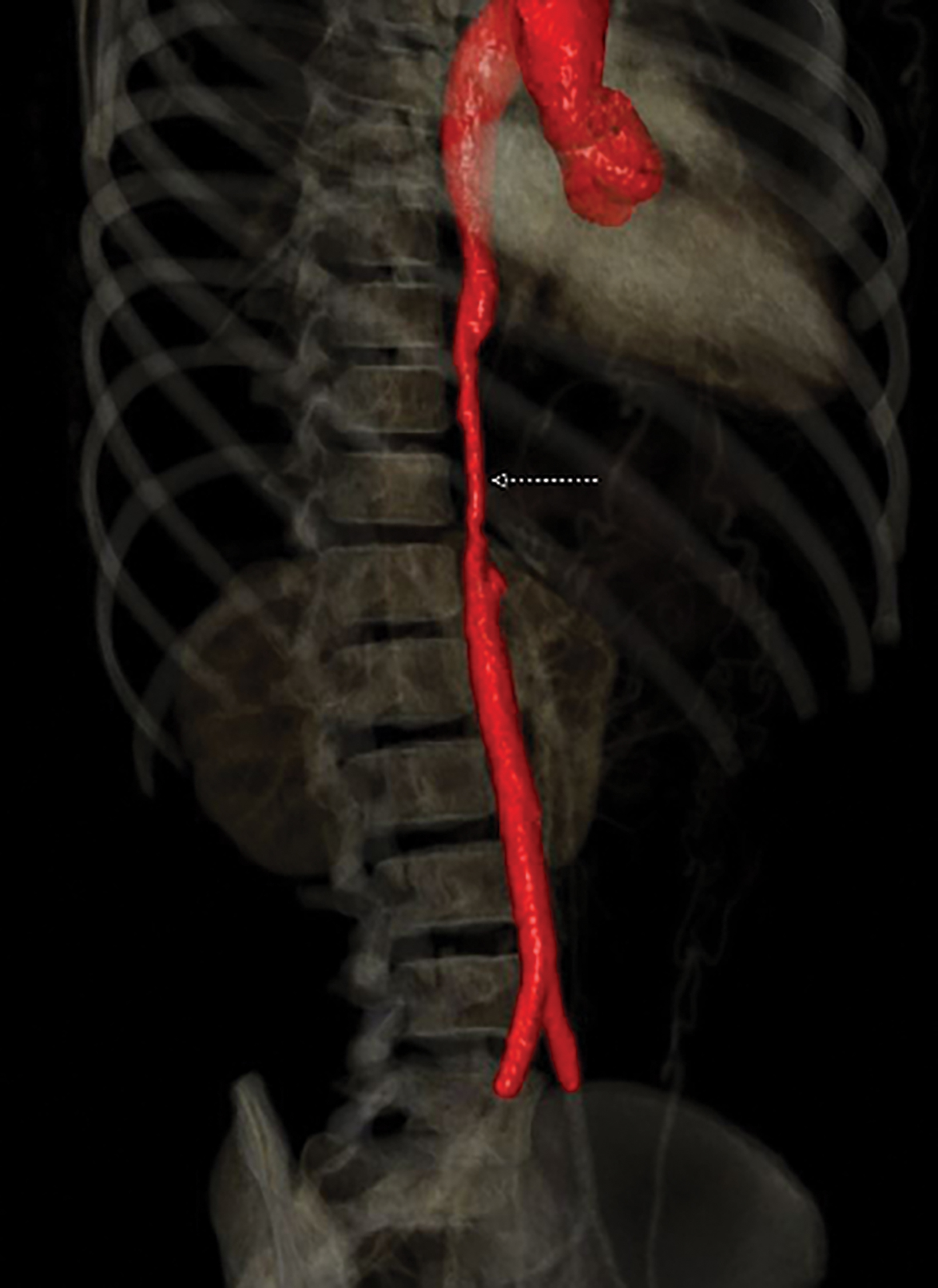
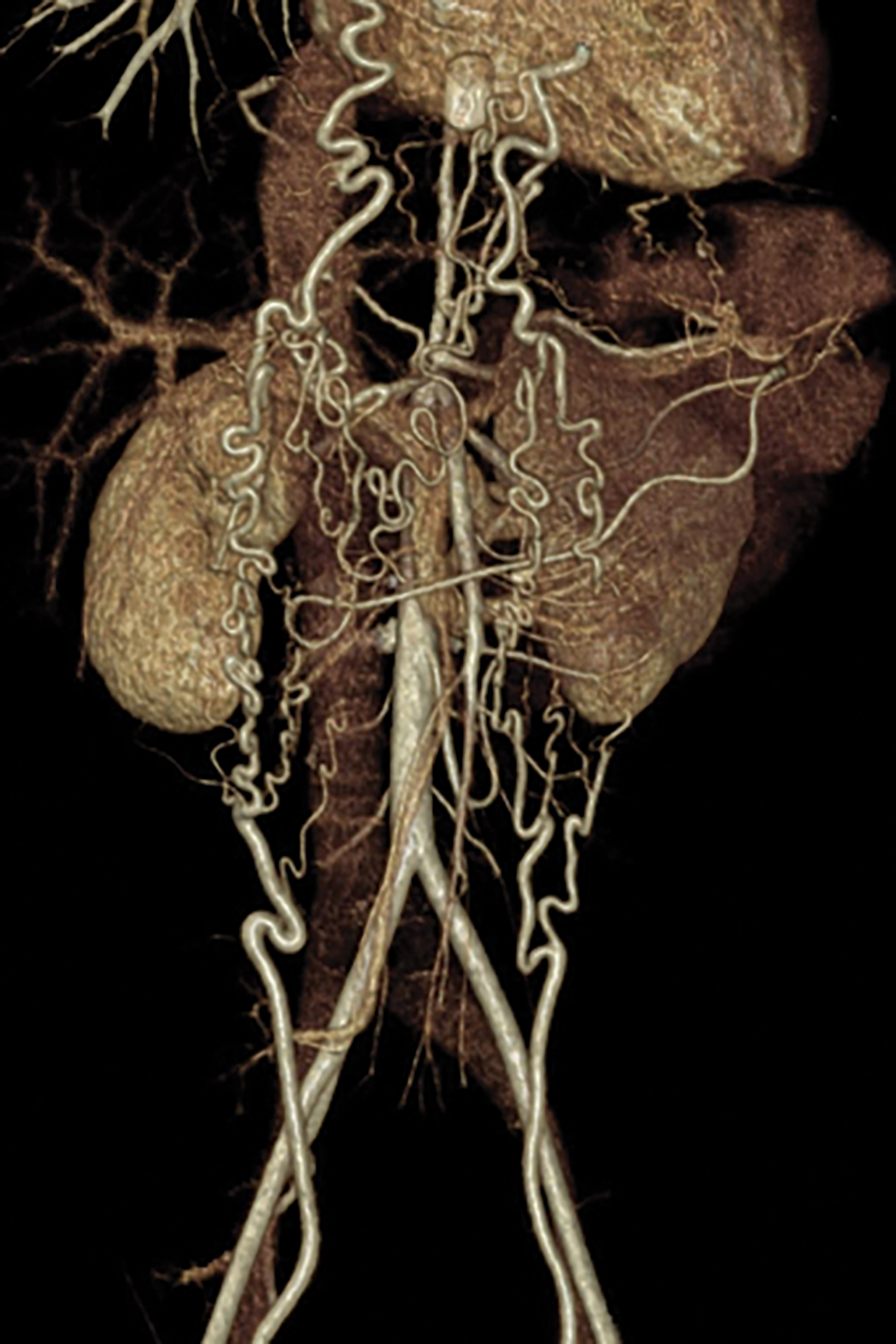
An axial CT angiogram (CTA, Figure 1) demonstrated a narrowed segment of the thoracoabdominal aorta measuring 3-4 mm in greatest diameter extending above the level of the celiac trunk and superior mesenteric arteries. Numerous collateral vessels were present in the anterior thoracic and abdominal wall.
Diagnosis
Middle aortic syndrome. Differential diagnosis includes primary hypertension resulting from renovascular hypertension, chronic renal disease, polycystic kidney disease, coarctation of the aorta, adrenal tumors, hyperthyroidism, medication-induced vessel scarring, and Kawasaki disease. Differential also includes aortic narrowing resulting from neurofibromatosis type 1, tuberous sclerosis, Williams syndrome, Alagille syndrome, and Takayasu arteritis.
Discussion
Middle aortic syndrome (MAS) is a rare vascular disorder that results in segmental narrowing of the abdominal or distal descending thoracic aorta. The stenosis is often accompanied by osteal stenosis of the aortic branches, especially the proximal renal and visceral arteries. The narrowing can be congenital or acquired. Fewer than 700 cases have been reported to date, 1 with MAS constituting approximately 0.5-2% of all cases of aortic stenosis in children and young adults. 2
Typically presenting in the pediatric population, MAS can result in significant morbidity secondary to stenosis of the aorta and its associated abdominal visceral branches. In a recent systematic review of 630 patients <18 years of age in 184 publications, the mean age at presentation was estimated to be 9.1 +/- 5 years. Children often presented with severe upper extremity or renovascular hypertension (>87%). Other presentations included abdominal bruit (23%), headache (13%), lower-extremity claudication (10%) and absent femoral pulses (7%). 1 Left untreated, patients can suffer from vascular sequelae such as left ventricular hypertrophy (30%), congestive heart failure (9%), cardiomegaly (7%), neurologic deficits (7%) or decreased renal function (6%).
