Macrocystic Lymphatic Malformation
Images
Case Summary
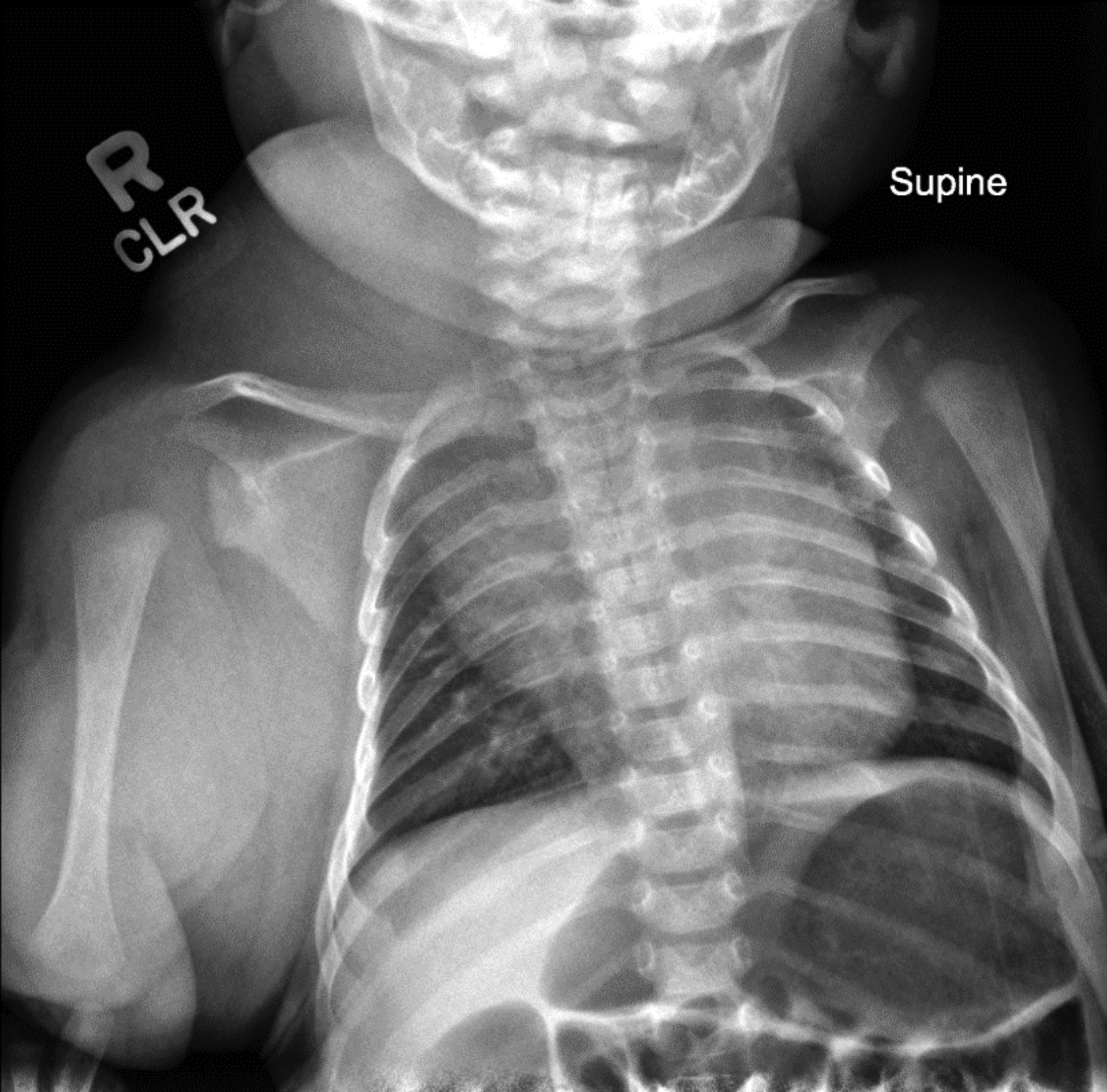
An infant presented with a large right-sided chest wall, axillary, and neck lymphatic malformation (LM), diagnosed prenatally. The patient had limited right upper extremity motion because of the large right chest wall and axillary mass.
