Interventional Stroke Management: An Update
Images


Editor’s note: This is the second part of a two-part series. The first part appeared in the September-October 2021 issue of Applied Radiology.
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Great strides have been made in the advancement of interventional stroke management over the past few years. Prior studies have looked at patient selection and assessment to determine whether medical management or endovascular treatment (EVT) is warranted. For those patients triaged into the EVT pathway, additional factors must be considered, as patient anatomy, thrombus composition, and thrombus location vary. Prior administration of the thrombolytic agent intravenous tissue plasminogen activator (IV-tPA) should not influence the decision to perform mechanical thrombectomy.
Distal occlusions are notoriously difficult to manage with EVT secondary to vessel tortuosity and small caliber. Additionally, variability in size and composition of thrombi, whether hard or soft, poses technical challenges. Some of the great leaps in EVT include new and continually improving retrievable stent technology that can be tailored for just about any scenario.
Of note, while continual improvements in guide catheters, distal thrombectomy suction catheters, and retrievable stent technologies allow for potentially safer attempts at thrombectomy, thorough patient workup is crucial before thrombectomy is attempted. Potential complications include vessel injury and hemorrhage, particularly in the setting of poor collateral vascular supply or when distal thrombi are present. In addition, anatomical access challenges can be mitigated with access via the radial artery or direct carotid arterial puncture. Understanding how and when to use these opportunities will help to prevent some of these complications and may also improve clinical outcomes over time.
Vascular Access
Endovascular procedures historically have utilized femoral artery catheterization owing to the artery’s ease of access and large size. However, complications related to arterial access may include pseudoaneurysm formation, retroperitoneal hematoma, arteriovenous fistula, and artery occlusion. Femoral access can also be painful and requires the patient to lie supine for several hours afterward.1
