Median Arcuate Ligament Syndrome
Images




Case Summary
An adolescent with a two-year history of abdominal pain and vomiting after meals presented with diffuse abdominal tenderness on palpation. The diagnostic workup consisted of a normal upper gastrointestinal series and esophagogastroduodenoscopy, as well as a celiac nerve block which did not relieve the postprandial pain. Subsequently, the patient underwent a surgical division of the median arcuate ligament (MAL), with resultant decreased intensity and frequency of the postprandial pain and vomiting.
Imaging Findings
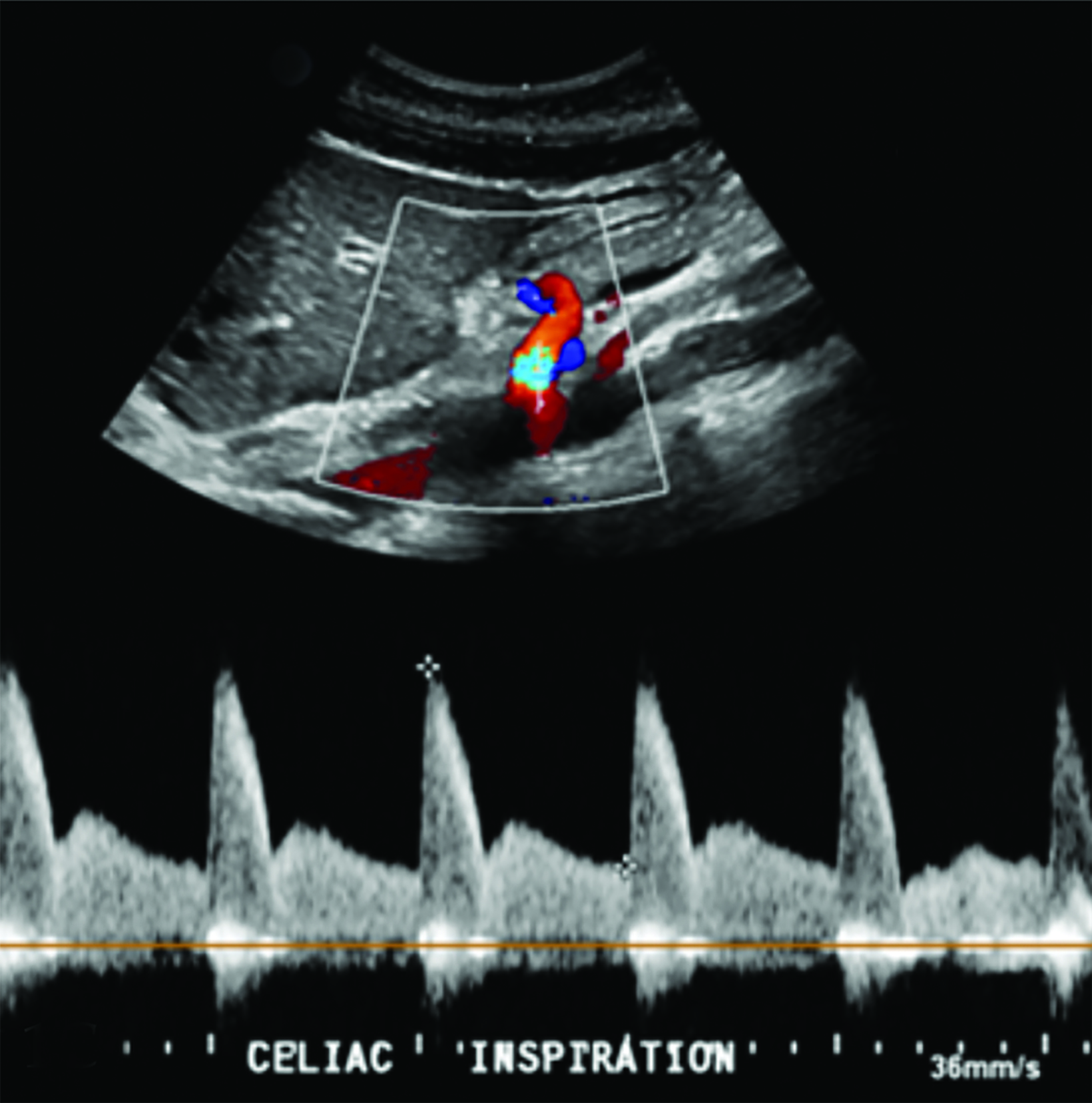
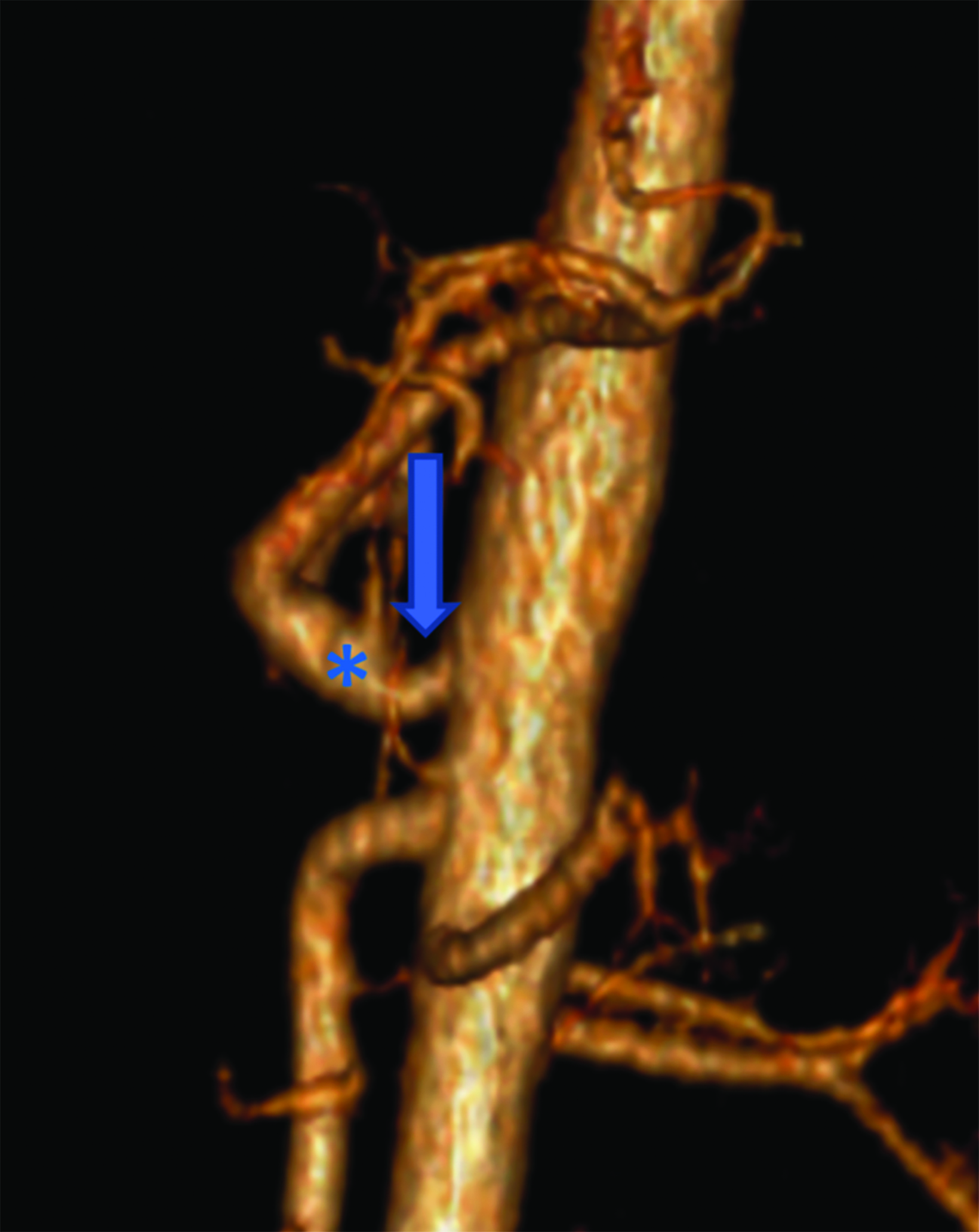
Abdominal ultrasound (US) of the aorta and celiac axis during inspiration and expiration (Figure 1) demonstrated a narrowed celiac origin on inspiration and a “hooked” appearance of the celiac origin with expiration. Duplex Doppler US images on inspiration and expiration demonstrated an abnormally increased peak systolic velocity of 318 cm/sec (normal < 200cm/second) during expiration and a deflection angle > 50 degrees (Normal < 50 degrees). An abdominal CT angiogram (Figure 2) with 3D reconstruction revealed arterial narrowing at the origin of the celiac axis with post stenotic dilatation.
Diagnosis
Median arcuate ligament syndrome.
The differential diagnosis includes gastritis, Crohn disease, inflammatory bowel syndrome, peptic ulcer disease, and gastroesophageal reflux disease (GERD).
