Imaging manifestations of primary and disseminated coccidioidomycosis
Images

Coccidioidomycosis was first described as a disease in an Argentinean soldier in 1892. It was identified as a fungal infection in 1900.1 The Coccidioides species are dimorphic fungi predominantly found in the southwestern United States, Mexico and Central and South America.2 The mycelial form grows in the soil of endemic regions and produces spores that become airborne following disturbances to the soil. Human infection occurs with the inhalation of airborne spores. A substantial proportion of infected individuals are minimally affected; however, some develop a significant primary pulmonary infection which may resolve or progress to a persistent pulmonary infection. A few patients develop disseminated coccidioidomycosis, which is associated with high morbidity and mortality. Disseminated infection can involve virtually any organ in the human body. This article describes the pathogenesis, epidemiology, clinical course, and diagnosis of coccidioidomycosis in the human body, while emphasizing the common radiologic findings of pulmonary and extrapulmonary disease.
Organism and pathogenesis
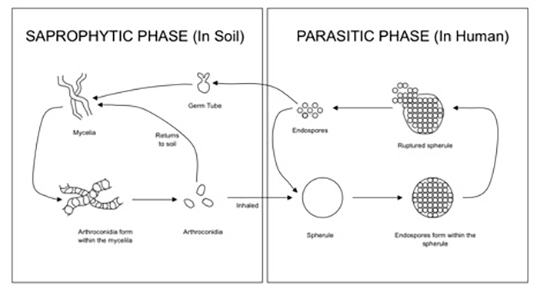
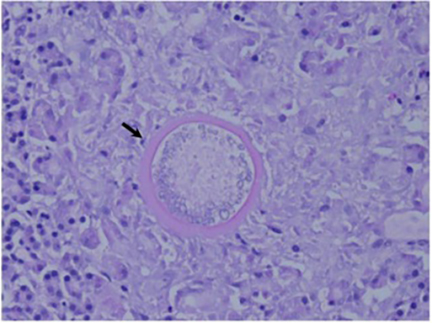
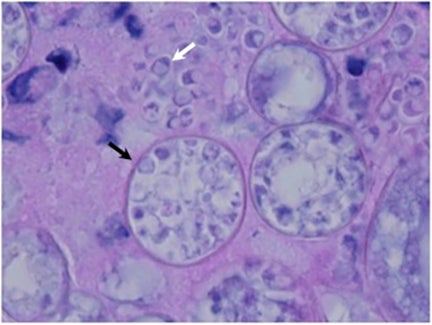
The Coccidioides species are dimorphic fungi that grow as mycelial strands within the soil (Figure 1). As the mycelia mature, arthroconidia are formed which become airborne and either return to the soil or are inhaled.2 The inhaled arthroconidia settle within the terminal bronchioles. Within hours to days, they develop into spherules that incite an inflammatory reaction in the region recruiting immune-mediated cells such as polymorphonuclear leukocytes and eosinophils.1 As the spherules mature, they produce and fill with hundreds to thousands of endospores (Figure 2). Ultimately the spherules rupture and release the endospores, each of which has the ability to develop into a mature spherule, propagating the cycle.3 Macrophages engulf some of the free endospores promoting an acute inflammatory reaction. If the infection is not entirely cleared, lymphocytes and histiocytes are recruited to the affected areas resulting in the formation of granulomas with giant cells.1
Epidemiology
The Coccidioides species are endemic to the desert soil of the western hemisphere. The two known species, Coccidioides immitis and Coccidioides posadasii, are identical morphologically and clinically but have distinct genetic and epidemiologic features.1 Coccidioides immitis is found primarily in California although it may also be found in Arizona.1, 4 Coccidioides posadasii is the predominant organism living outside of California, inhabiting the southwestern United States, Mexico, and Central and South America.1 Of these regions, the San Joaquin Valley of central California and south central Arizona are the areas of greatest endemicity.4
The incidence of coccidioidal infection is highest in late summer and early fall. Natural events that disturb the soil such as dust storms, landslides, and earthquakes put those exposed at increased risk. The incidence is higher in individuals with occupational or recreational exposure to dust and soil. Interestingly, many cases have been acquired from a single exposure by driving through an endemic region.3
It is estimated that 100,000 infections occur each year in the United States with 30-60% of the cases being subclinical. In the highly endemic areas, the infection rate is approximately 2-4% per year and the prevalence is approximately 30-40%.1 Intermittent epidemics have occurred in the past 30 years with the most recent episodes occurring in the San Joaquin Valley in the 1990s and in Los Angeles following the 1994 Northridge earthquake. As populations continue to grow within these endemic regions, the incidence of infection continues to increase.5 Additionally, with the recent increase of population mobility and travel, this fungal infection is more likely to be encountered outside of these endemic areas.