In interventional cardiology, radial artery catheterization has been utilized over a longer time period, with fewer complications.1 Radial access can have a steep learning curve associated with accessing the cerebral vasculature. It also may be challenging to insert larger catheters into the radial artery. Peterson, et al, performed a meta-analysis of thrombectomy via transradial access in acute stroke. They found no significant differences in puncture time to reperfusion, mortality, radiographic reperfusion, or clinical outcomes.2
For tortuous or otherwise difficult anatomy, occasionally direct trans-carotid access may be performed under ultrasound guidance. Although most interventionalists are inexperienced in accessing the carotid artery directly, Scoco, et al, found trans-carotid approaches to be safe and effective with careful attention to technique and knowledge of anatomy.3 Complications include neck hematomas.
Posterior Circulation
While the first clinical trials that demonstrated the efficacy of endovascular thrombectomy analyzed anterior circulation proximal occlusions in patients that presented <4.5 hours after last known well, stent retrievers have also been successfully used to revascularize the posterior circulation. Several studies have achieved thrombolysis in cerebral infarction (TICI) grade 2b or grade 3 recanalization, indicating complete filling of the expected vascular territory but slower-than-normal or complete reperfusion, respectively. Compared to clinical outcomes for anterior circulation thrombectomies, however, posterior circulation thrombectomies generally have poorer outcomes, however the natural history of basilar artery occlusion is worse compared to the anterior circulation.4
Guide Catheter
Although not specifically recorded, most procedures in the first group of randomized control trials for EVT of acute large-vessel occlusion likely utilized only stent retriever thrombectomy technology, wherein a stent is placed temporarily during the procedure and retrieved at the conclusion. Since then, analyses of the additional use of balloon guide catheter technologies for thrombectomies have been performed.5
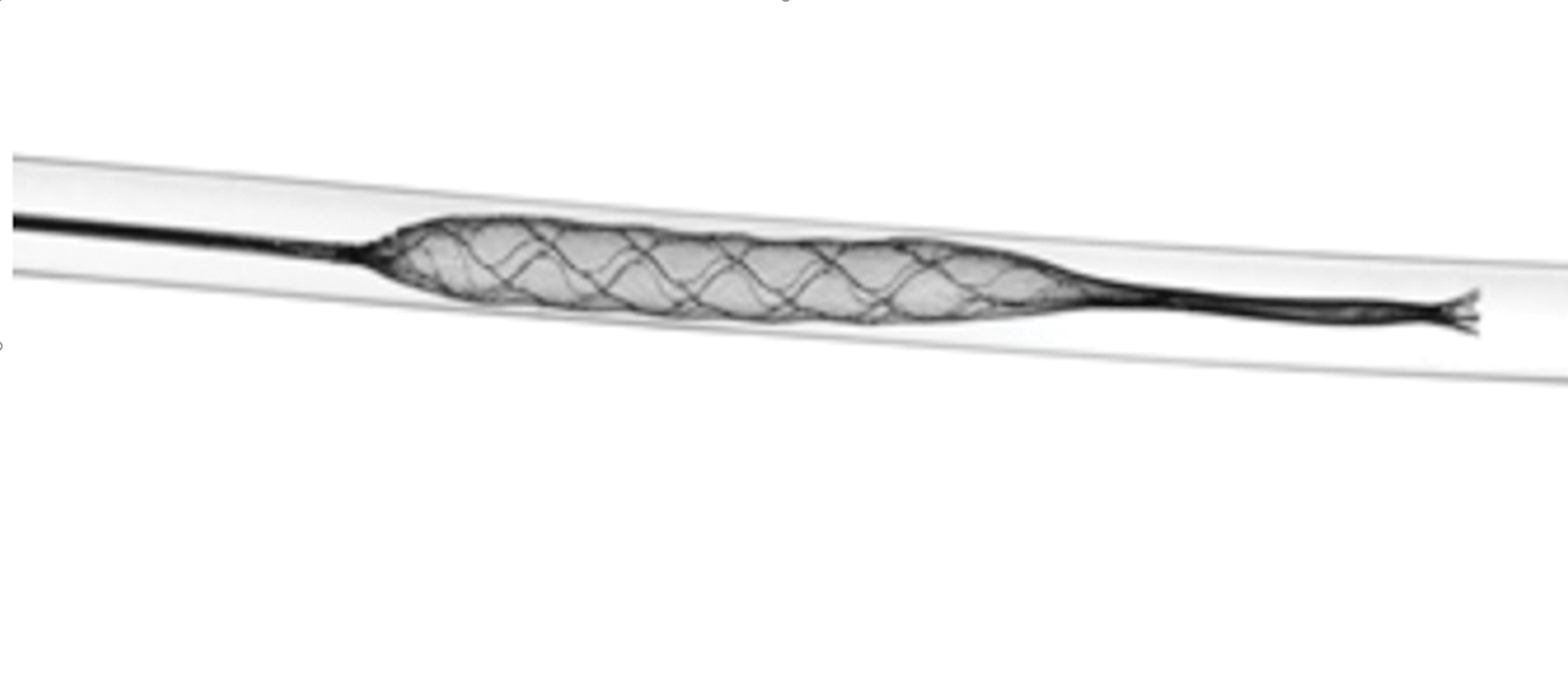
