Imaging of the pancreas: Part 2



In Part 2 of this two-part article, the authors discuss imaging manifestations of various abnormalities affecting the pancreas.
Diagnosis of pancreatic conditions is a challenge to physicians due to the anatomical location of the organ deep within the abdomen.
Imaging with multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) along with the use of 3-dimensional(3D) imaging play a critical role in the evaluation of pancreatic diseases.
Specific types of pancreatitis
Groove pancreatitis
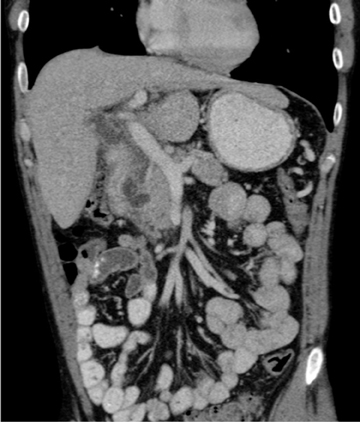
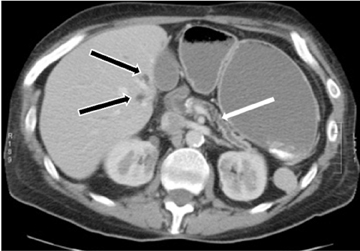
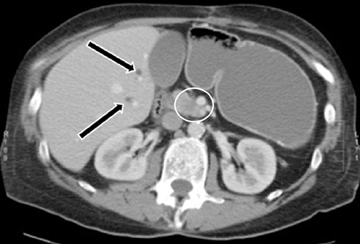
This rare form occurs in the spaces between the head of the pancreas, the second portion of the duodenum, and the CBD. The inflammatory involvement results in scar-tissue formation in the pancreatico-duodenal groove and subsequent cystic dystrophy of heterotopic pancreatic tissue in the duodenal wall.6,16 Imaging features include inflammatory involvement of the pancreas and medial wall of the duodenum,with cystic changes limited mostly to the pancreatico-duodenal groove (Figure 10). Groove pancreatitis can occasionally mimic pancreatic adenocarcinoma given its focal nature, its propensity to cause strictures of the bile and pancreatic ducts, and its fibrotic character, which leads to decreased enhancement and T1 hypointensity. Findings favoring groove pancreatitis over adenocarcinoma include cystic changes within the lesion, smooth rather than abrupt narrowing of the pancreatic and CBD, and a sheet-like mass rather than a rounded mass.
Autoimmune pancreatitis
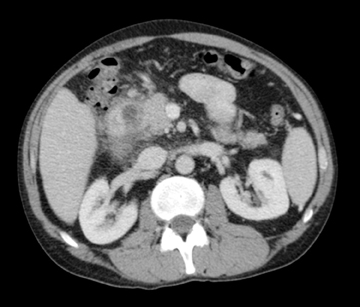
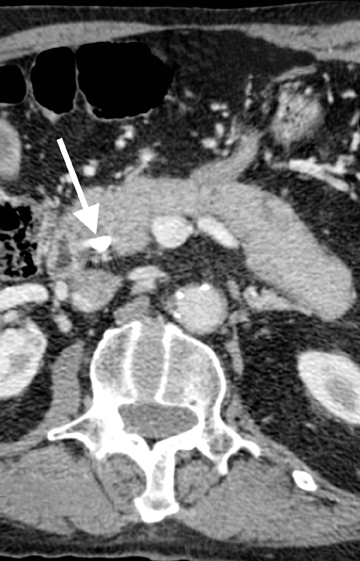
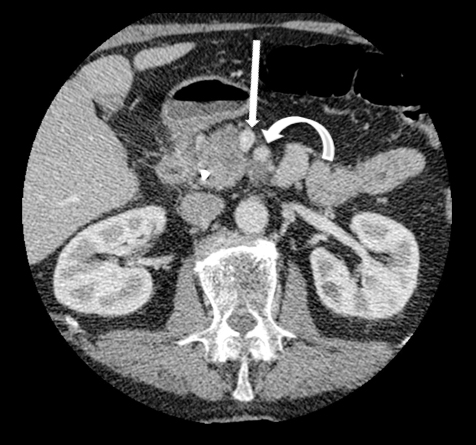
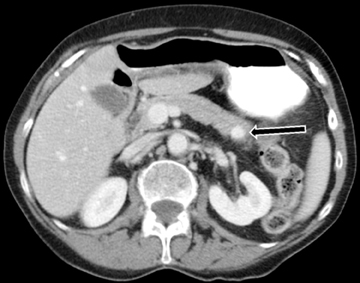