Clinical course
With the primary pulmonary infection, approximately 60% of infected individuals are asymptomatic or minimally symptomatic.2 The remaining 40% of individuals develop nonspecific symptoms such as fever, headache, sore throat, cough, fatigue, and pleuritic chest pain, which typically occur one to three weeks after infection.5 The presence of a diffuse maculopapular rash, erythema nodosum, or erythema multiforme may help point to the specific diagnosis of coccidioidomycosis.1, 6 The syndrome of “valley fever” or “desert rheumatism” develops in about 25% of those infected and comprises the triad of arthralgia, fever, and skin rash.1, 7 Most cases resolve within a few weeks even without treatment. However, treatment is recommended for specific populations of patients, especially pregnant women, patients who are immunocompromised and patients with significant comorbidities such as diabetes and cardiopulmonary disease.1
In approximately 5% of patients a persistent pulmonary infection, defined as respiratory infection lasting longer than six weeks, ensues.8,9 Clinical manifestations include productive cough, hemoptysis, and weight loss.5 Antifungal treatment is recommended and may be effective; however, elderly and diabetic patients have a poor prognosis.1
In less than 1% of infected individuals, the primary pulmonary infection disseminates to other parts of the lung and body by lymphohematogenous spread. The most common sites of extrapulmonary involvement are the central nervous system (CNS), bones and joints, and soft tissues.1 Individuals at increased risk for disseminated disease include African-Americans, Filipinos, pregnant women, and immunosuppressed patients.3 Males are at higher risk than females.10 Dissemination usually becomes apparent within a few weeks of the primary pulmonary infection, but can occur up to more than two years later.1 A minority of patients presenting with disseminated disease have no known history of a primary pulmonary infection.4 Generalized symptoms include night sweats, dyspnea at rest, fever, and weight loss.1 Patients with disseminated pulmonary disease may present with profound hypoxemia, acute respiratory distress syndrome, and respiratory failure.11 Commonly seen symptoms related to central nervous system involvement include headache, nausea, vomiting, visual changes and altered mental status.1 Patients with wide-spread dissemination may develop septic shock or fungemia. Overall, disseminated infection is associated with a significant morbidity and mortality requiring long term treatment with antifungal medications and surgical intervention in appropriate cases.1, 11 The treatment of coccidioidal meningitis usually requires the addition of intrathecal drugs.1
Diagnosis
Multiple methods exist for the diagnosis of coccidioidal infection, including microscopic detection of endospore containing spherules within tissue or fluid samples, molecular detection using nucleic acid amplification with polymerase chain reaction, cultures on fungal or bacterial media, and serologic testing. Serum IgM antibodies are detectable within 1-3 weeks of infection. Serum IgG antibodies are detectable by complement fixation (CF) at a later time.12 Quantitative complement-fixing antibody titers using IgG can be used to monitor disease activity over time as they decrease with disease regression or resolution. In early disease, the CF titer is usually around 1:8 or 1:16.7 A CF titer above 1:32 or an increasing titer indicates progression of disease and possibly dissemination.12 It is important to note that a negative serologic result does not exclude the diagnosis of coccidioidomycosis, and repeat testing should be considered if the clinical index of suspicion is high.1 Skin testing was used in the past; however, it is no longer available in the United States.12