Balloon guide catheters are used to reduce clot fragmentation and distal embolization (Figure 1). The largest contributor to distal embolization during mechanical thrombectomy is when the clot becomes embedded in the retriever and is brought into the catheter. Components of, or the entire clot itself, can be sheared off the device as the clot enters the receiver. Balloon guide catheters placed proximally can momentarily block anterograde flow, preventing the emboli from traveling distally. However, balloon guide catheters can be more difficult to handle, may injure the parent artery during balloon inflation, and may be incompatible with other devices.6
The necessity of balloon guide catheters is still being debated. A retrospective analysis by Velasco, et al, demonstrated improved angiographic results and shorter procedure duration when they were used.7 Conversely, Bourcier, et al, reviewed data from the Endovascular Treatment in Ischemic Stroke registry and found that reperfusion and clinical results with and without balloon guide catheters did not differ significantly from thrombectomy via contact aspiration and stent retrievers.5
New Retrievable Stent Technologies
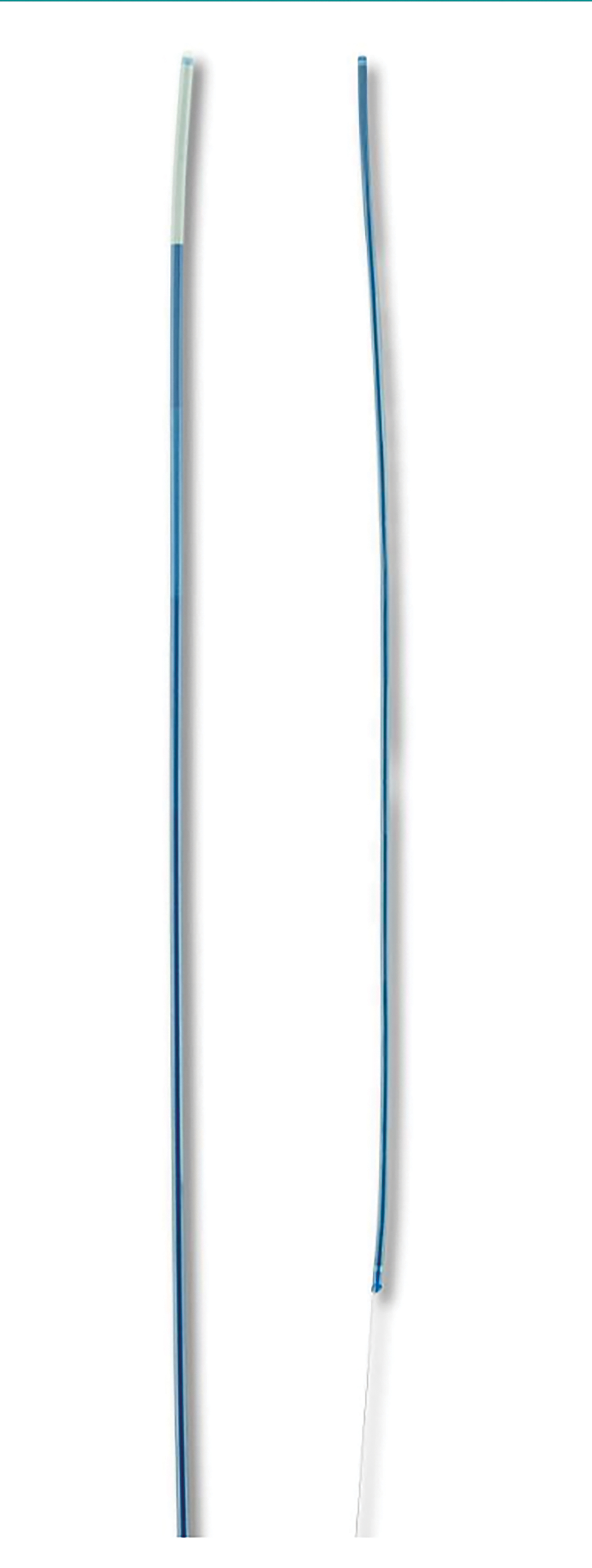
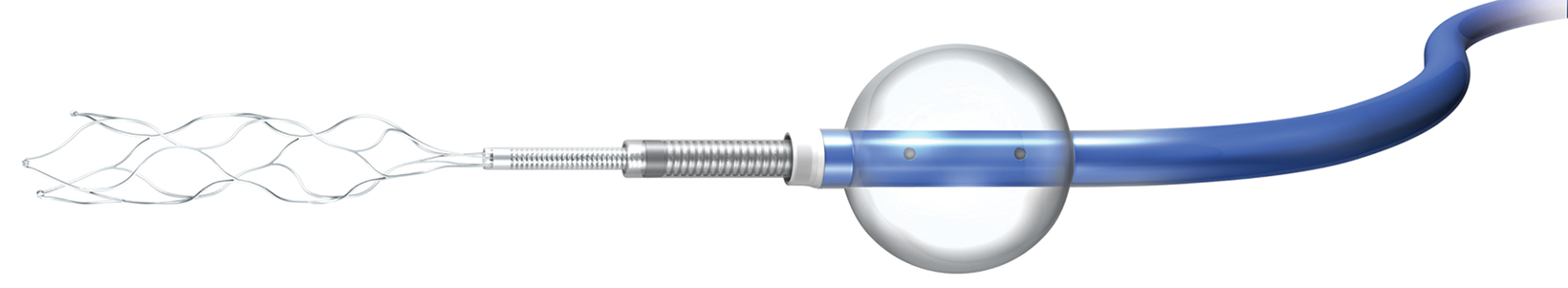
Numerous devices have been developed to improve the efficacy of retrievable stents. The Lazarus Effect Cover (Medtronic) employs a protective sheath around the stent to protect against distal embolization (Figure 2). The Q aspiration catheter (MIVI Neuroscience Inc.) consists of a control wire on the proximal catheter shaft (Figure 3). This allows the distal end of the catheter to function as an extension to increase suction of the guide catheter.4
The longer length of the stent retriever may play a role in its efficacy by allowing better placement and increasing the margin of error in patients with tortuous anatomy. It may also increase device stability distal to the clot, thus increasing the chances for successful thrombectomy.8
Vascular tortuosity can affect success rates in mechanical thrombectomy. Traveling around a sharp curve, the stent can become elongated, resulting in clot dislodgement. Fragmented or segmented stents can remain patent in tortuous anatomy, improving thrombectomy success rates.9
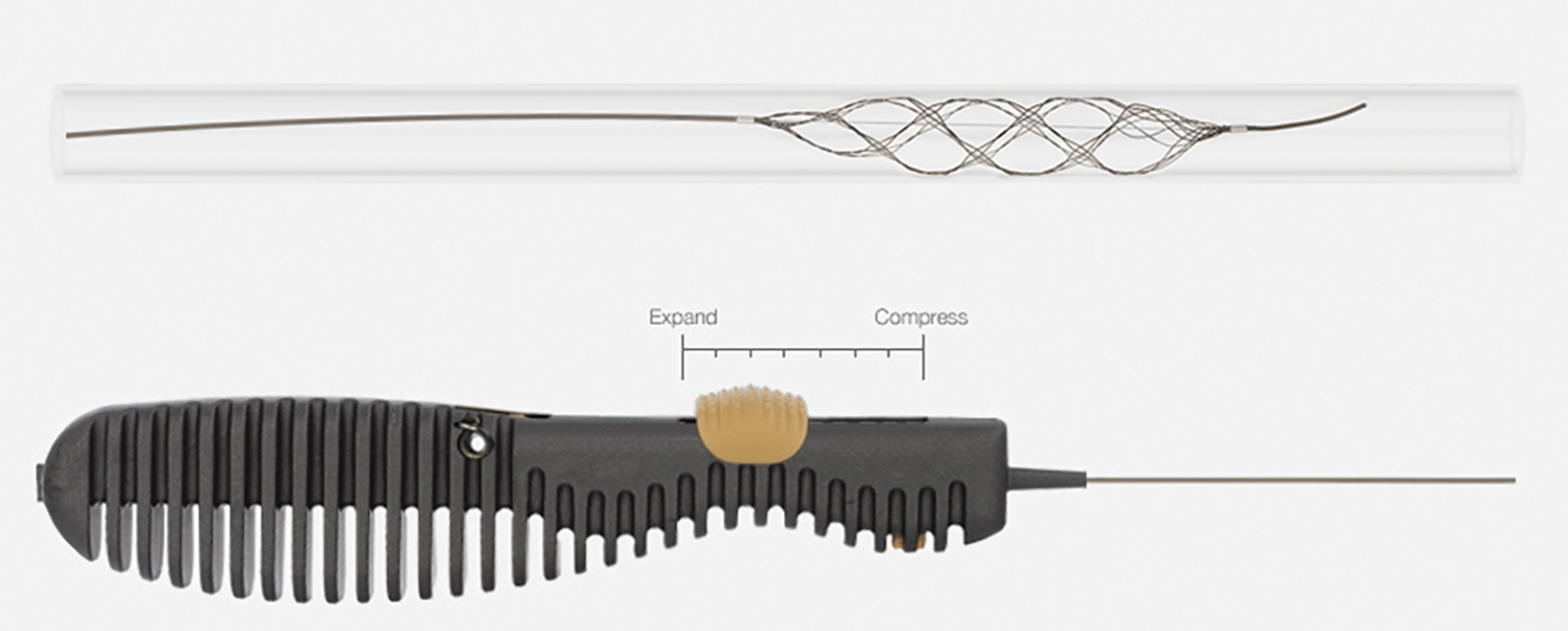
The Tigertriever (Rapid Medical) stent allows a manual, stepwise increase in radial force during device expansion (Figure 4). This permits slight overexpansion of the device compared to the affected artery and safer, controlled, graded expansion compared to other self-expanding stent retrievers. The device has demonstrated reperfusion rates and a safety profile compared to similar devices.10