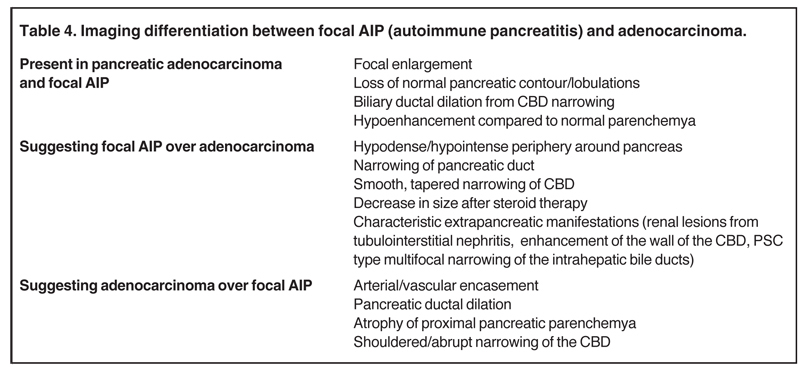
Autoimmune pancreatitis, first described in 1995, is pathologically characterized by dense inflammatory infiltrates of lymphocytes and plasma cells around the small- to medium-sized pancreatic ducts with associated fibrosis. The clinical presentation is similar to pancreatic cancer, with weight loss and obstructive jaundice, but presents with the absence of severe attacks of abdominal pain seen with acute pancreatitis. Extrapancreatic manifestations are observed in 19% to 50% of cases and include multifocal biliary strictures, renal lesions from tubulointerstitial nephritis, retroperitoneal fibrosis, Sjogren’s syndrome, and inflammatory bowel disease (Figures 11 and 12). Serum IgG4levels are characteristically elevated and a level > 135 mg/dL is 95% accurate and 97% specific in making the diagnosis. Elevation of IgG4is rare in pancreatic cancer and is observed only in 10% of cases. A combination of imaging findings, serologic, or histologic criteria are necessary to diagnose autoimmune pancreatitis (Table 3).17-19
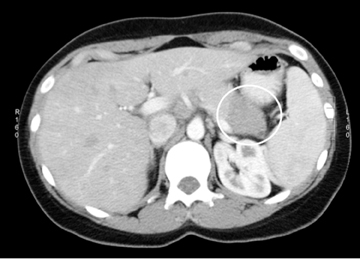
Autoimmune pancreatitis has typical imaging features. Classically it is described as diffuse pancreatic enlargement, which becomes featureless secondary to loss of normal pancreatic lobulations, foreshortening of the pancreatic tail and a peripheral rind or ‘wrapping’ around the pancreas, which appears hypodense on CT and as hypointense on both T1 and T2 images (Figure 13). Pancreatic ductal irregularities are often seen as ductal strictures or diffuse narrowing.20 Autoimmune pancreatitis can also present as a focal mass, which often poses a diagnostic dilemma (Figure 14). The differences and similarities in imaging features of focal autoimmune pancreatitis and pancreatic adenocarcinoma are listed in Table 4.