With regard to the diagnosis of central nervous system involvement, isolation of Coccidioides immitis from infected cerebrospinal fluid (CSF) is difficult, occurring in less than 50% of patients. Diagnosis of active disease in the central nervous system is therefore often based on the presence of complement-fixing antibodies in the CSF.13-15
Pulmonary manifestations
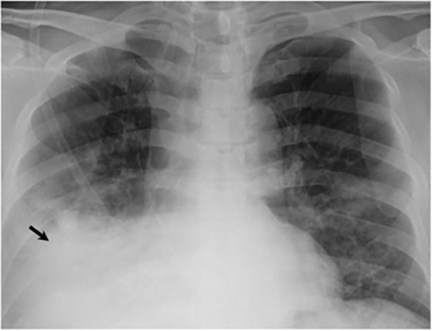
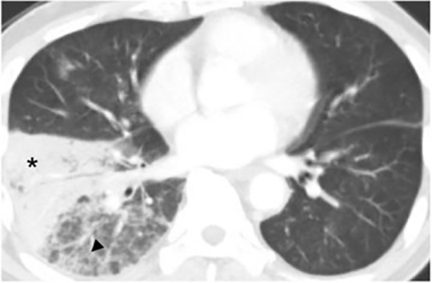
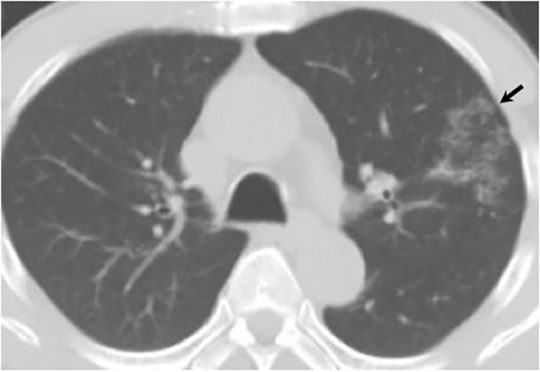
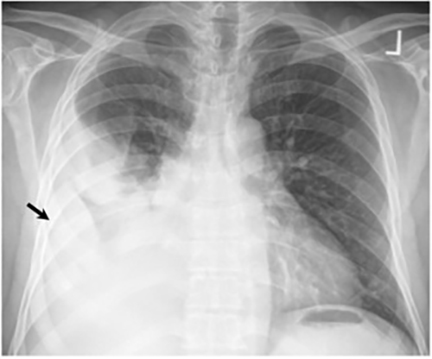
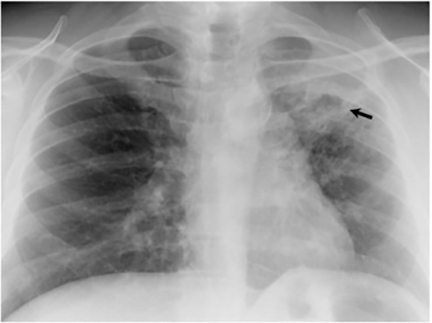
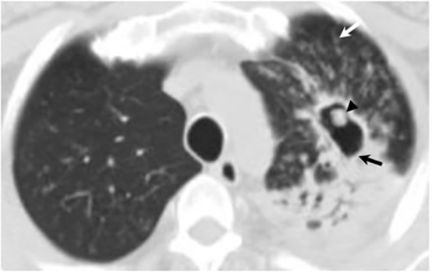
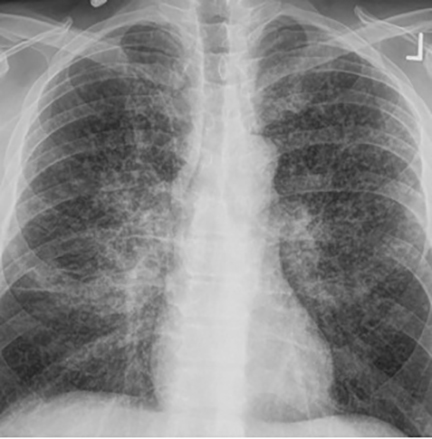
The most common radiologic manifestation of primary pulmonary infection is parenchymal consolidation, which is found in approximately 75% of symptomatic individuals.16 The consolidation can be segmental, lobar, or patchy in distribution.8 It is usually unilateral and perihilar or basilar (Figures 3,4). The consolidation may be transient, resolving in one area and recurring in another. These fleeting consolidations are referred to as “phantom infiltrates.” Acute pulmonary infection may also manifest radiologically as small nodules measuring 5 mm to 2.5 cm in size. These nodules may be well-circumscribed, resembling metastases or patchy resembling bronchopneumonia16 (Figure 5). Capone et al found that the most common manifestation on CT for acute pulmonary infection was multiple pulmonary nodules located primarily in the lung bases.17
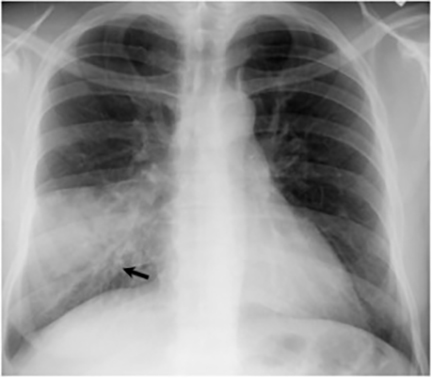
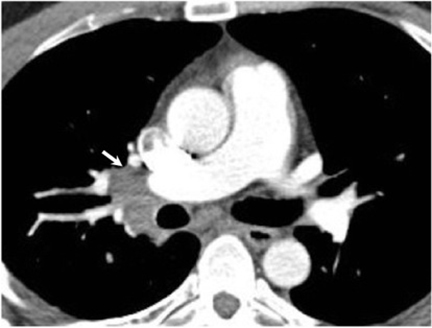
Hilar lymphadenopathy is seen concurrently with a pulmonary parenchymal abnormality in 20% of patients with acute infection (Figure 6). However, these two findings are not always positively correlated. For example, lymphadenopathy may continue to progress while pulmonary lesions regress.16
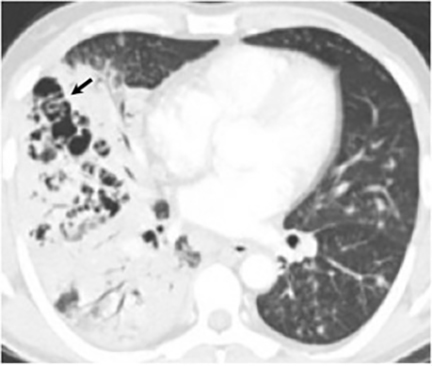
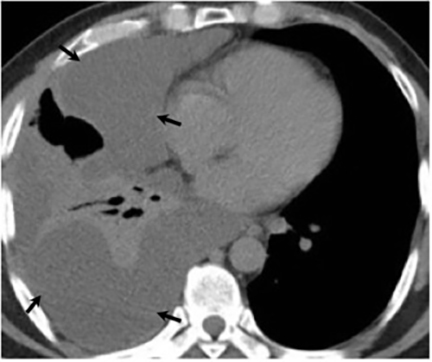
Pleural effusions are seen in approximately 20% of patients with acute respiratory infection and are usually small and unilateral. Most often they resolve rapidly; however, in 2% of cases the effusions are large and may persist for weeks to years (Figure 7). The fluid is often exudative with pleural biopsy revealing granulomas and fungal organisms.16 Pleural fluid can be seen with or without a pulmonary parenchymal finding. Pleural thickening can also be seen without a prior effusion.8
Persistent pulmonary coccidioidomycosis occurs when a pulmonary parenchymal abnormality persists for greater than 6 weeks.8,9 Persistent segmental or lobar pneumonia can be seen.8 Small pulmonary nodules, referred to as coccidiomas, may develop in sites of prior consolidation.16 They average 1.5 cm in diameter and are usually round, solitary, well-circumscribed and located more than 5 cm from the hilum.8,16 The nodules may resolve, remain stable, or break down and disseminate.16 The differential diagnosis for a coccidioma presenting as a solitary pulmonary nodule includes granuloma from another fungal disease, tuberculoma and neoplasm.