Clot Properties
The properties of clots may have clinical implications. Intravenous administration of tPA has been shown most effective in small thrombi with red blood cell (RBC)-rich composition. Gunning et al suggest that that RBC content above 20% may dramatically increase friction properties of the clot, which could affect mechanical thrombectomy success rates.11 For thrombectomy efficacy, the proportion of fibrin may be relevant in determining the probability of successful retrieval, as fibrin-rich thrombi are firm, tough, and sticky, and therefore less likely to deform during mechanical thrombectomy. Furthermore, stent retrievers with high radial force, designed to capture rather than penetrate the thrombus, may perform better on firm thrombi. Clot properties may also determine whether a thrombus is prone to fragmentation during thrombectomy.12
Different neurointerventional techniques may be more useful for thrombectomy, depending on clot properties. With softer clots, the size and type of stents have less of an effect; therefore, small-caliber stents may be used to reduce the likelihood of vessel injury. However, with harder clots, a large-caliber aspirator, larger stent, and/or employing the “push-and-fluff” technique (which maximizes device expan sion), may be required. Subsequent direct aspiration following the use of a stent retriever may also be needed with harder clots.13
Micro-guide wires are used in mechanical thrombectomies to gain access distal to the thrombus. The round tip of the catheter aims to prevent unintended entry into perforating branches, and its modified pigtail shape helps it to move past the thrombus. Experiments with simulated clots have shown that the wire retains its shape with a soft clot; however, the wire can become stuck and deform when encountering a hard clot. This makes renavigating the micro-guidewire or reusing it for further thrombectomy attempts difficult.13
Distal Occlusions
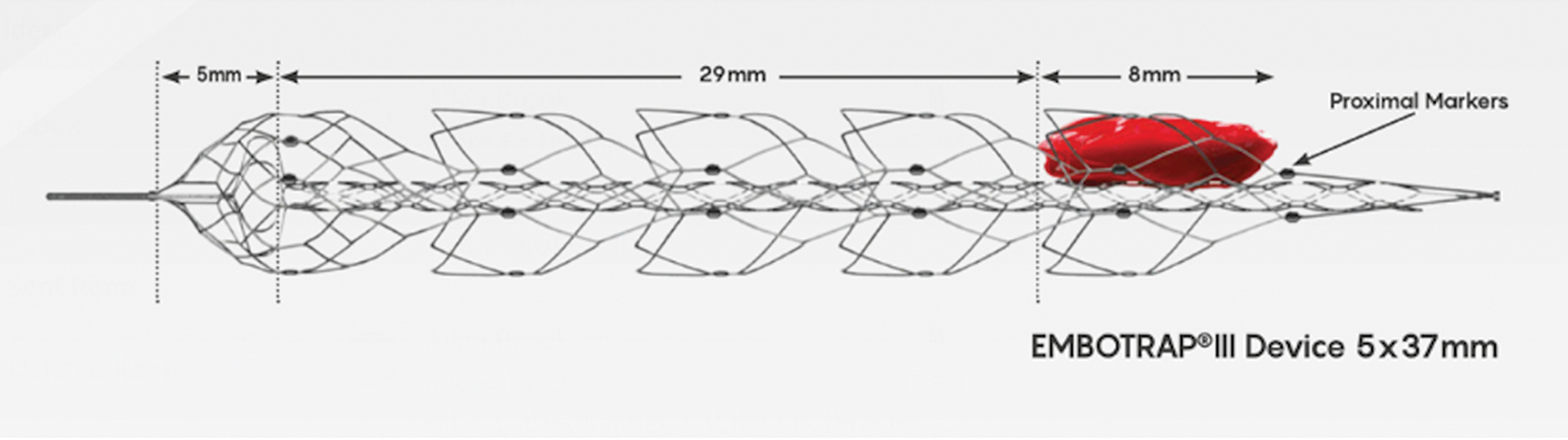
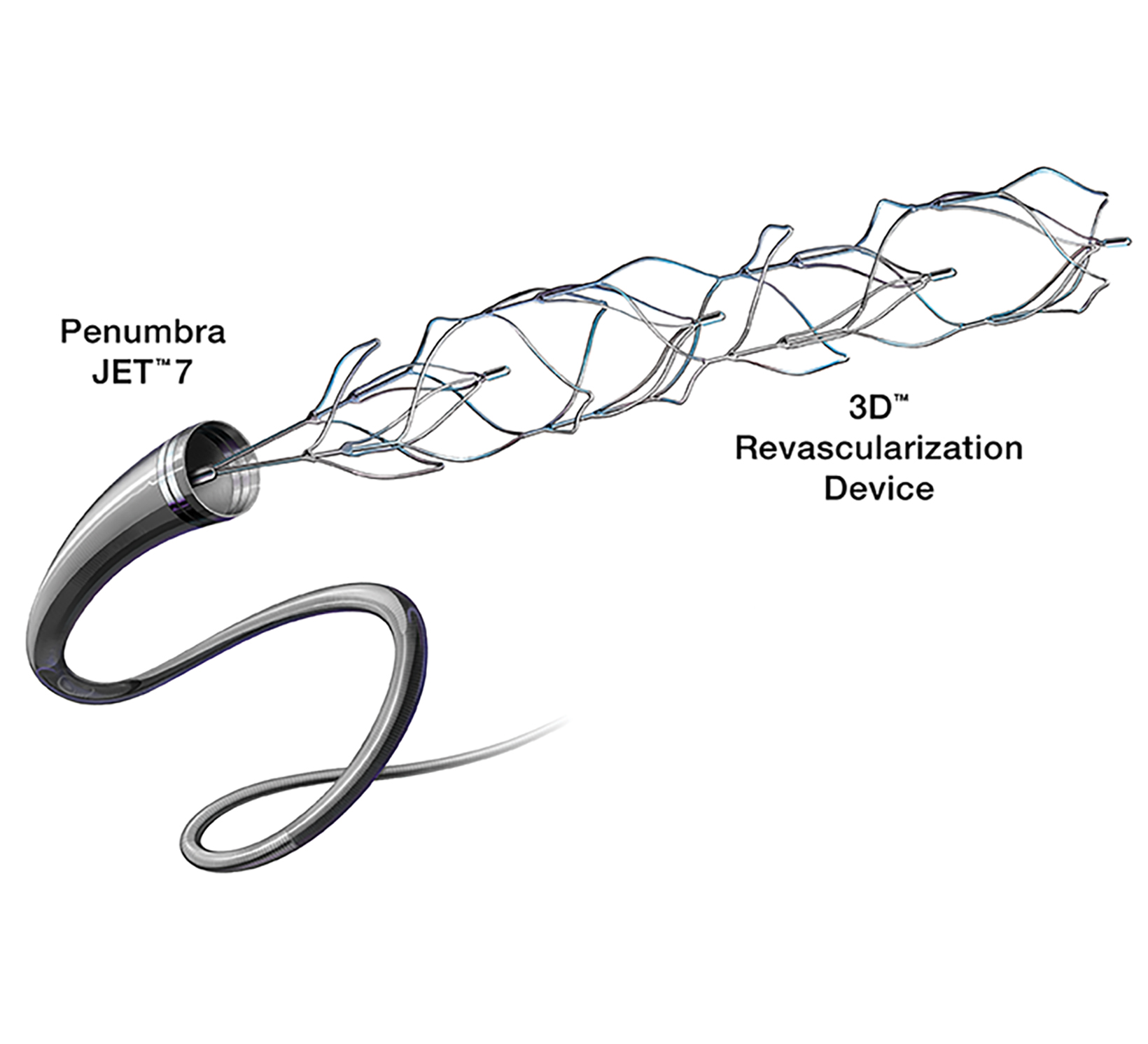
New stent retrievers can reach more distal vasculature than earlier devices and have additional capabilities, including smaller versions of commercially available stent retrievers and dynamically expanding stents.14