Tropical pancreatitis
A chronic form, tropical pancreatitis has unique clinical, epidemiological and imaging features. It characteristically presents at a young age (mean age 12.5 years, M:F, 1.6 to 5:1), leads to early development of diabetes, has a specific geographic distribution (India, Asia, South America), and is associated with malnutrition, which is thought to be a cause, not an effect. Another potential etiology is a mutation of the serine protease inhibitor Kazal type 1 (SPINK1) gene.21 On imaging, there is significant atrophy of the pancreas, marked dilatation of the pancreatic duct, and large intrapancreatic ductal stones, which have been reported up to 5 cm in size. This disease is associated with a marked predisposition to pancreatic adenocarcinoma, which occurs at an early average age of 45 years.6
Pancreatic neoplasms
Cystic pancreatic lesions
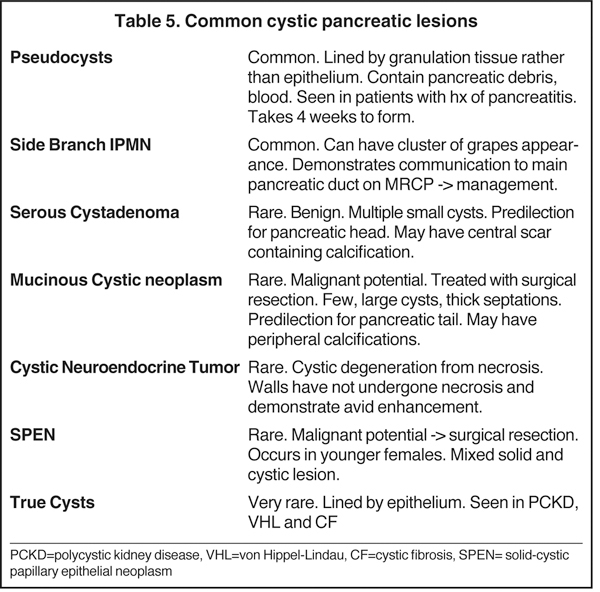
Increased utilization of cross-sectional imaging, such as MDCT and MR, has led to an increased detection of cystic pancreatic lesions with nearly a third of cases being diagnosed in asymptomatic patients (Tables 5 and 6) and a prevalence of incidental cystic lesions on CT being present in 2.6% of examinations.22 Despite the significant overlap in the imaging features of pancreatic cystic lesions, MDCT andMRI are fairly accurate in the characterization of these lesions. Occasionally, EUS with or without aspiration/biopsy might be necessitated for characterization prior to surgical intervention.
Intraductal papillary mucinous neoplasm (IPMN)
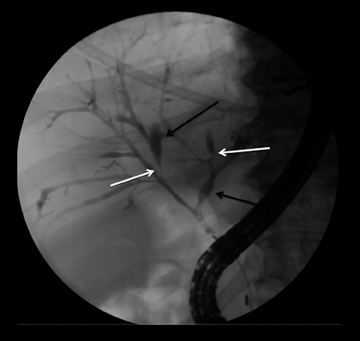
IPMNs are commonly encountered cystic lesions of the pancreas and can be classified based on involvement of the main duct and side branch. There is the main duct IPMN, side branch IPMN, and combined IPMN (involving both main duct and side branch). Main duct IPMNs cause diffuse enlargement of the pancreatic duct and do not present as cystic lesions of pancreas. Up to 40% of main ducts IPMNs have malignant features at diagnosis and they are often surgically resected. The features on imaging suggestive of malignancy in main duct IPMNs include enhancing mural nodules or main duct dilatation > 1 cm. On the other hand, side branch IPMNs (also known as branch duct IPMNs) are less likely to be malignant and are often followed up on serial imaging. Side branch IPMNs have a macrolobulated or ‘cluster of grapes’ appearance on imaging. The most characteristic imaging feature is the communication of the lesion with the main pancreatic duct, which differentiates them from mucinous cystic neoplasms. A typical finding on ERCP is the so called ‘bulging papilla’ secondary to increased mucin production by the lesions. Demonstration of communication with the pancreatic duct with MRCP or ERCP is of paramount importance in diagnosing IPMNs as this limits the differential diagnosis of a cystic lesion to IPMN and pseudocysts, which are the only other cystic lesions to demonstrate ductal communication. Surgical resection is performed in symptomatic patients or in those lesions that demonstrate suspicious features on follow up imaging, such as enhancing mural modules, a size > 3cm and concomitant main pancreatic ductal dilatation.2,23 In asymptomatic patients with side branch IPMNs measuring < 3 cm without solid nodules, follow-up imaging is recommended for detection of suspicious features.
