Lower Dose Prostate Cancer Therapies Prove Effective and Better Tolerated
Images
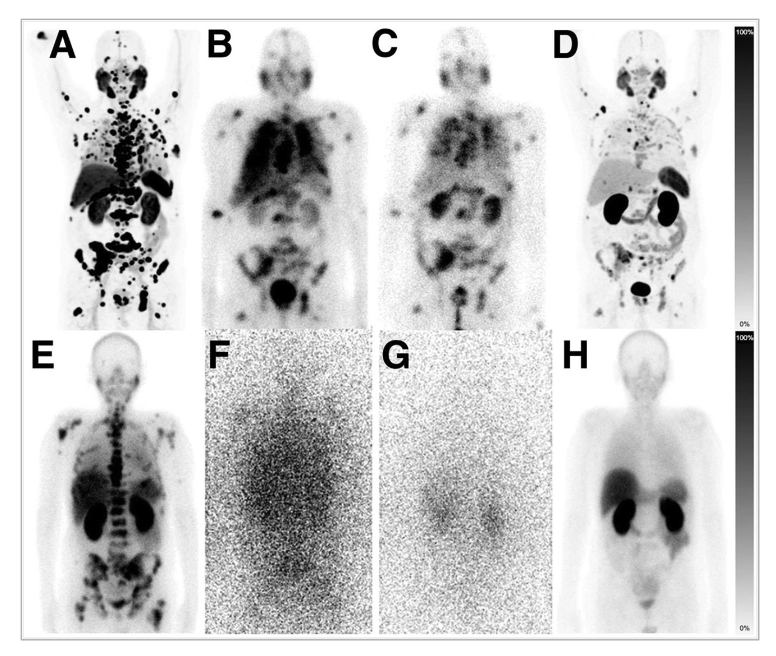
New research published in the July issue of The Journal of Nuclear Medicine shows that two reduced dose radiopharmaceutical therapies for advanced-stage metastatic castrate-resistant prostate cancer have been shown to be just as effective as the standard dose. Treatment with deescalated 225Ac-PSMA-617 or a cocktail therapy of 177Lu/225Ac-PSMA-617 resulted in similar median overall survival and prostate specific antigen (PSA) response rates as the standard 225Ac-PSMA-617 dose and was better-tolerated among patients.
The standard dose for 225Ac-PSMA targeted radiopharmaceutical alpha-therapy is 100 kBq per kilogram of body weight or an approximation of eight MBq. After multiple treatment cycles of this dose, salivary gland toxicity often increases and patients experience uncomfortable dry mouth. For some patients this impact on their quality of life causes them to discontinue treatment.
“Preliminary data from other studies has shown that reduced doses of PSMA treatment result in lower rates of dry mouth while still maintaining promising anti-tumor activity,” said Hendrik Rathke, MD, from the Department of Nuclear Medicine at Heidelberg University Hospital in Heidelberg, Germany. “In our study we aimed to determine the tolerability, PSA response rate, and overall survival observed in patients who received a regimen of less than 100 kBq of 225Ac-PSMA or an 177Lu/225Ac-PSMA-617 cocktail therapy.”
Researchers conducted a retrospective analysis of 233 patients who were treated with 225Ac-PSMA at Heidelberg University Hospital from 2014-2022; 104 received a median of six MBq of 225Ac-PSMA monotherapy and 129 received an 177Lu/225Ac-PSMA-617 cocktail therapy. Baseline characteristics, PSA response, and overall survival were compared with the most appropriate historical controls.
Of the patients who received 225Ac-PSMA monotherapy, 55 patients (53 percent) presented with a best PSA response of at least 50 percent. In the 177Lu/225Ac-PSMA-617 cocktail group, a best PSA response of at least 50 percent was observed in 74 patients (57 percent). The median overall survival was nine months in the 225Ac-PSMA monotherapy and was 15 months in the 177Lu/225Ac-PSMA-617 cocktail group. If adjusted for prognostic baseline characteristics, the efficacy of both regimens was not significantly different.
“The baseline prognostic characteristics of patients in this study are worse than patients who were recruited to the VISION clinical trial, yet the median overall survival and PSA response rates are equivalent,” noted Rathke. “This leads to the assumption that patients with late stage prostate cancer can benefit from targeted radiopharmaceutical alpha-therapy.”
The authors of “Deescalated 225Ac-PSMA-617 Versus 177Lu/225Ac-PSMA-617 Cocktail Therapy: A Single-Center Retrospective Analysis of 233 Patients” include Hendrik Rathke, Department of Nuclear Medicine, Heidelberg University Hospital, Heidelberg, Germany, and Department of Nuclear Medicine, Bern University Hospital, Bern, Switzerland; Erik Winter, Uwe Haberkorn, and Clemens Kratochwil, Department of Nuclear Medicine, Heidelberg University Hospital, Heidelberg, Germany; Frank Bruchertseifer and Alfred Morgenstern, Joint Research Centre, European Commission, Karlsruhe, Germany; Manuel Rörich, Department of Nuclear Medicine, Heidelberg University Hospital, Heidelberg, Germany, and Department of Nuclear Medicine, Mainz University Hospital, Mainz, Germany; and Frederik Lars Giesel, Department of Nuclear Medicine, University Hospital Duesseldorf, Duesseldorf, Germany.