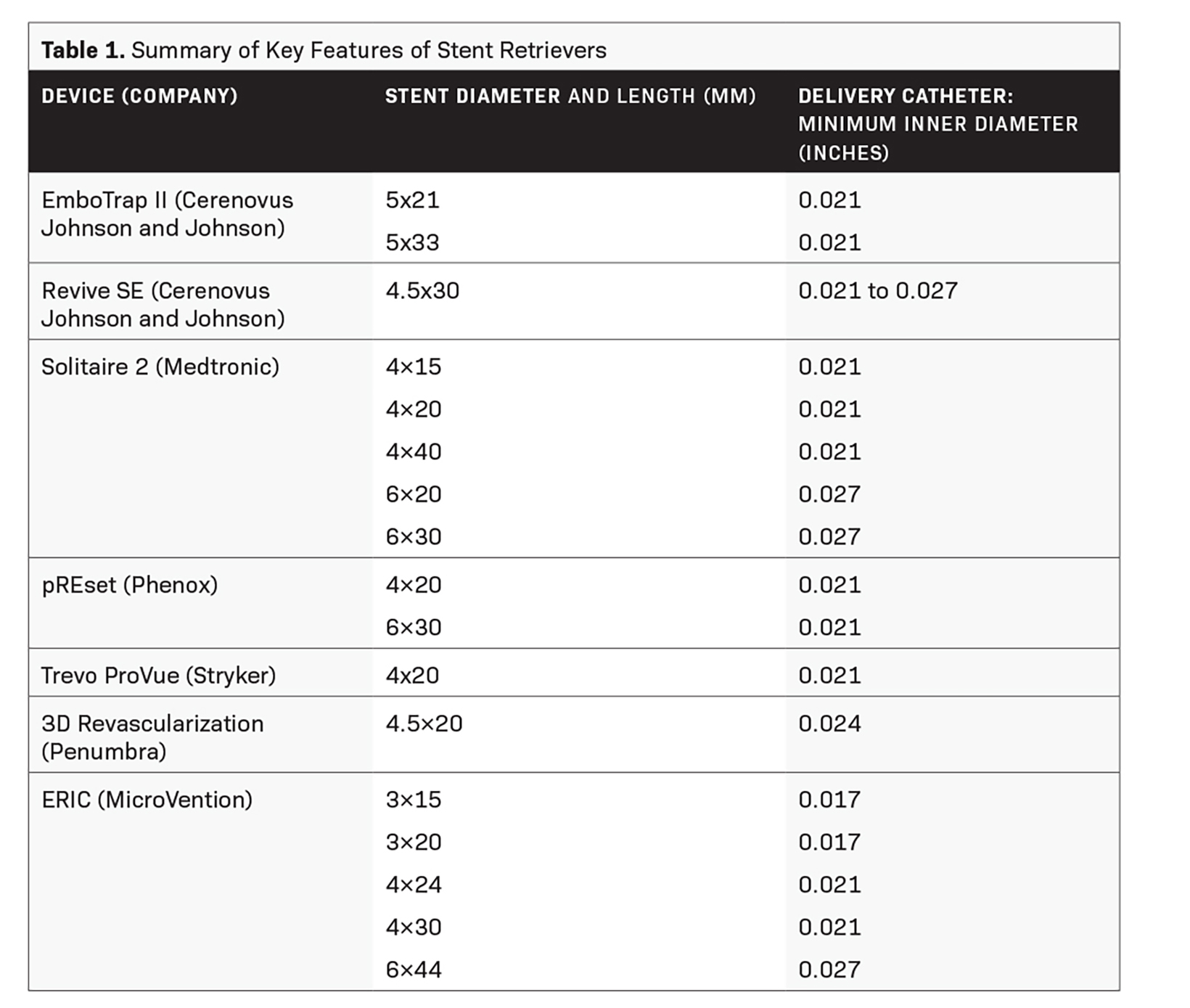
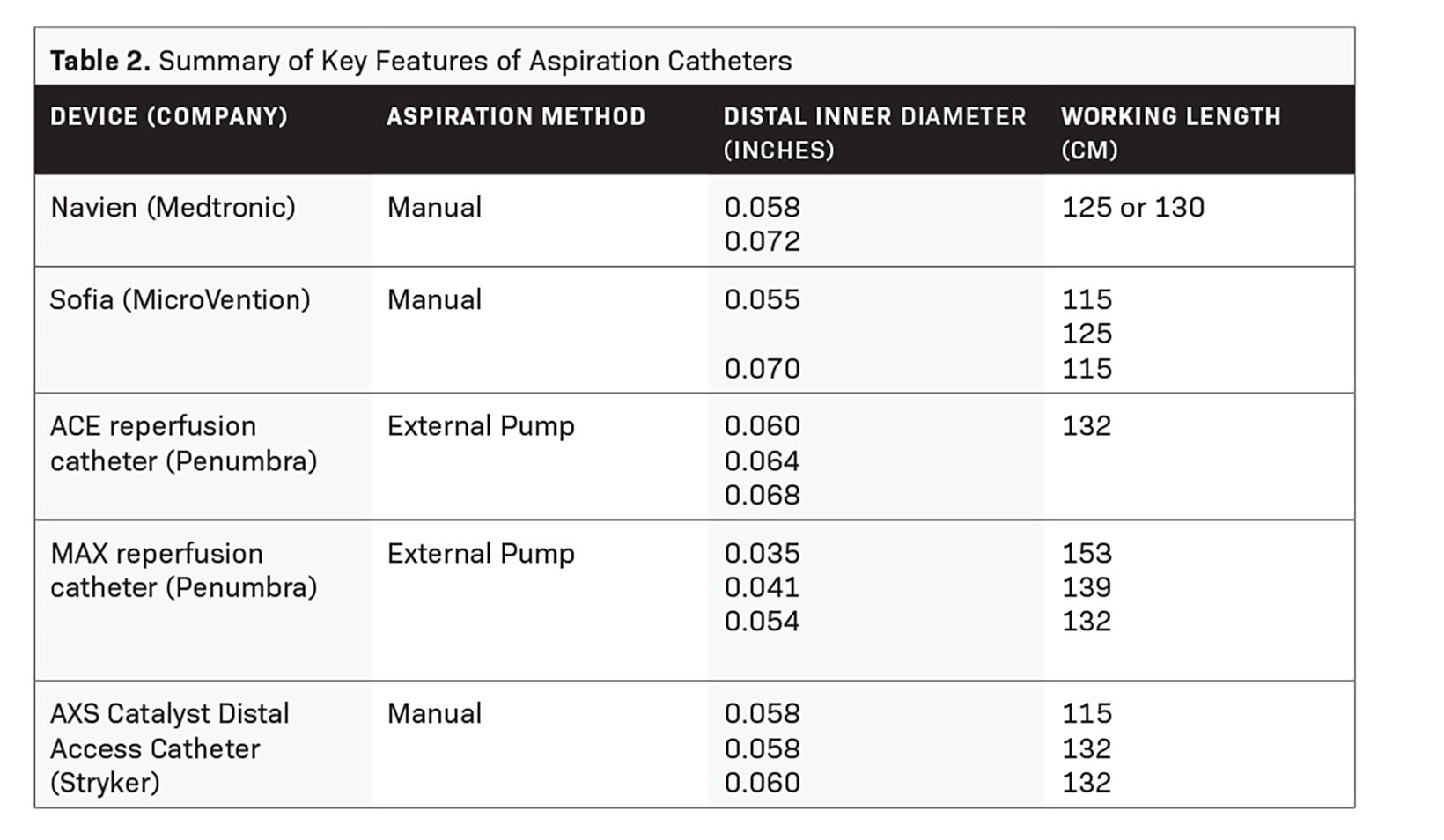
Many newer stent retrievers (pREsetTM /pREset LITETM , phenox GmbH; SolitaireTM , Medtronic; Trevo, Stryker; EmbotrapTM , Neuravi; 3D Revascularization Device, Penumbra) have resulted in TICI 2b or better recanalization with vessel diameters below 2 mm (Figures 5-8). Although recanalization of distal branches can reduce resultant infarct size, the procedure increases the risk of focal subarachnoid hemorrhage.15
Selection for Thrombectomy
Several studies have explored the possibility of expanding the criteria of treatment candidates for mechanical thrombectomy. Earlier studies included patients with small ischemic cores, large penumbras, and significant neurological deficit. Newer studies have shown favorable out comes in patients with core sizes up to 100 ml. Retrospective analyses have shown efficacy in patients with more mild stroke symptoms.14
A retrospective analysis of patients with anterior circulation large-vessel occlusion with admission National Institutes of Health stroke scale (NIHSS) scores lower than 6 treated with mechanical thrombectomy demonstrated similar efficacy for mechanical thrombectomy compared to more traditional selection criteria. The study did, however, demonstrate an increased risk of asymptomatic intracranial hemorrhage.16
Although most thrombectomy studies have evaluated patients with large-vessel occlusions, Cimflova, et al, reviewed current opinions on the use of mechanical thrombectomy for medium-vessel occlusions (MeVO). The majority of responding physicians stated they would directly treat these patients with endovascular thrombectomy. These physicians were more likely to treat younger patients with greater stroke severity or smaller core volume.17 Respondents were also more likely to perform endovascular thrombectomy in patients with MeVO involving middle cerebral artery segments (M2/3, M3) and posterior cerebral artery segments (P2/3) than in those involving anterior cerebral artery (A3) segments.17
General Anesthesia vs Sedation