Thin- or thick-walled cavitary lesions, most of which are identified in the upper lungs, may develop from excavation of consolidation or nodules. Although cavitary lesions usually resolve spontaneously within 2 years, they have been associated with complications including bacterial superinfection, mycetoma formation, and rupture into the pleural space resulting in an empyema, pneumothorax, or bronchopleural fistula.16 The “air crescent sign” (the finding of a mobile mass within a cavity), which is most commonly associated with Aspergillus, has been seen in cavitary lesions associated with coccidioidomycosis18 (Figure 8). Biapical fibronodular changes with cavities and scarring, mimicking tuberculosis or histoplasmosis, can occur but is not common.10,16 End-stage disease includes fibrosis, bronchiectasis and calcification, although calcification related to chronic coccidioidomycosis is less common than seen in association with tuberculosis or histoplasmosis.16
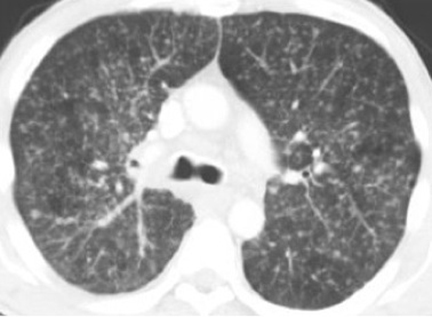
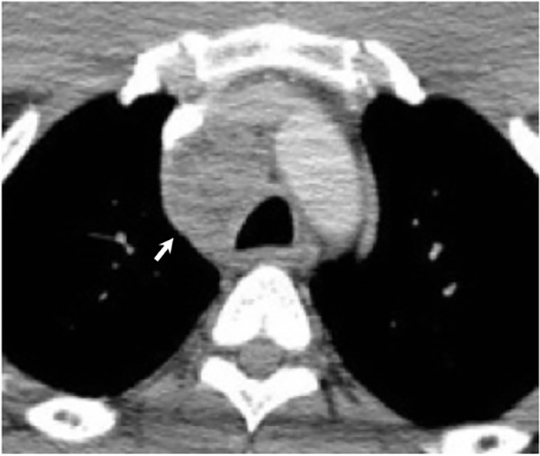
Disseminated pulmonary infection can manifest as a miliary or reticulonodular pattern (Figure 9). The presence of paratracheal or mediastinal lymphadenopathy should suggest disseminated disease (Figure 10). In addition, disseminated coccidioidomycosis can cause pericardial effusions resulting in the development of cardiac tamponade or restrictive pericarditis.8
Extrapulmonary manifestations
Central nervous system
Central nervous system involvement is the most severe and frequent manifestation of disseminated coccidioidomycosis, occurring as a result of lymphohematogenous spread from primary infection in the lungs to the meninges.13,14 Common manifestations of coccidioidal meningitis include hydrocephalus, cerebral infarction, vasculitis, parenchymal or parameningeal masses and abscesses, periventricular white matter abnormalities, and spinal arachnoiditis.15,19-21 Subarachnoid hemorrhage from vasculitis or ruptured mycotic aneurysm and intrasellar granuloma formation are among the rarer complications that have been reported in the literature.22,23 Hydrocephalus is the most common complication of coccidioidal meningitis and is associated with the highest mortality, reaching 38.7% in one study.21,24 This study also reported an overall mortality rate of 26% for patients with coccidioidal meningitis, a rate similar to other published series.24