Imaging Findings
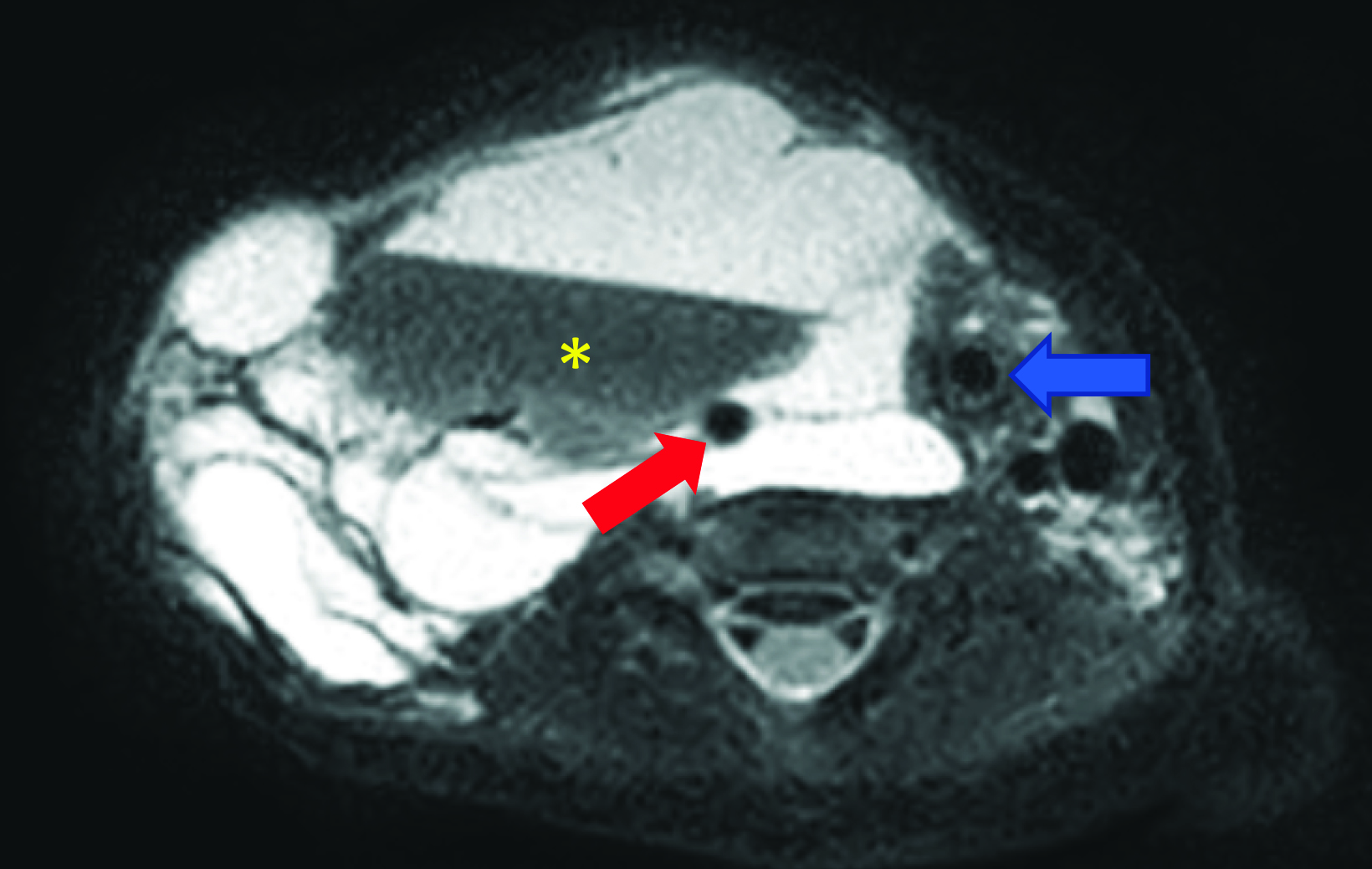
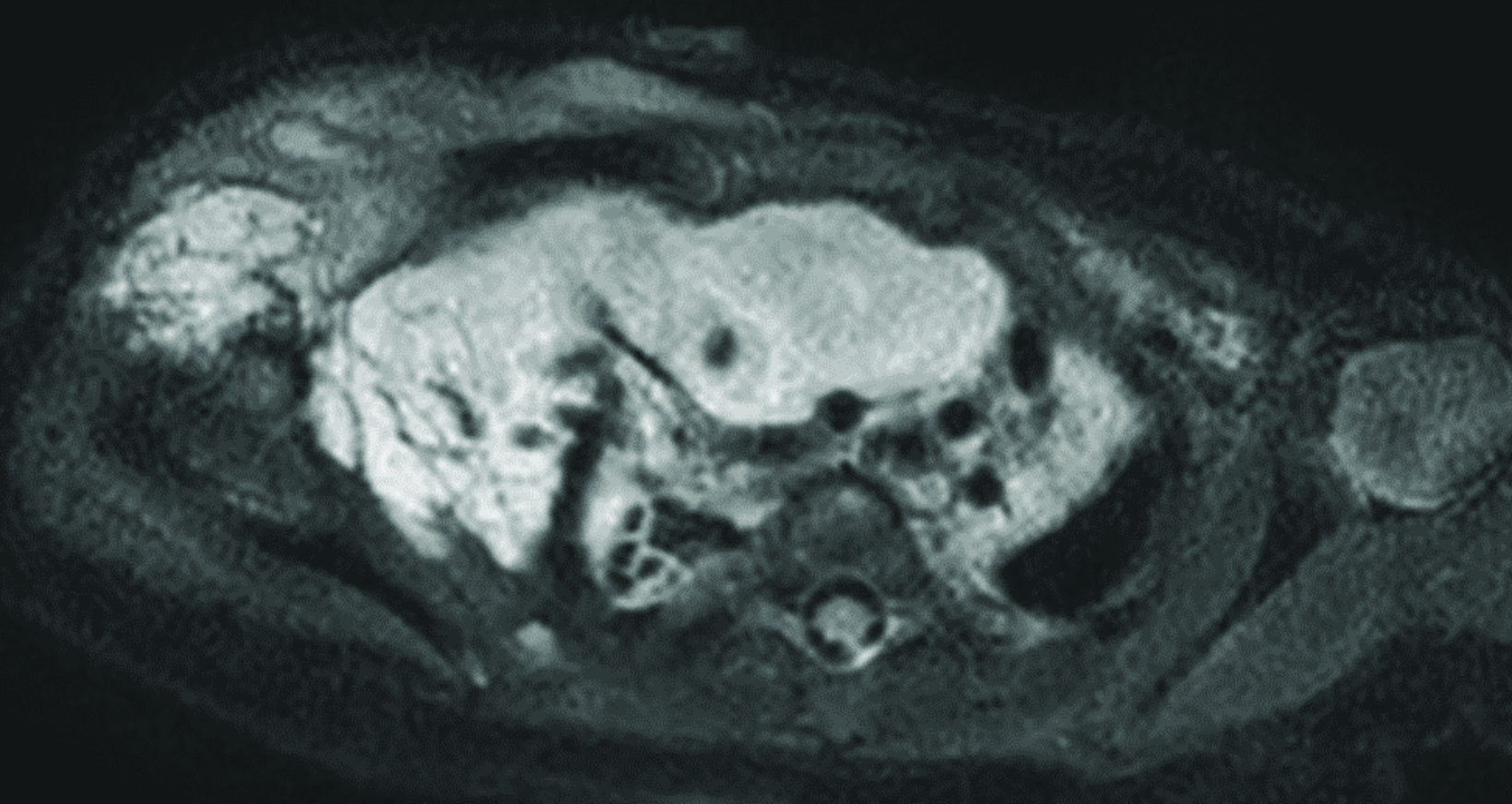
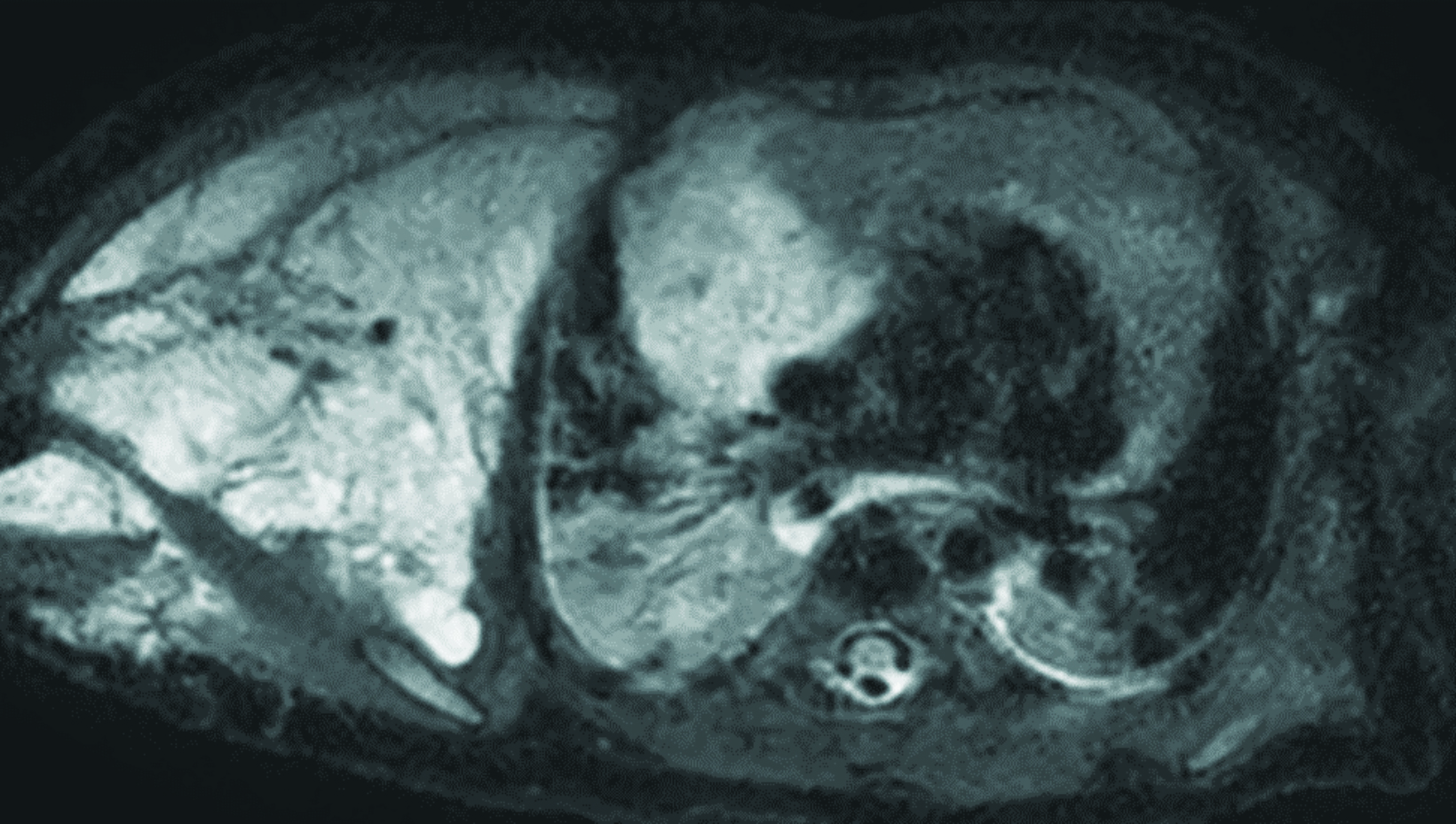