The Sedation versus Intubation for Endovascular Stroke Treatment trial showed no difference in post-thrombectomy NIHSS scores between patients receiving general anesthesia compared to those receiving conscious sedation. The Anesthesia During Stroke trial compared conscious sedation to general anesthesia and showed that maintaining blood pressure required significantly more vasoactive drugs in the general anesthesia group than those under sedation. However, there was no difference in functional outcome at three months between the two groups. Blood pressure management pre- and post-thrombectomy and during induction of anesthesia are key focus points that must be well controlled.
Conversely, the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke trial found that for every 100 patients treated under general anesthesia, 18 had worse functional outcomes compared to the non-general anesthesia group. This may be because those in the anesthesia group were more likely to be unconscious, agitated, and/or vomiting. In the randomized control trials that demonstrated no difference in sedation, participants were suitable candidates for either anesthesia or conscious sedation.18
Vacuum Aspiration
The vacuum force required for suction thrombectomy can be created manually using a large-volume syringe or via vacuum pumps. Vacuum pumps work on the principle of Poiseuille’s Law which states that the vacuum force exerted on the clot depends directly on the pressure generated within the lumen and the radius of the distal catheter tip. Several medical pumps are now commercially available from companies such as Penumbra, Stryker, and Microvention. One study found the Penumbra Jet Engine was able to reach and maintain the highest peak aspiration pressures. Simple manual aspiration with a 60cc syringe has been shown to create vacuum pressures similar to vacuum pumps and is more cost effective compared to suction pumps, canisters, and tubing.19
Direct Aspiration
Several randomized trials have demonstrated the efficacy of thrombectomy in large-vessel occlusions. Because these trials predominantly used stent retrievers, established stroke guidelines specifically recommend the use of these devices.20
A direct aspiration first-pass technique attempts to remove a thrombus without a stent retriever. If unsuccessful, a stent retriever may then be used.20