Serous cystadenoma (SCA)
Also known as microcystic adenomas, serous cystadenomas do not have malignant potential. Therefore, resection is only indicated when they become symptomatic due to mass effect. They typically affect older females and are associated with von Hippel-Lindau disease. SCAs favor the pancreatic head and are composed of multiple tiny cysts (> 6 cysts measuring 1 mm to 2 cm). Characteristically, these lesions will have a central scar, which can sometimes calcify and has a predilection for the pancreatic head. These lesions may grow up to 4 mm per year. The diagnosis of a serous cystadenoma can be made with confidence when a lesion demonstrates the classic imaging appearance of a multi-lobulated external border, thin enhancing septa in a honeycomb-like appearance, and a central scar, which occasionally calcifies. Atypical appearances can be seen. For example, occasionally the cysts can be too small to resolve even with MRI or high-frequency EUS and appear to have a solid appearance. Additionally, these lesions, instead of having numerous tiny cysts, can have a few larger cysts (oligocystic or macrocystic variant),lack a central scar and can be located in the pancreatic body or tail, and therefore mimic a mucinous cystic neoplasm. EUS-guided aspiration can confirm the diagnosis in atypical cases by establishing the presence of glycogen rich epithelial cells and absence of mucin.2,24
Mucinous cystic neoplasm
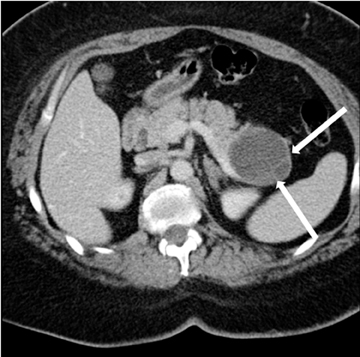
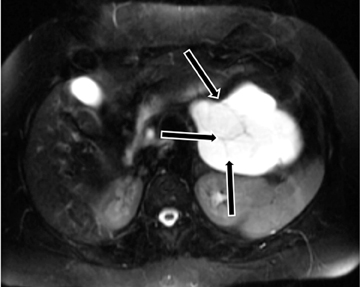
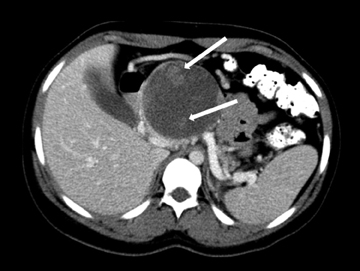
Also known as mucinous macrocystic neoplasm, these lesions, unlike their microcystic counterparts, have malignant potential or are frankly malignant at diagnosis. Therefore, surgical resection is indicated. These lesions demonstrate a 9:1 female-to-male incidence. They are more common in the pancreatic tail and contain a few large mucin-containing cysts (< 4 cysts larger than 2 cm). On imaging, these lesions tend to have a smooth outer contour and a few septae, which can occasionally be enhancing and thick (Figures 15 and 16). Enhancing nodular and papillary projections may occasionally be seen. When suspected, EUS aspiration will be performed before surgical excision to exclude other cystic lesions, including a pseudocyst and serous cystadenoma. The fluid aspirated will contain thick mucin and an elevated level of tumor markers, such as carcinoembryonic antigen (CEA) and CA-19-9.1,24,25
Cystic neuroendocrine tumors
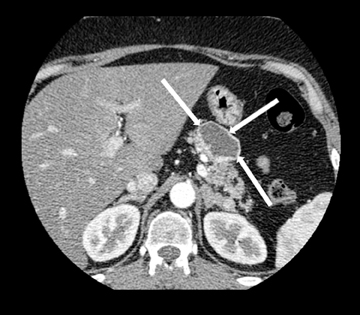
Neuroendocrine tumors, as discussed below, can sometimes undergo cystic degeneration and mimic a cystic neoplasm. The outer wall of the lesion will often be spared from cystic degeneration and therefore demonstrate features consistent with neuroendocrine tumor, specifically avid early enhancement (Figure 17).