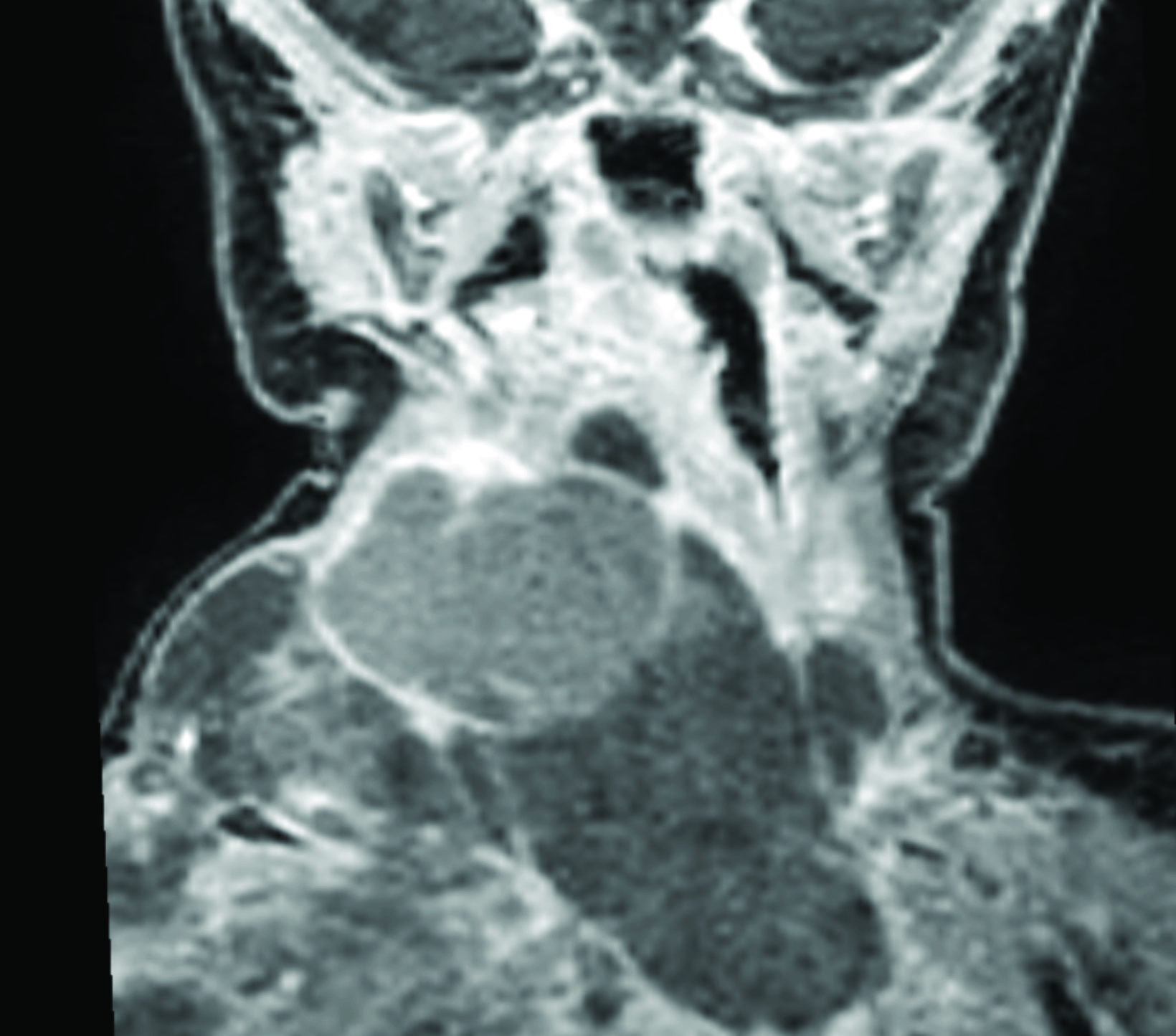
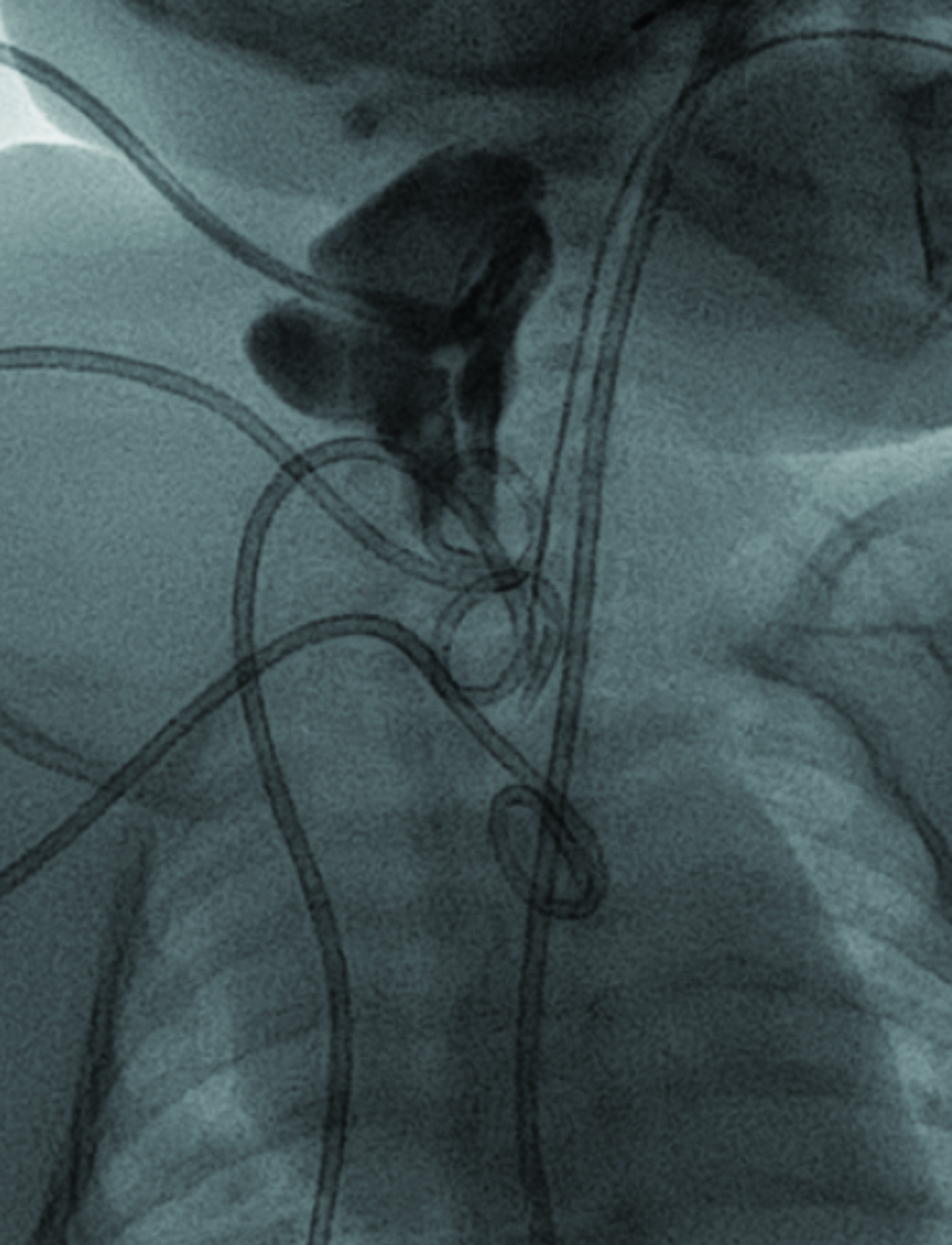
Chest radiograph (Figure 1) shows a soft-tissue mass involving the right neck and upper extremity. Chest MRI (Figure 2) revealed a large macrocystic mass extending from the base of the neck to the right upper extremity and into the mediastinum. Some of the cystic spaces had fluid-fluid levels. The LM was treated with image-guided (ultrasound and fluoroscopic) drainage and sclerotherapy (Figure 3).