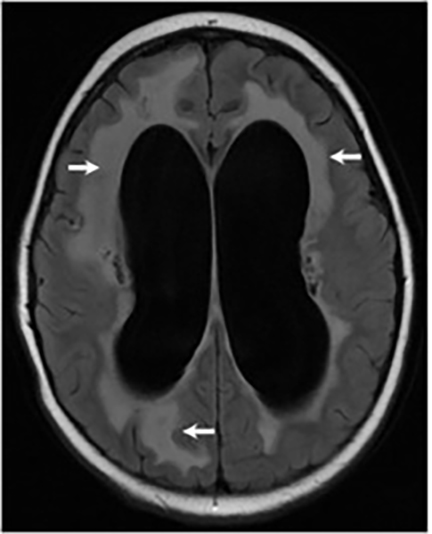
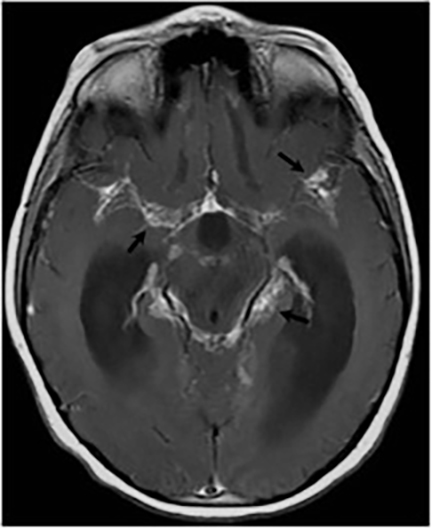
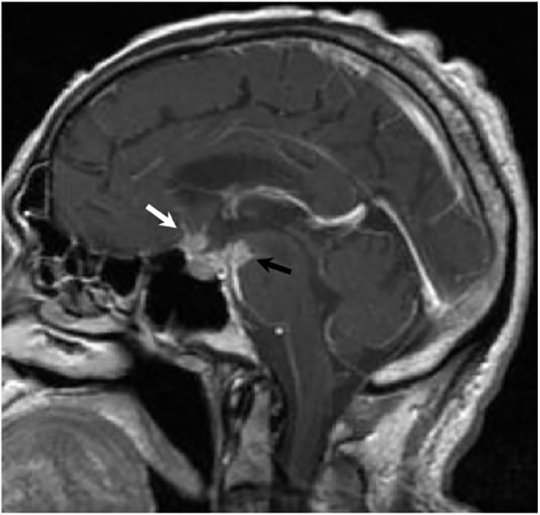
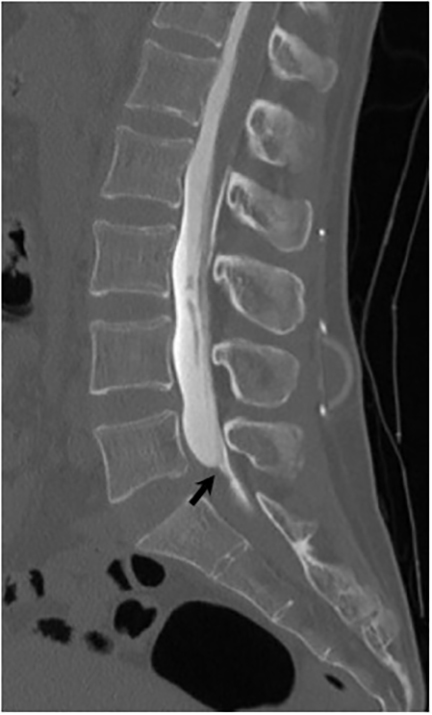
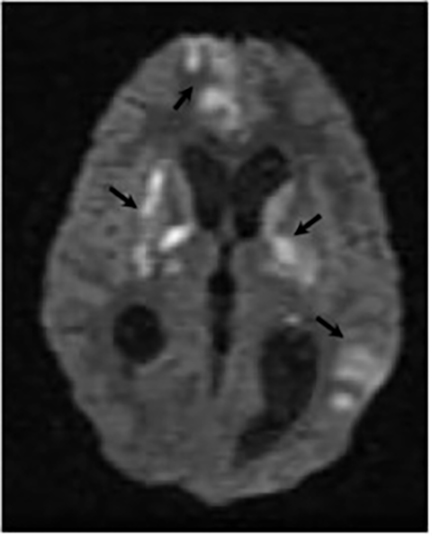
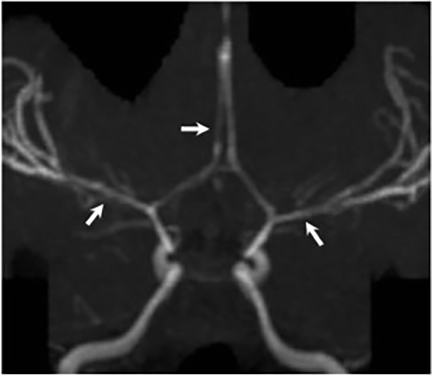
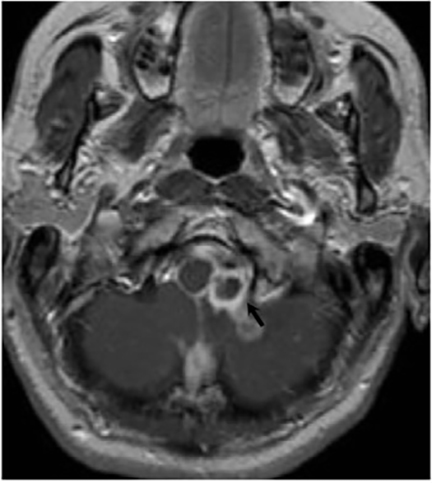
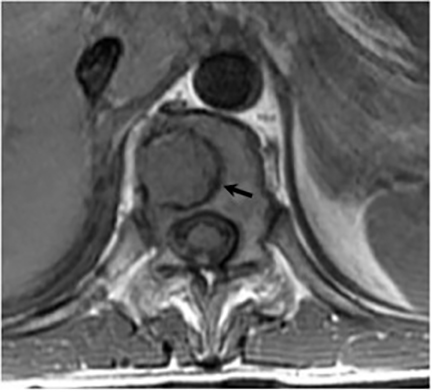
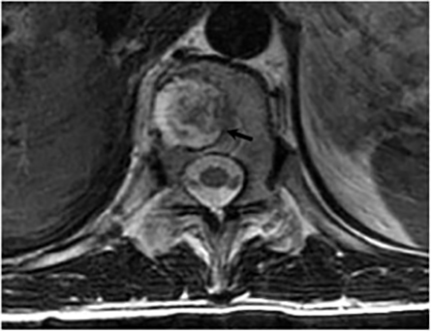
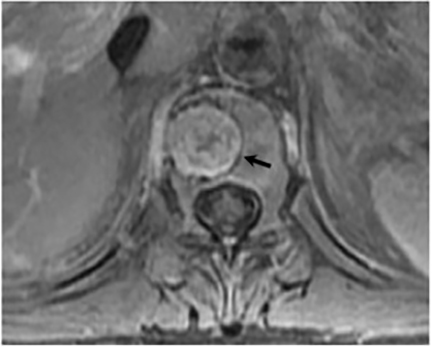
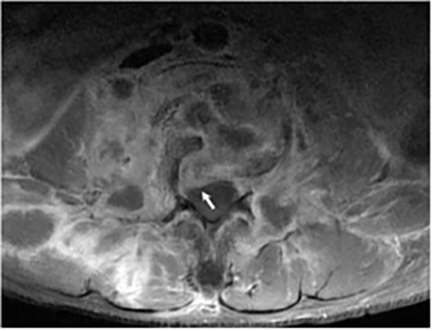
Magnetic resonance (MR) imaging is superior to computed tomography (CT) in the evaluation of central nervous system coccidioidomycosis.24 Frequently, diffuse or focal leptomeningeal enhancement (predominantly involving the basilar cisterns, craniocervical junction, or pericallosal regions) is seen on postcontrast gadolinium images25,26 (Figures 11,12). The basilar cisterns may appear isointense to CSF or isointense to brain parenchyma on T1-weighted images, and may appear normal or hypo- to isointense relative to brain parenchyma on T2-weighted images.25 Abnormal enhancement may extend inferiorly along the leptomeninges surrounding the spinal cord and spinal nerves, potentially causing arachnoiditis and subarachnoid block20 (Figures 13,14). Communicating hydrocephalus may result due to occlusion of basilar cisterns by inflammatory exudate and/or fibrosis preventing CSF from being absorbed by arachnoid granulations or non-communicating hydrocephalus may result from obstruction at the level of the outlet foramina of the fourth ventricle27 (Figure 11). In addition, there may be evidence of acute infarctions and edema involving the brain stem, thalamus, cerebellum, or basal ganglia, likely resulting from vasospasm or vasculitis of smaller to medium-sized meningeal and perforating vessels associated with meningitis21, 27 (Figure 15). Ischemia from vasculitis may further manifest itself as diffuse periventricular white matter abnormalities.21 Focal enhancing parenchymal lesions and abscesses have also been reported but are seen less frequently21, 24 (Figure 16). Mass lesions and abscesses can additionally occur in the spinal subarachnoid space, usually as a result of direct extension from contiguous bony infection or, less frequently, as a result of isolated deposition from primary lung infection.20, 28