The etiology of MAS is not well understood; approximately 64% of cases are idiopathic. Congenital MAS is caused by a developmental anomaly in the fusion and maturation of the paired embryonic dorsal aortas and typically is diagnosed early in life. Acquired causes of MAS include neurofibromatosis type 1, Williams syndrome, Alagille syndrome, fibromuscular dysplasia, retroperitoneal fibrosis, mucopolysaccharidosis, and arteritides such as Takayasu arteritis. 3-7
While catheter angiography was previously considered as the gold standard for diagnosing idiopathic MAS, 8 the condition now can be effectively diagnosed with computed tomography angiography (CTA) or magnetic resonance angiography (MRA). No guidelines exist on the optimal workup for MAS, but detection of aortic stenosis by ultrasound has also been described, with patients often receiving a subsequent CTA or MRA to further delineate the involved vessels. In the aforementioned systematic review, segmental or diffuse narrowing of the abdominal aorta was noted in 97% of cases, with thoracic involvement in 3%, renal artery involvement in 66.2%, superior mesenteric artery involvement in 29.5%, and celiac axis involvement in 22.4%. 1
A multidisciplinary approach is critical to managing patients with MAS. Treatment strategies vary depending upon the nature of the aortic stenosis, the extent of visceral involvement, and the presence of secondary co-morbidities. Medical management has been utilized in uncomplicated cases, with varying results. In addition to medical management, patients can be treated with endovascular interventions such as angioplasty with or without stent placement or surgery.
Surgical options depend on location of the stenosis and the affected vessels. Potential options include aorto-aortic bypass, reconstruction patch graft, or renal auto-transplantation. Indications for surgical or endovascular intervention include high risk of renal failure, refractory hypertension, claudication, and intestinal ischemia. 8 Blood pressure control following endovascular intervention with and without subsequent medication is reported to be 36% and 18%, respectively. After surgical intervention, control with and without subsequent medication is reported to be 55% and 25%, respectively. 1
Conclusion
MAS may be congenital or acquired. Medical therapy may control blood pressure but does not treat the underlying aortic stenosis. Currently, no therapeutic approach is curative. Surgery is the primary treatment for congenital causes of MAS, with balloon angioplasty or cutting balloon angioplasty a consideration in other select instances.
References
- Rumman RK, Nickel C, Matsuda-Abedini M, et al. Disease beyond the arch: a systematic review of middle aortic syndrome in childhood. Am J Hypertens. 2015;28(7):833-846. DOI: 10.1093/ajh/hpu296
- Cohen JR, Birnbaum E. Coarctation of the abdominal aorta. J Vasc Surg. 1988; 8:160–164.
- Connolly JE, Wilson SE, Lawrence PL, Fujitani RM. Middle aortic syndrome: distal thoracic and abdominal coarctation, a disorder with multiple etiologies. J Am Coll Surg. 2002; 194:774–781.
- Criado E, Izquierdo L, Luján S, Puras E, del Mar Espino M. Abdominal aortic coarctation, renovascular, hypertension, and neurofibromatosis. Ann Vasc Surg. 2002;16:363–367.
- Raas-Rothschild A, Shteyer E, Lerer I, Nir A, Granot E, Rein AJ. Jagged1 gene mutation for abdominal coarctation of the aorta in Alagille syndrome. Am J Med Genet. 2002; 112:75–78.
- Radford DJ, Pohlner PG. The middle aortic syndrome: an important feature of Williams’ syndrome. Cardiol Young. 2000;10:597–602.
- Taketani T, Miyata T, Morota T, Takamoto S. Surgical treatment of atypical aortic coarctation complicating Takayasu’s arteritis-experience with 33 cases over 44 years. J Vasc Surg. 2005;41:597–601.
- Sethna CB, Kaplan BS, Cahill AM, Velazquez OC, Meyers KE. Idiopathic mid-aortic syndrome in children. Pediatr Nephrol.2008; 23:1135–1142. doi: 10.1007/s0067-008-0767-4
References
Citation
K Z, RB T, CM S, SA J, AJ T. Middle Aortic Syndrome. Appl Radiol. 2021;(6):21-23.
November 5, 2021