The recent Cardiovascular Outcomes for People using Anticoagulation Strategies trial was a multicenter, randomized, non-inferiority trial that compared aspiration as first pass with initial stent retriever thrombectomy.20 The trial showed that patients presenting within 6 hours of onset of anterior circulation large-vessel occlusion and an Alberta Stroke Programme Early CT Score (ASPECTS) greater than 6 (therefore more likely to have a worse functional outcome) who were treated with direct aspiration thrombectomy had non-inferior functional outcomes compared to those treated with first-line stent retriever.
Complications Following Thrombectomy
Hemorrhagic transformation after mechanical thrombectomy occurs at a rate of up to 11.6%.21 Risk factors include a history of smoking, a low ASPECTS score, unfavorable collateral vasculature on angiography, and thromboembolic migration. Various chemokines and growth factors are involved in reperfusion following ischemic stroke. A robust collateral flow may help mitigate this acute reperfusion stress and minimize the risk of hemorrhagic transformation.21
Subarachnoid hemorrhage or arterial perforation has been correlated with TICI scores below 2b. M2 segment and carotid terminus occlusions have also been associated with higher risk of subarachnoid hemorrhage. This may be because of technical difficulties associated with removing these lesions. Arterial dissection incidence ranges from .6 to 7% and is also correlated with revascularization scores below TICI 2b.22
Some studies have shown gender, hypertension, age, diabetes, and history of smoking were not associated with subarachnoid hemorrhage, clot embolization, or dissection. Owing to different definitions, rates of intracranial hemorrhage vary greatly in the literature, ranging from 3 to 35% of cases. Higher NIHSS at onset, ongoing antiplatelet therapy, diabetes, and longer groin-to-reperfusion time were associated with higher risk of symptomatic intracranial hemorrhage.22
Mechanical Thrombectomy
The Options
Devices frequently used for mechanical thrombectomy currently fall into two main categories: stent retrievers and aspiration devices. These devices have been shown to increase the odds of good functional outcomes more than fourfold in randomized clinical trials and large series.1 They work by restoring perfusion in occluded, ischemic, but not yet fully infarcted brain tissue. Though these devices achieve similar goals, their biomechanical mechanisms of action differ slightly.