Diagnosis
Macrocystic lymphatic malformation (formerly known as cystic hygroma).
Discussion
Lymphatic malformations are congenital masses resulting from errors in the development of the lymphatic endothelium and vasculature. They may form in any location, and be separate from the primitive lymphatics from which they derive.1 The most common location for this anomaly is within the head and neck. The axilla, chest, and perineum are the second-most common sites. Lymphatic malformations are particularly problematic because they tend to grow with the child, expand and enlarge over time, and may recur after treatment.
Lymphatic malformations may be divided into macrocystic and microcystic lesions. This is important because the drugs used for therapy differ. Most LMs contain macrocystic and microcystic components, with no histological difference between the two. Macrocystic lesions measure > 2 cm in diameter and present at birth as large, translucent, soft, compressible masses covered by normal skin.
Microcystic LMs are made up of smaller cysts or soft-tissue enlargement without cyst formation. They are congenital and may be noted at birth or appear in the first few years of life, presenting as a cluster of translucent or hemorrhagic vesicles that may intermittingly leak lymphatic fluid. Both types grow with the child and manifest in a waxing and waning course, with changes in size associated with increased hormonal activity, infections, intralesional hemorrhage, inflammation, and trauma.
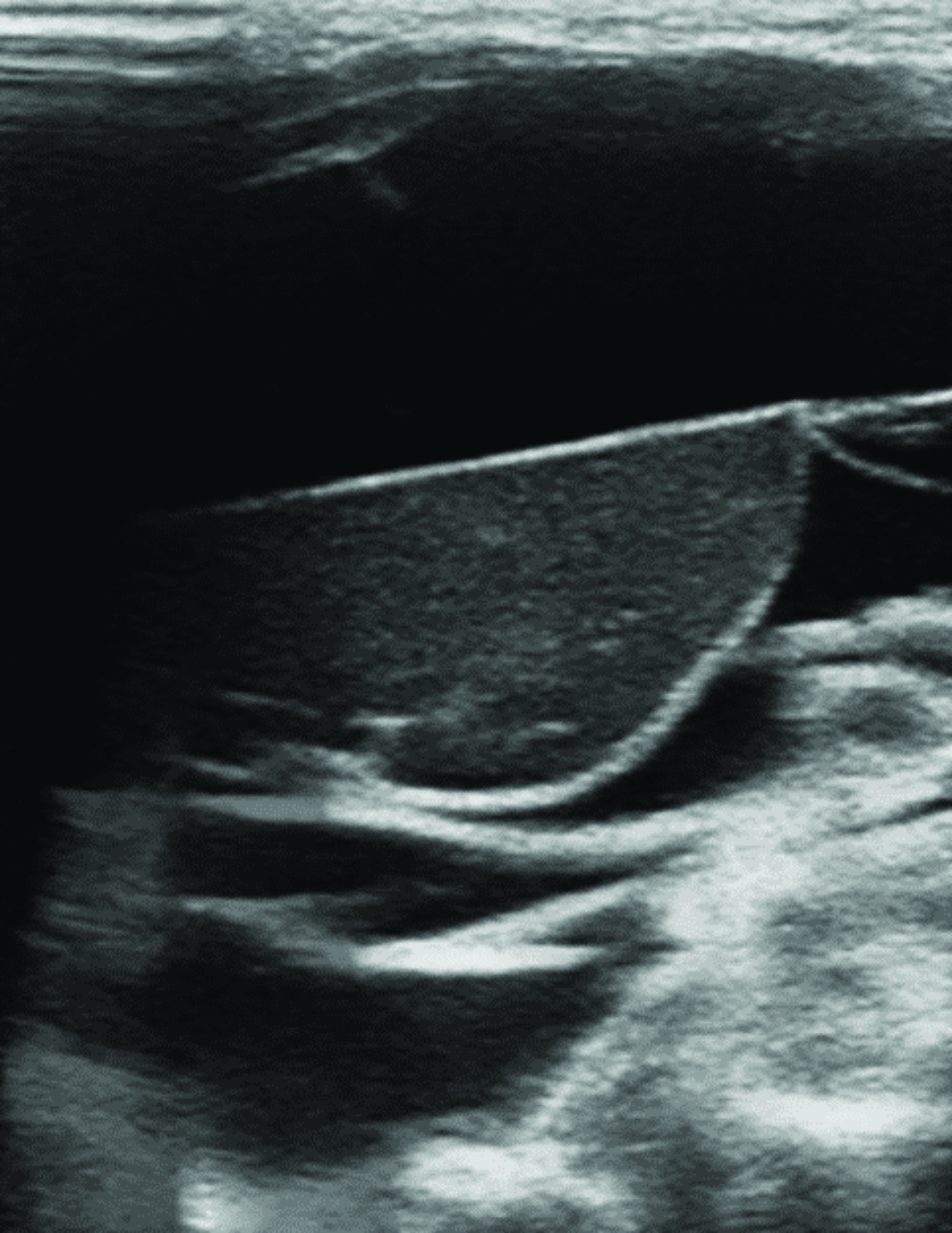
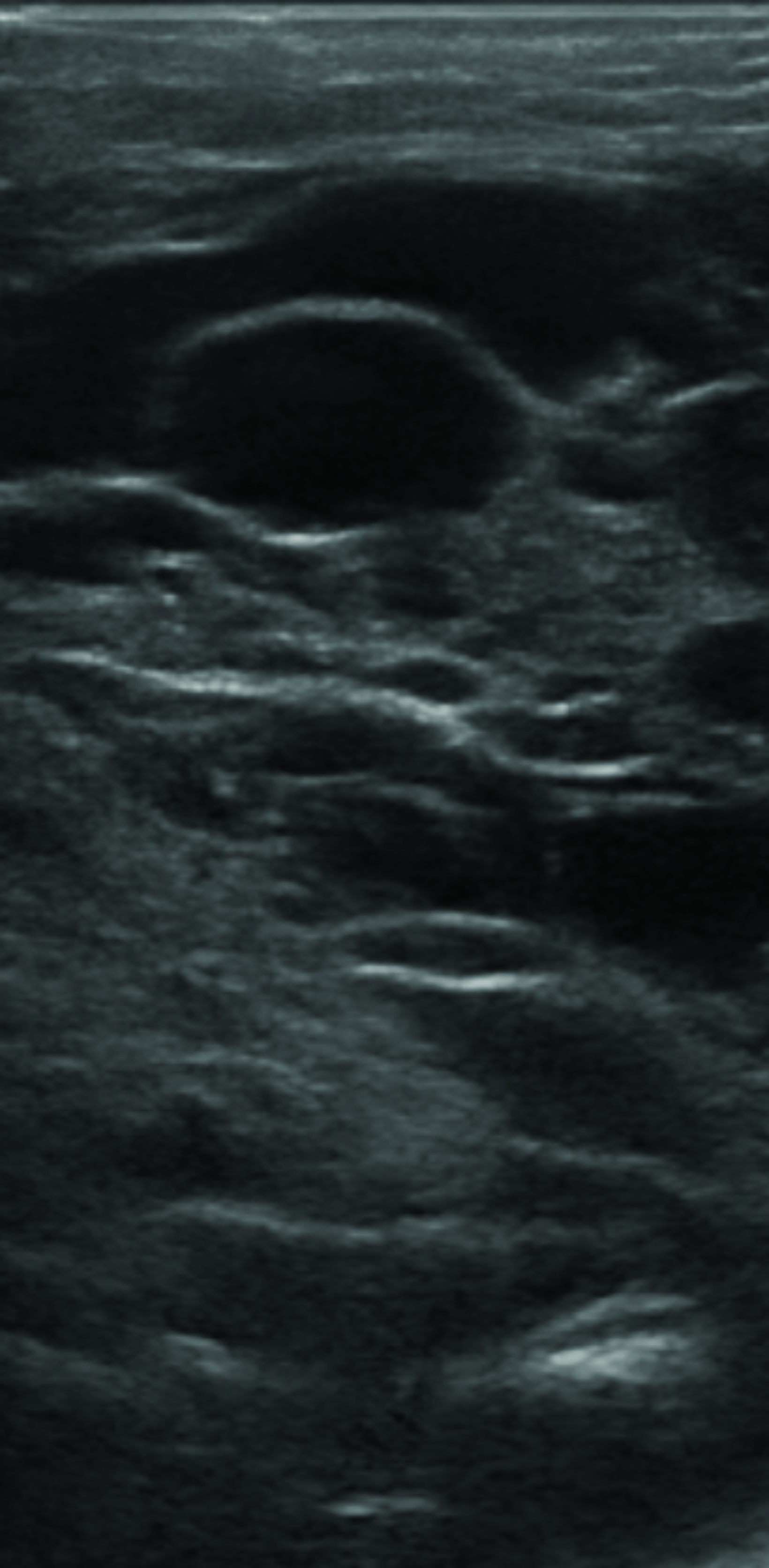
On ultrasound, macrocystic LMs appear as well-defined, multilocular, cystic masses with internal septa of varying thickness. The cystic contents are usually anechoic but may be hyperechoic if debris, infection, or hemorrhage complicate the malformation. These lesions are of low flow, with absent Doppler signal owing to the lack of flowing blood. The lesions are compressible and may have mobile internal debris. Microcystic LMs are frequently more poorly defined and may appear solid because of the dominant soft-tissue component.