Bone and joint
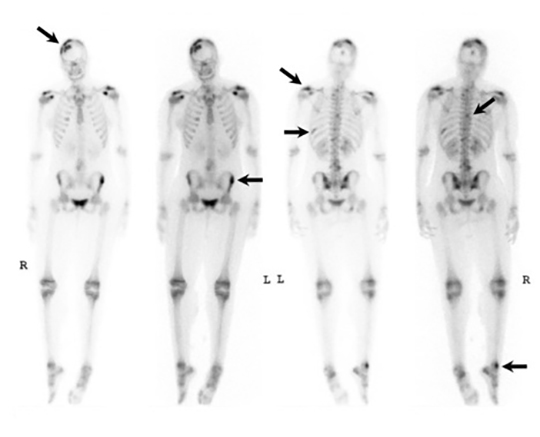
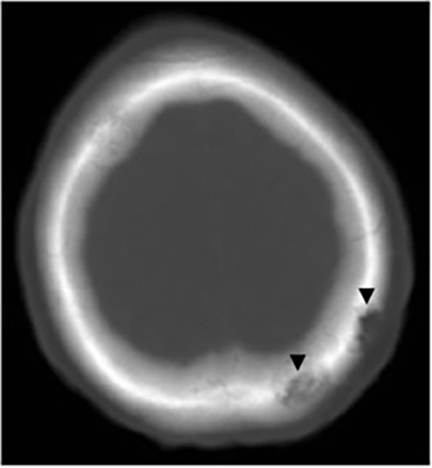
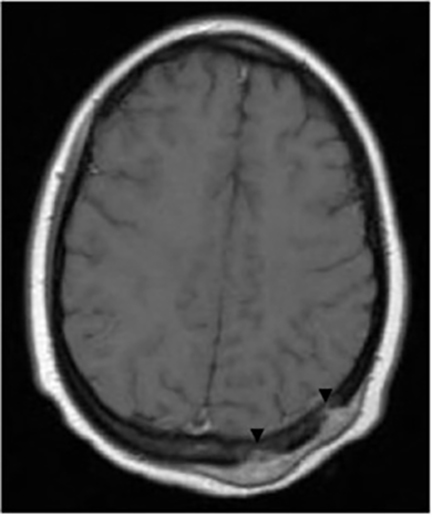
It has been reported that up to 50% of patients with disseminated disease demonstrate bone and joint involvement8 (Figure 17). Coccidioidomycosis can affect almost any bone in the body, although the axial skeleton is most frequently involved. Multiple bone lesions are often present. The most common radiographic pattern seen is multiple punched-out lytic lesions with circumscribed margins. This type of lesion is usually seen in the long and flat bones. Permeative bone destruction is another recognized pattern of osseous involvement which often has associated periosteal reaction and soft tissue disease.29 Soft tissue abscesses within the extremities without associated osteomyelitis have also been described.30 Skull lesions are often present with coccidioidomycosis and are seldom seen with other granulomatous infectious diseases including tuberculosis.28 On CT, the osseous lesions are often expansile and of low density. On MR imaging, the osseous lesions are T1 hypointense and T2 hyperintense.29 Abnormalities of the bones and associated soft tissues generally enhance after the intravenous administration of gadolinium.31
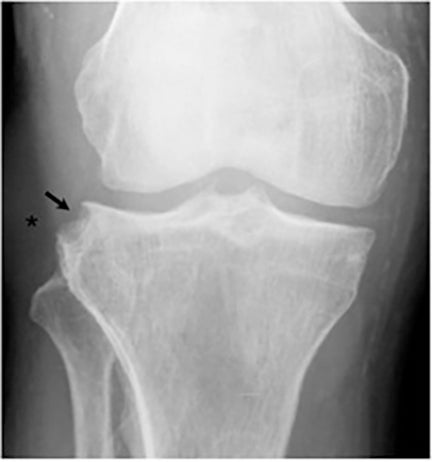
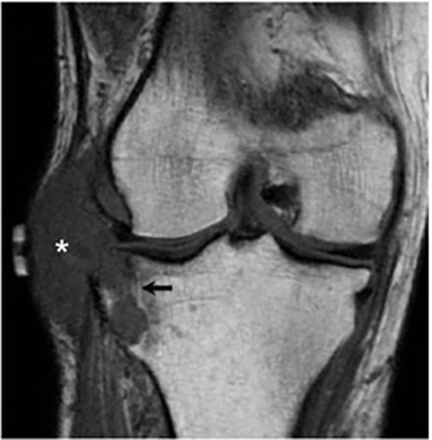
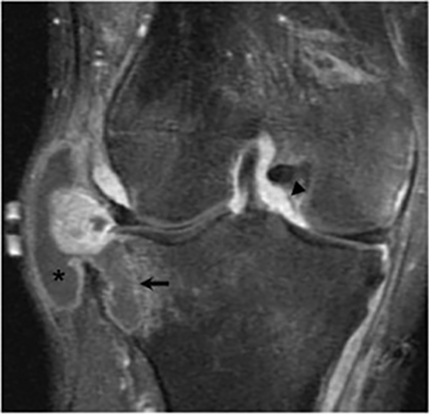
Joint involvement is usually monoarticular, most commonly affecting the ankle or knee. Coccidioidomycosis arthritis usually results from extension of adjacent osteomyelitis, although direct hematogenous spread may rarely occur.32 The findings of articular involvement include synovitis, joint effusion, periarticular bony destruction, well-defined erosions, juxtaarticular osteopenia, and relative joint space preservation early in the disease29 ,32 (Figure 18).
