Intraoperative MRI Guides Precision Treatment in Parkinson Disease Clinical Trial
Images
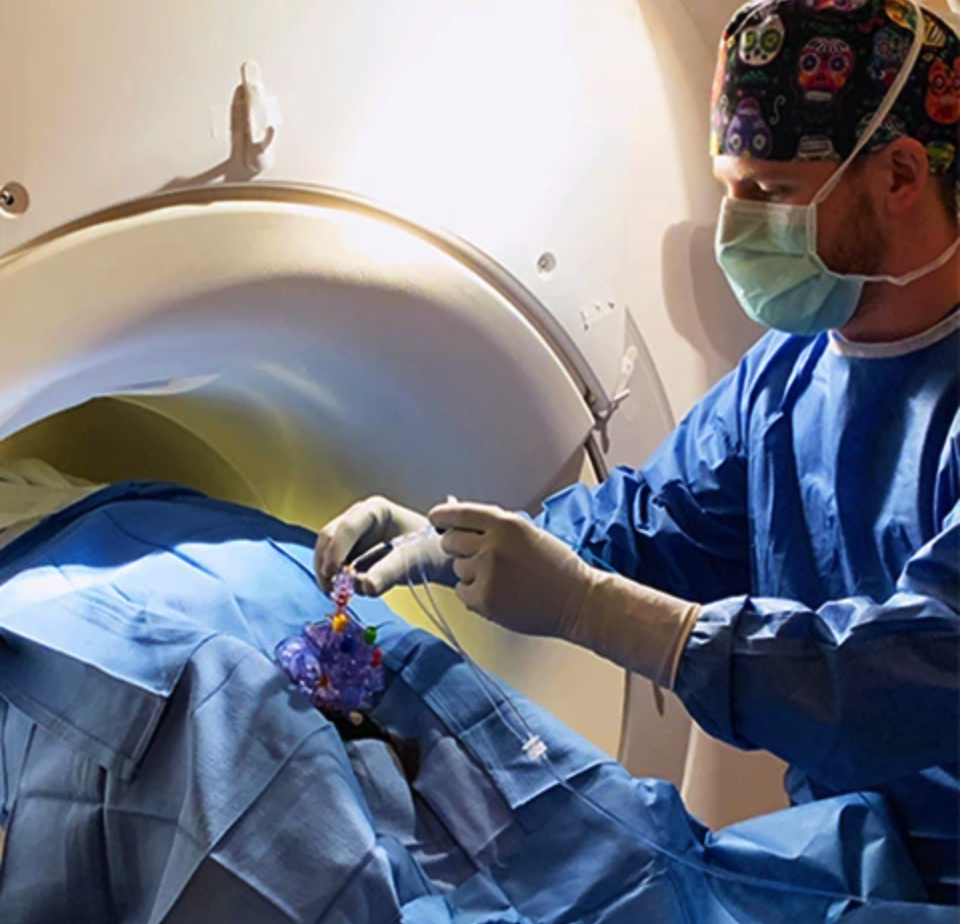
The MRI-guided ClearPoint Navigation System from Aspen Neuroscience is being utilized for all patients enrolled in the recently launched ASPIRO Phase 1/2a clinical trial for transplantation of dopaminergic neuron precursor cells (DANPCs) in patients with Parkinson Disease (PD). ASPIRO is an open label trial to assess safety and tolerability of ANPD001, an autologous, dopaminergic neuron cell replacement therapy for participants with moderate to severe PD.
"By the time of diagnosis, it is common for people with Parkinson to have lost the majority of dopaminergic neurons, leading to progressive loss of motor and neurological function," explained Edward Wirth III, MD, PhD, Chief Medical Officer of Aspen Neuroscience. "To replace these lost cells, we must target a very specific area of the brain with a high degree of surgical precision. Utilizing the latest advances in intraoperative MRI guided techniques provided by the ClearPoint system, the patient's new cells are transplanted, one microliter at a time, to the exact area where they are most needed."
The Autologous-derived Study of a Parkinson's Investigational Regenerative therapy in an Open-label trial (ASPIRO) is a Phase 1/2a clinical trial to assess the safety, tolerability, and potential efficacy of ANPD001 in patients with moderate to severe Parkinson disease. The dose escalation study includes patients 50–70 years of age and excludes patients with cognitive impairment and other comorbidities that could preclude treatment. All enrolled patients are under the care of a movement disorder specialist.
The primary study endpoint is safety and tolerability of ANPD001. Secondary endpoints include improvement in "on" time, when patients experience periods of symptom control, and improvements in motor symptoms and quality of life based on standard Parkinson disease rating scales.
ANPD001 is an investigational autologous neuronal replacement therapy being studied as a regenerative therapy for PD. Aspen's personalized approach means that patients do not require immunosuppressive drugs to counteract the body's immune response against foreign cells.
DANPCs are transplanted to the putamen, a small section located in the mid-brain, in a single transplantation procedure under ClearPoint MRI guidance, using the SmartFlow Cannula and the Aspen Metered Delivery Syringe (AMDS). This surgical approach was developed by the trial's lead neurosurgeon and renowned MRI-guided stereotactic neurosurgery pioneer Paul Larson, MD, FAANS, professor of neurosurgery at the University of Arizona College of Medicine – Tucson and neurosurgeon at Banner University Medical Center, Tucson.
"We are honored to be a part of such an important and groundbreaking study to demonstrate the ability of personalized medical approaches for treating neurodegenerative conditions," explained Jeremy Stigall, Chief Business Officer at ClearPoint Neuro. "The ClearPoint Neuro Navigation System is being used successfully in more than 80 centers worldwide for multiple applications, and investigational gene and cell therapy trials. We are thrilled to partner with Aspen to support the first multi-center trial for an autologous neuron replacement therapy for Parkinson disease."
The ClearPoint System utilizes intraprocedural MR images to provide real-time navigational instruction for the neurosurgeon, and confirmation that the desired anatomical target has been reached with submillimetric accuracy. Combined with The SmartFlow® Cannula, which is less than 2 millimeters in diameter, this allows minimally-invasive delivery of therapeutic agents in a patient's brain.
Related Articles
Citation
Intraoperative MRI Guides Precision Treatment in Parkinson Disease Clinical Trial. Appl Radiol.
July 2, 2024