Stent retrievers (Table 1) are stent-like mesh, self-expanding wires that are deployed within a thrombus, entangling it within the stent structure. Both are then withdrawn into the delivery catheter. This offers immediate flow restoration, more effective capture and clearance of a target thrombus, less fragmentation and embolism of thrombi, and reduced trauma to the vessel wall.2
Aspiration Devices
Aspiration catheters (Table 2) are flexible devices with a large inner diameter that break clots into smaller pieces that can be aspirated using external pump or manual suction. While manual aspiration risks clogging the catheter tip, adding an in-bore separator wire with a bulbous tip that can be advanced and retracted, such as that in the Penumbra system, allows clot disruption and extraction ahead of the catheter.
Conclusion
Interventional stroke management continues to evolve. Factors including patient anatomy, thrombus composition, and thrombus location impact device selection. Understanding the advantages and limitations of the available systems may help prevent complications and improve patient outcomes.
References
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973.
- Jahan R. Solitaire flow-restoration device for treatment of acute ischemic stroke: safety and recanalization efficacy study in a swine vessel occlusion model. AJNR Am J Neuroradiol. 2010;31(10):1938-1943.
- Khanna O., et al. A comparison of radial versus femoral arte`ry access for acute stroke interventions. J Neurosurg. 2020: 1-6.
- Peterson C, and Waldau B. Transradial access for thrombectomy in acute stroke: A systematic review and meta-analysis. Clin Neurol Neurosurg. 2020. 198: 106235.
- Scoco, A.N., et al., Trans-carotid and trans-radial access for mechanical thrombectomy for acute ischemic stroke: A systematic review and meta-analysis. Cureus. 2020; 12(6): e8875.
- Munich SA, Vakharia K, Levy EI, Overview of mechanical thrombectomy techniques. Neurosurgery. 2019; 85(suppl 1): S60-S67.
- Bourcier R, et al. Balloon guide catheter is not superior to conventional guide catheter when stent retriever and contact aspiration are combined for stroke treatment. Neurosurgery. 2020; 88(1): E83-E90.
- Chueh JY, et al. Role of balloon guide catheter in modern endovascular thrombectomy. J Korean Neurosurg Soc. 2020; 63(1): 14-25.
- Velasco A, et al. Comparison of a balloon guide catheter and a non-balloon buide catheter for mechanical thrombectomy. Radiology. 2016; 280(1): 169-176.
- Haussen DC, et al. Longer stent retrievers enhance thrombectomy performance in acute stroke. J Neurointerv Surg. 2019; 11(1): 6-8.
- Kaneko N, et al. Stent retrievers with segmented design improve the efficacy of thrombectomy in tortuous vessels. J Neurointerv Surg. 2019; 11(2): 119-122.
- Will L, et al. Mechanical thrombectomy in acute ischemic stroke using a manually expandable stent retriever (Tigertriever) : preliminary single center experience. Clin Neuroradiol. 2021; 31(2): 491-497.
- Gunning, GM, et al. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg, 2018; 10(1): 34-38.
- De Meyer SF, et al. Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int J Stroke. 2017; 12(6): 606-614.
- Ohshima e.a. Relationship between clot quality and microguidewire configuration during endovascular thrombectomy for acute ischemic stroke. World Neurosurgery. 2017; 107: 657-662.
- Liaw N. and Liebeskind D. Emerging therapies in acute ischemic stroke. F1000Res. 2020. 9.
- Kurre W, et al. Stent retriever thrombectomy of small caliber Intracranial vessels using pREset LITE: safety and efficacy. Clin Neuroradiol. 2017; 27(3): 351-360.
- Goyal N, et al. Medical management vs mechanical thrombectomy for mild strokes: an international multicenter study and systematic review and meta-analysis. JAMA Neurol. 2020. 77(1):16-24.
- Cimflova P, et al. Factors influencing thrombectomy decision making for primary medium vessel occlusion stroke. J Neurointerv Surg. 2021.
- Wang A, Abramawicz A. Endovascular thrombectomy in acute ischemic stroke: new treatment guide. Curr Opin Anesthesiol. 2018. 31: 473-480.
- Yaeger K, et al., A technical comparison of thrombectomy vacuum aspiration systems. J Neurointerv Surg. 2020. 12(1): 72-76.
- Turk AS, 3rd, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019; 393(10175): 998-1008.
- Otsu Y, et al. Strategies to prevent hemorrhagic transformation after reperfusion therapies for acute ischemic stroke: A literature review. J Neurol Sci. 2020; 419:117217.
- Salsano G, et al. Complications of mechanical thrombectomy for acute ischemic stroke: Incidence, risk factors, and clinical relevance in the Italian Registry of Endovascular Treatment in acute stroke. Int J Stroke. 2020: doi:1747493020976681.
References
Citation
AR H, S B, A F, A B, D K, A B. Interventional Stroke Management: An Update. Appl Radiol. 2021;(6):8-14.
November 6, 2021