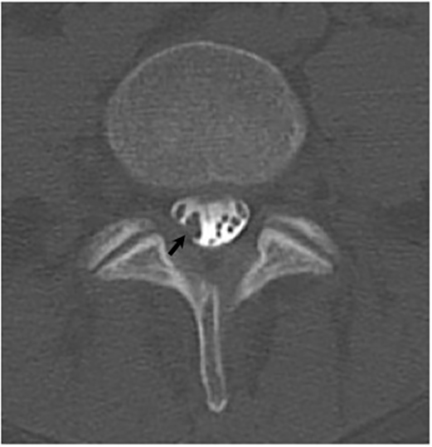
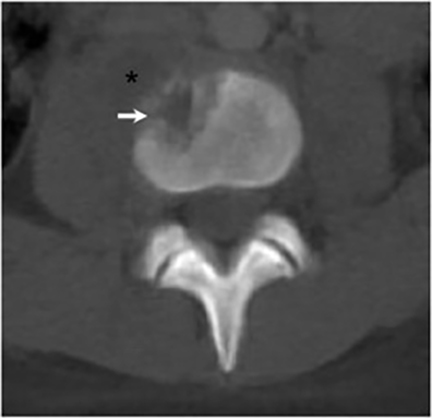
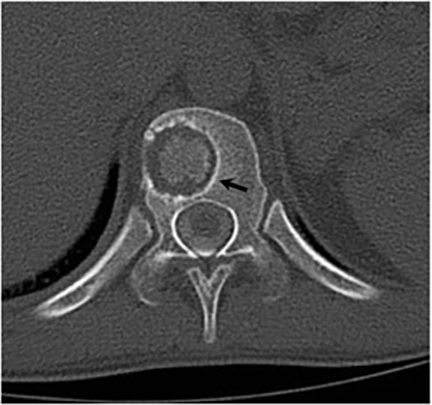
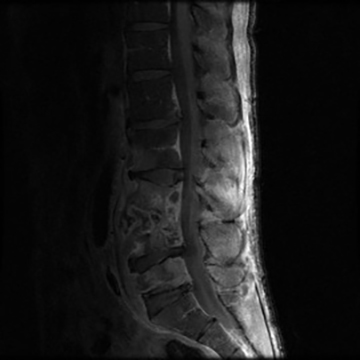
Vertebral osteomyelitis is common and seen in approximately 25% of patients with disseminated disease31 (Figures 19, 20). Both circumscribed lytic lesions and permeative lesions have been reported in the spine with involvement of the vertebral bodies, pedicles, and laminae. Near complete vertebral destruction has been described.29 Extensive paraspinal soft tissue disease with abscess and phlegmon formation is common. While disk spaces may appear preserved on radiographs, disk space involvement is often present and demonstrated on MR images as abnormal signal intensity or enhancement. The degree of disk space narrowing is minimal compared to the degree of vertebral and soft tissue disease. Epidural disease, subligamentous spread of infection, nerve root impingement, and cord compression are frequently seen.31 Gibbous deformity of the spine, commonly seen in tuberculosis, is an unusual finding in coccidioidomycosis.29 Disk space disease with minimal disk space narrowing and significant soft tissue involvement may also help favor coccidioidal disease over other granulomatous processes.31
Radionuclide scanning with both gallium-67 citrate and technetium-99m methylene-diphosphonate is useful in detecting osseous involvement with demonstration of increased tracer activity within the coccidioidal lesions.8 It is recommended that both gallium and bone scintigraphy be performed when disseminated coccidioidomycosis is suspected as the lesions may be detected on one exam and not the other.33 Radionuclide scans are useful in the detection of lesions which are clinically occult or not visible by radiography.8 MR imaging, however, is most sensitive for detecting early disease when radiographs, CT, and bone scans may be falsely negative.31 CT and MR imaging are useful in demonstrating the extent of bony involvement and any associated soft tissue extension.29 MR imaging is essential when vertebral osteomyelitis is suspected or present to evaluate the extent of marrow involvement, intervertebral disks, spinal canal, and adjacent soft tissues.31
Skin
The skin manifestations of erythema nodosum, a maculopapular rash, or erythema multiforme represent a reaction to the primary pulmonary infection.34 Skin infection with the fungal organisms commonly occurs with hematogenous dissemination. A variety of cutaneous lesions have been described including papules, nodules, verrucous lesions, superficial abscesses, pustules, and scars. Lesions commonly occur on the head (particularly the nasolabial fold), neck, and chest.35
Head and neck
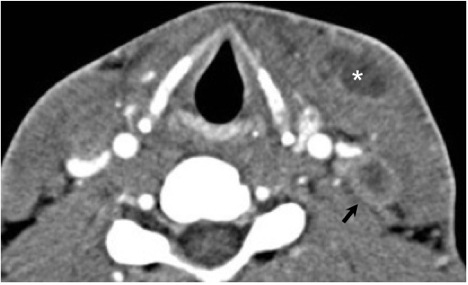
Numerous possible head and neck manifestations of disseminated disease have been described including laryngitis, thyroiditis, cervical lymphadenitis, and soft tissue abscesses7, 36 (Figure 21). There are case reports of neck masses due to cervical lymphadenopathy representing the only manifestation of disseminated disease.37
Abdomen and pelvis
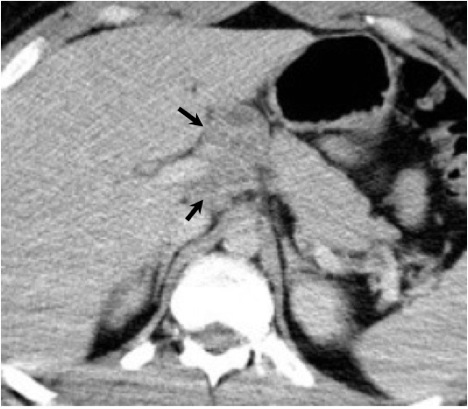