Discussion
Median arcuate ligament syndrome (MALS), also known as Dunbar Syndrome, is an uncommon condition where the MAL compresses the celiac trunk. The condition was first discovered in 1917 by Benjamin Lipshutz while dissecting cadavers; he noted the MAL overlapping and compressing the celiac artery.1 Many cases of MALS are asymptomatic, owing to typically minor compression and/or development of collateral circulation, especially involving the arterial arcades of the pancreas.2 In symptomatic cases, the symptoms are often attributed to other abdominal pathologies. It is usually diagnosed after other etiologies have been excluded. MALS typically affects women 20-40 years of age, most commonly at a ratio of 2:1 or 3:1 that of the general population. The incidence of MALS is approximately 2 per 100,000.3
As the first major branch of the descending abdominal aorta, the celiac artery usually originates just below the crus of the diaphragm. The vessel supplies blood to the liver, stomach, abdominal esophagus, spleen, and the superior portions of the duodenum and the pancreas. The MAL crosses over the aorta just above the celiac trunk, with its primary role to attach the diaphragm to the spine.
In patients with MALS, the ligament is lower than normal, compressing the celiac trunk and reducing blood flow to the abdominal organs, especially the stomach and liver.4 This hypoperfusion leads to many of the manifestations of MALS, which may rarely even include necrosis of the stomach and liver if left untreated.5 The most prevalent signs and symptoms are postprandial nausea, diarrhea, vomiting, and food aversion. In addition, a bruit may be present in the epigastric region. Food aversion often leads to significant weight loss, typically more than 20 pounds.
The pain is believed to be caused by compression of the celiac plexus, the collection of nerves located directly over the celiac artery.6
Other common etiologies such as GERD, gastritis, and Crohn disease must first be excluded before MALS can be diagnosed. Patients in suspect cases will undergo Doppler ultrasound and CT examination.7 In those with MALS, Doppler ultrasound of the celiac artery will reveal lower blood flow, suggesting celiac artery stenosis. As stenosis and/or compression occur the peak systolic velocity (PSV) increases, serving as a useful parameter for diagnosis. The PSV must be obtained at end inspiration, as in 13-51% of healthy individuals the celiac artery is compressed during expiration, although this is clinically insignificant.3 During inspiration, the celiac trunk moves inferiorly in the abdominal cavity, relieving some of the compression and resulting in a lower PSV and potentially creating a false negative result. A peak systolic velocity of ≥200cm/s within the artery during expiration has a sensitivity of 75% and a specificity of 89% for MALS.3
Computed tomography scans with sagittal reconstruction are also useful in diagnosing MALS. Classic findings are narrowing and inferior displacement of the proximal celiac artery, forming a “J” shape or “hooking” of the artery. This helps to differentiate MALS from atherosclerosis in older adults. Catheter angiography is the gold standard for detecting arterial pathology, although CT angiography may obviate invasive imaging in most cases. Angiography may reveal post-stenotic dilation.
Management of MALS revolves around decompressing the celiac artery and the celiac plexus to restore blood flow and alleviate pain. Traditionally, open surgical decompression was the gold standard. The surgeon would dissect and separate the MAL fibers that were infringing on the celiac artery.8 While the procedure had a 0% 30-day mortality rate, it had a 16.1% complication rate. The most common complications were chylous ascites, pleural effusion, and wound dehiscence.9
More recently, laparoscopic surgery has become the preferred approach to treatment, as the procedure is minimally invasive, faster, (average 136 minutes), and requires a shorter hospital stay (average 3.8 days). Complications do occur with laparoscopic surgery, although at a lower rate than open surgery (5.1%).
A celiac plexus block (CPB) is another treatment option. In a study of 103 cases using CPB, 84% of patients reported lower post procedure pain scores, with the average score dropping from 6.3 to 0.9 on a 0-10 scale.10 Nausea decreased from 37.9% before the procedure to 11.6% afterward. However, although CPB has a lower complication rate (0.97%) than laparoscopic surgery, it is not recommended for patients with ischemia, which cannot be corrected with the interventional procedure.10
Conclusion
Median arcuate ligament syndrome is important to keep in mind when presented with patient complaints of generalized abdominal symptoms, as it can lead to debilitating signs and symptoms, including marked weight loss. Obtaining Doppler ultrasound and sagittal CT scans at the end of inspiration is the most effective means of visualizing celiac artery compression. Laparoscopic surgery is typically the therapeutic option.
References
Citation
J T, CM S, AJ T, RB T. Median Arcuate Ligament Syndrome. Appl Radiol. 2024;(3):13-16.
May 7, 2024