Solid pseudopapillary tumor (SPT)
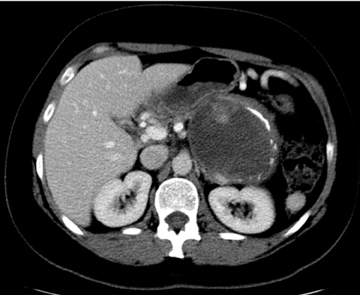
Previously known as solid-cystic papillary epithelial neoplasms (SPEN), these lesions are unique among the cystic pancreatic neoplasms in that they tend to affect young patients (20-30 yrs). Affected patients tend to be African American or Asian and female. The appearance of these lesions is varied, but they are classically large, well encapsulated, and of variable internal characteristics with areas of hemorrhagic necrosis (Figures 18 and 19). The prognosis is good with a 5-year survival estimated at 97% after surgical resection.26
True cyst
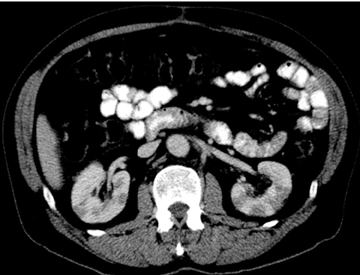
Unlike cystic lesions seen in other solid organs in the abdomen, true cysts are rare and almost never occur in a normal patient population. When present, true cysts are often multiple rounded and well defined lesions. Evidence of underlying conditions, such as von Hippel-Lindau disease, polycystic kidney disease, and cystic fibrosis, are often apparent on imaging (Figure 20).
Solid pancreatic tumors
Pancreatic adenocarcinoma
With an estimated 43,140 new cases of pancreatic adenocarcinoma in the U.S. in 2010 and a 5-year-survival rate of < 5%, pancreatic adenocarcinoma is the fourth-leading cause of cancer death in the U.S. Pancreatic adenocarcinoma is primarily a disease of the elderly with80% of cases affecting patients in the sixth or eighth decade and accounts for 95% of all malignant pancreatic lesions.27 Surgical resection of localized tumors allows cure, but only about 15% to 20% of new cases are candidates for surgical resection at presentation. Complete resection with negative margins without lymph nodal involvement gives the best possible 5-year survival rates of up to 25% to 30%. CA19-9 may be elevated in up to 80% of cases, but has limited sensitivity to identify patients with small tumors amenable to surgical resection.CA-19-9 is mainly useful in follow-up of patients undergoing treatment and when a rise in CA-19-9 precedes imaging manifestation. While there is an association between chronic pancreatitis, diabetes, and pancreatic adenocarcinoma, the causal relationship is uncertain. Smoking has been shown to be associated with 2 times the increased risk of developing pancreatic adenocarcinoma. While rare, it is interesting to note that rare genetic syndromes, such as familial atypical multiple mole melanoma syndrome (FAMMM), hereditary pancreatitis, and Putz-Jeghers syndrome are associated with a 20- to 130-fold increased risk of pancreatic cancer.1
Imaging findings
Multiphasic MDCT of the pancreas is the modality of choice for diagnosis and staging of pancreatic carcinoma and involves acquisition of images in the pancreatic phase instead of the arterial phase. MDCT has a dual role in pancreatic cancer which includes lesion detection, localization and characterization; and secondly, in the determination of tumor resectability. Although ultrasound and MRI may playa role in the initial diagnosis of pancreatic carcinoma, MDCT is preferred for diagnosis and staging. On US, they are seen as ill-defined hypoechoic lesions within the pancreas. On T1-weighted MRI, the mass will be hypointense to normal the pancreas. On both MDCT andMR, pancreatic cancer appears as a hypoenhancing mass with focal contour abnormality. The hypoenhancement is accounted for by the desmoplastic and hypovascular nature of the tumor. On dynamic images, the pancreatic phase shows the greatest attenuation differentiation and is therefore more sensitive than the portal-venous phase in detection of these lesions. However, in up to 10% of cases, pancreatic cancer can be iso-attenuating to pancreatic parenchyma, and therefore secondary signs provide a clue to diagnosis. These secondary signs include pancreatic ductal dilatation and parenchymal atrophy distal to the lesion with abrupt change in ductal caliber at the site of lesion (Figure 21).While MDCT is about 95% accurate in diagnosing pancreatic cancer, approximately 5% of patients undergo surgical resection for benign disease, such as chronic pancreatitis or focal AIP. On T2W MR imaging, the lesion itself will often be inconspicuous, but secondary signs of upstream pancreatic ductal dilatation or focal contour abnormality of the pancreas may be seen.