Lymphatic malformations are often extensive and involve multiple tissue planes. They have a variable appearance on MRI because of the variable protein content within the cystic spaces. On T1 sequences, components of the malformation can be either hypointense or hyperintense. On T2 sequences, LMs are typically hyperintense. Fluid-fluid levels may be observed in macrocystic LMs due to dependent proteinaceous material, internal hemorrhage, or infection. Contrast enhancement is limited to the wall of the cyst and septations.
LMs are treated when disfiguring, symptomatic (eg, from airway compression) and complicated by bleeding or infection. Sclerotherapy is currently the first-line therapy for both types. Sclerotherapy of macrocysts involves aspiration of the cyst contents, followed by injection of an inflammatory sclerosant (absolute alcohol, sodium tetradecyl sulfate) that causes injury to the cell lining of the cyst. Cyst wall injury is followed by thrombosis and scarring, leading to collapse of the macrocyst that occurs up to 6-8 weeks post sclerotherapy.
Sclerotherapy of microcystic LMs (doxycycline, bleomycin) consists of injecting a sclerosing agent into multiple microcysts. Owing to the progressive nature of this disease, residual LMs may grow larger. In general, one can expect a better result from sclerotherapy of macrocysts.
Sclerosants utilized for LMs in the United States include doxycycline, bleomycin, sodium tetradecyl sulfate (STS), and ethanol. Doxycycline has been shown very effective and safe, with an additional theoretical benefit of reducing postoperative infection.2,5 However, doxycycline has potential to affect the enamel of the teeth in young children.
Bleomycin is used to treat microcystic LMs and is preferred where a reactive inflammatory response must be avoided, such as in intra-orbital microcystic LMs. Although an effective sclerosant, STS carries a higher rate of localized tissue swelling and is less effective than ethanol.
Ethanol is the most effective sclerosant but carries the highest complication rate, with large volumes (> 1cc/kg) to be avoided due to the risk of local and systemic complications, including central nervous system depression from alcohol intoxication, thromboembolism, and arrhythmias.4
Ethanol also can injure nerves and should not be used near important structures such as the cranial nerves.6
The most common complication of sclerotherapy is skin ulceration, which is more frequent with superficial lesions and the use of ethanol.4 Skin ulceration is managed with local wound care.
Sclerotherapy alleviates symptoms and has superior efficacy, less disfigurement, and lower complication rates compared to surgical resection.2 Resection may be indicated for small, well-localized LMs that can be removed for a cure, or symptomatic LMs after pretreatment with sclerotherapy.1,7
Additionally, when considering resection, the postoperative scar/deformity should be weighed against the preoperative appearance of the lesion. Wound healing may be a problem if an incomplete resection is performed because of continuous fluid leakage. For diffuse malformations, subtotal resections are preferred to complete removal, as total resections may cause a deformity worse than the initial lesion and come with a high rate of LM recurrence (35%–64%).1,7
Sirolimus (rapamycin) has recently emerged as an effective agent for the medical management of LMs. Sirolimus works as an mTOR inhibitor. mTOR acts as a master switch of numerous cellular processes, including cellular catabolism and anabolism, cell motility, angiogenesis, and cell growth. A 2018 systematic review by Wiegand et al found 60 of 63 patients from 20 studies of different LM subtypes had a treatment response to sirolimus.8
However, many of the studies included did not quantify the treatment response. Further randomized, controlled studies are required to analyze the efficacy and long-term adverse effects of sirolimus before evaluating its potential as a medical intervention.
Conclusion
Lymphatic malformations are rare, congenital, vascular malformations that progress over time, grow with the child, and rarely regress spontaneously. They are characterized as macrocystic, microcystic, or mixed lesions. Their progression can compress nearby structures and they can be complicated by hemorrhage or infection.
Sclerotherapy is the current first-line therapy; sclerosants cause an inflammatory reaction that leads to LM collapse. Ultrasound plays an important role in the diagnosis, characterization, and image-guided treatment of LMs.
References
Citation
DD A, RB T, CM S, AJ T. Macrocystic Lymphatic Malformation. Appl Radiol. 2023;(4):30-33.
June 23, 2023