True determination of resectability of borderline tumors will be made at the time of laporotomy. The signs of potential resectability at laparotomy include demonstration of a normal fat plane between the celiac axis or SMA, and a patent SMV and portal vein. The definition of unresectable is ever-changing and dependent on surgeons and institutional protocols. The following are the current CT findings that are consistent with nonresectable pancreatic cancer: The first sign of unresectability is distant metastases. While MDCT will often detect larger hepatic metastases,it is limited in detecting small hepatic metastases as well as peritoneal metastatic deposits. Therefore, patients often undergo diagnostic laparoscopy for inspection of the omentum prior to surgical resection. The second sign is locally advanced arterial or venous involvement. SpecificMDCT findings consistent with locally advanced unresectable pancreatic cancer include arterial encasement of the celiac trunk, hepatic artery,or SMA. Arterial encasement > 180 degree would not only make tumor resection technically impossible but is also associated with a high rate of neoplastic involvement within the mesenteric neural plexus.8 Therefore, even if resection of the main mass were feasible, residual disease would make it oncologically unsound; however, this concept is being challenged in a recent paper.28 Venous involvement will only preclude potential respectability in cases where surgical reconstruction is technically impossible.8
There are tumors which are considered by CT criteria to be borderline resectable. Findings on CT consistent with borderline resectability include tumors that about < 180 degrees of the circumference of SMA or celiac artery (Figure 22). With short segment encasement of the common hepatic artery surgeons can often perform resection with grafting, a technically feasible, and oncologic sound technique. While clearly enlarged and distant lymph nodes are consistent with nonresectable tumors, imaging alone is inaccurate in assessment of lymph nodal involvement with metastases. Additionally, positive lymph nodes within the resection bed may be removed at the time of surgery leaving the patient with no residual disease.
Using these parameters, CT is about 95% accurate in precluding truly unresectable patients from undergoing an unnecessary attempted Whipple’s procedure. However, only about 50% of cases thought to be resectable at CT are truly resectable at the time of laparotomy. In cases in which the pancreatic cancer is determined either by CT or laparotomy to be borderline but unresectable, attempts made to convert the patient to a surgical candidate by giving neoadjuvant chemotherapy and/or external beam radiation therapy and then restaging.
Pancreatic endocrine tumors
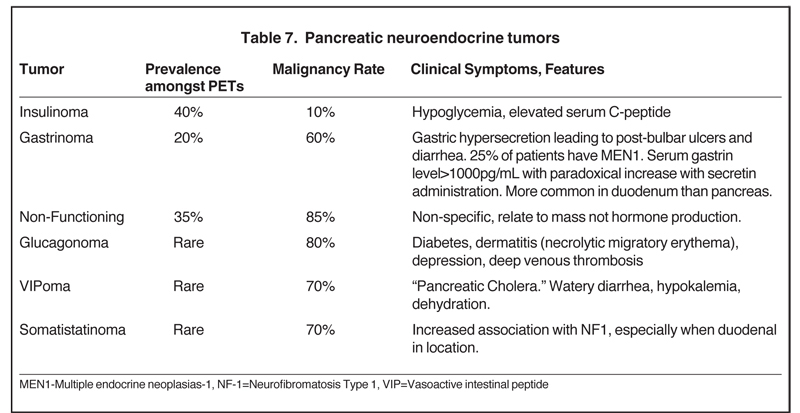
Also referred to as pancreatic islet cell tumors, these arise from pancreatic ductal cells and despite resemblance to normal islet cells histologically, they are appropriately termed as pancreatic endocrine tumors. There are 7 different types of pancreatic endocrine tumors, the most common of which are insulinomas, gastrinomas, and nonfunctional tumors (Table 7). Pancreatic endocrine tumors account for only 1% to 2% of all pancreatic neoplasms and affect only 1 in 100,000 persons. The typical age of onset is in the fourth or fifth decade.Most of these pancreatic endocrine tumors occur sporadically; however, certain syndromes, such as an MEN1, Von Hippel-Lindau,neurofibromatosis, and tuberous sclerosis, increase the likelihood of developing these tumors at a younger age. Pancreatic endocrine tumors are considered malignant based on biologic behavior (extra-pancreatic invasion or metastatic disease), not pathologic findings. The so-called functioning pancreatic endocrine tumors often produce characteristic clinical symptoms depending on the hormone excreted.
Nonfunctioning pancreatic endocrine tumors produce symptoms solely due to mass-effect, such as abdominal pain, weight loss, and jaundice.29,30
Imaging findings
Pancreatic endocrine tumors are highly vascular lesions leading to the characteristic finding of early and avid contrast enhancement.
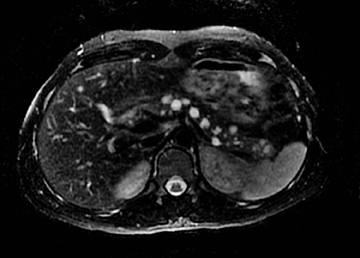
The homogeneous enhancement is the rule for smaller lesions, while larger lesions may show areas of necrosis and cystic degeneration and have heterogeneous or only peripheral enhancement (Figures 23 and 24). Unlike pancreatic adenocarcinomas, pancreatic endocrine tumors are more conspicuous on the early arterial phase and stand out as hyperattenuating compared to the normal pancreas. Most tumors appear well circumscribed, but larger and malignant tumors may demonstrate poorly defined borders. Nonfunctioning pancreatic endocrine tumors, either because of their inherently aggressive behavior or delay in presentation, are seen with metastasis in about 70% of cases. On MRI, these tumors are T1 hypointense and characteristically, but variably, T2 hyperintense. Indium-111 octreotide scans can be used and are most helpful in identifying subtle foci of metastatic disease in patients whose tumor is known from pathologic examination tobe well differentiated and contain somatostatin receptors. Pancreatic endocrine tumors may sometimes be peripancreatic, such as duodenal, in location.30 The overall sensitivity of Indium-111 octreotide scintigraphy for diagnosis of pancreatic neuroendocrine tumors is high with 80% to 100% sensitivity for carcinoids and 60% to 90% for pancreatic NETs.
Conclusion
Optimization of scanning protocols as well as knowledge of the various maladies of the pancreas and the role of imaging in the clinical management of patients with pancreatic disorders allows the radiologist to play a large role in the diagnosis and management of inflammatory as well as neoplastic disorders of the pancreas.
References
- Hrubran R, Wilentz R. The Pancreas. In: Kumar V, Abbas AK, Fausto N, eds. Robbins and cotran pathologic basis of disease 7th Ed. Philadelphia, PA: Elsevier Saunders; 2005:939-953.
- Leyendecker JR, Oliphant M. Pancreas. In: Dalrymple NC, Leyendecker JR, Oliphant M, eds. Problem solving in abdominal imaging. Philadelphia, PA: Mosby Elsevier; 2009.
- Marino C, Gorelick F. Panceatic and salivary glands. In: Boron W, Boulpaep E eds. Medical physiology: A cellular and molecular approach. Philadelphia, PA: Elsevier; 2005: 908-926.
- Mulkeen A, Yoo, P, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006;12:3180-3185.
- Roth CG, Deshmukh S. MRI of the pancreaticobiliary system. In: Roth CG ed. Fundamentals of body MRI. Philadelphia, PA: Elsevier Saunders; 2012: 129-199.
- Shanbhogue A, Fasih N, Surabhi V, et al. A Clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009;29: 1003-1026.
- Pezzilli R. Pancreas divisum and acute or chronic pancreatitis. JPancreas. 2012;13:118-119.
- Brennan DD, Zamboni G, Raptopoulos V, Kruskal J. Comprehenisve preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics. 2007;27: 1653-1666.
- Balthazar EJ. Acute pancreatitis: Assessment of severity with clinical and CT Evaluation. Radiology. 2002;223:603-613.
- Singla A, Csikesz N, Simons J, et al. National hospital volume in acute pancreatitis: Analysis of the nationwide inpatient sample 1998-2006. HBP (Oxford). 2009;11:391-397.
- Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417.
- Balthazar EJ, Robinson DL, Megibow AJ, Ransson JH. Acute pancreatitis: Value of CT in establishing prognosis. Radiology. 1990;174:331-336.
- Banks PA. Infected necrosis: Morbidity and therapeutic consequences. Hepatogastoenterology. 1991;38:116-119.
- Leung TK, Lee CM, Lin SY, et al. Computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World J Gastroenterol. 2005;11:6049-6052.
- Braganza J, Lee S, McCloy R, McMahon M. Chronic pancreatitis. Lancet. 2011;377:1184-1197.
- Blasbalg R, Baroni RH, Costa DN, Marchado MC. MRI features of groove pancreatitis. AJR Am J Roentgenol. 2007;789:73-80.
- Pezzilli R, Imbrogno A, Fabbri D. Autoimmune pancreatitis management: reflections on the past decade and the decade to come. Expert Rev Clin Immunol. 2012; 8:115-117.
- Psarras K, Baltatzis ME, Pavlidis ET, et al. Autoimmune pancreatitis versus pancreatic cancer: A comprehensive review with emphasis on differential diagnosis. Hepatobiliary Pancreat Dis Int. 2011;10:465-473.
- Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis. J Gastroenterol. 2006;41:626-631.
- Kawamoto S, Siegelman SS, Hrubran RH, Fishman EK. Lymphoplasmacytic sclerosing pancreatitis (Autoimmune Pancreatitis): Evaluation with multidetector CT. Radiographics. 2008;28:157-170.
- Lee F, Raleigh J. Tropical calcific pancreatitis. N Engl J Med. 2011; 13;365:1425.
- Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807.
- Augustin VT, Vandermeer TJ. Intraductal papillary mucinous neoplasm: A clinicopathologic review. Surg Clin North Am. 2010;90:377-398.
- Sahani DV, Kadavigere R, SaokarA, et al. Cystic pancreatic lesions: A simple imaging-based classification system for guiding management. Radiographics. 2005;25:14711484.
- de Jong K, Bruno MJ, Fockens P. Epidemiology, diagnosis, and management of cystic lesions of the pancreas. Gastroenterol Res Pract. 2012;147465. Epub 2011.
- Igbinosa O. Pseudopapillary tumor of the pancreas. An algorithmic approach. JOP. 2011;12: 262-265.
- Saif MW. Pancreatic neoplasm in 2011: An update. JOP. 2011;12:316-321.
- Bachellier P, Rosso E, Lucescu I, et al. Is the need for an arterial resection a contraindication to pancreatic resection for locally advanced pancreatic adenocarcinoma? A case-matched controlled study. J Surg Oncol. 2011;103:75-84.
- Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-781.
- Lewis R, Lattin GE, Paal E. Pancreatic endocrine tumors: Radiologic-clinicopathologic correlation. Radiographics. 2010;30:1445-1464.