The abdomen and pelvis are other potential sites for dissemination. Liver and spleen involvement may be demonstrated on CT as diffuse hepatosplenomegaly or focal low density parenchymal lesions.8, 38 A radionuclide liver-spleen scan may demonstrate patchy tracer uptake in the liver. Ultrasound or CT may also demonstrate enlarged abdominal lymph nodes8 (Figure 22). Peritoneal coccidioidomycosis has been described, but is rare. Reported findings on CT include ascites, large omental masses, and low density peritoneal lesions with peripheral enhancement mimicking peritoneal carcinomatosis or pseudomyxoma peritonei.39, 40 Female genital infection may affect the uterus, fallopian tubes or ovaries. A complex adnexal mass may be seen on ultrasound and CT with a differential diagnosis of tubo-ovarian abscess or ovarian carcinoma. Peritoneal disease is often present concurrently with a complex adnexal mass in coccidioidal pelvic inflammatory disease which may falsely raise the suspicion for advanced ovarian cancer41,42. Dissemination to the male genital tract may affect the testes, epididymis, or prostate. On ultrasound, coccidioidal orchitis and epididymitis may manifest as testicular and epididymal masses.43
Conclusion
Coccidioidomycosis is an uncommon infection usually encountered in patients with a history of exposure to endemic areas. A large percentage of patients with primary pulmonary infection are asymptomatic or minimally symptomatic. Those that develop a noticeable illness usually have nonspecific symptoms and present radiologically with consolidation or nodules. The coexistence of a diffuse maculopapular rash, erythema nodosum, or erythema multiforme may aid in the specific diagnosis of coccidioidomycosis. Although the primary infection usually resolves spontaneously over time, a small percentage of patients progress to a persistent pulmonary infection which may manifest radiographically as pneumonia, a solitary pulmonary nodule, cavitary lesions, or fibrosis. Calcification is not common, in contrast to tuberculosis or histoplasmosis. Disseminated infection, although infrequent, can affect almost any organ system in the body with central nervous system and osseous involvement being the most common. Coccidioidomycosis has been labeled “the other great imitator,” as it can mimic other infectious, inflammatory, and neoplastic processes. Given the extremely variable presentation, a high level of suspicion for the diagnosis must be maintained in patients who have been in endemic areas as infection can occur following even a solitary exposure. Radiologists can assist in the early recognition and treatment of this disease by familiarizing themselves with the radiologic manifestations of coccidioidal infection.
References
- Oppenheimer AP, Arsura EL, Hospenthal DR. Coccidioidomycosis (Infectious Diseases). eMedicine. 2010. Available at: http://emedicine.medscape.com /article/215978-overview. Accessed November 10, 2010.
- Stevens DA. Coccidioidomycosis. N Engl J Med. 1995;332:1077-1082.
- Kirkland TN, Fierer J. Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis. 1996;2:192-199.
- Johnson RH, Baqui S. Coccidioidomycosis. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. 1st ed. Totowa, New Jersey: Humana Press; 2007:295-315.
- Parish JM, Blair JE. Coccidioidomycosis. Mayo Clin Proc. 2008;83:343-348.
- Galgiani JN. Coccidioidomycosis. West J Med. 1993;159:153-171.
- Arnold MG, Arnold JC, Bloom DC, et al. Head and neck manifestations of disseminated coccidioidomycosis. Laryngoscope. 2004;114:747-752.
- McGahan JP, Graves DS, Palmer PE, et al. Classic and contemporary imaging of coccidioidomycosis. AJR Am J Roentgenol. 1981;136: 393-404.
- Gotway MB, Berger WG, Leung JWT. Pulmonary Infections. In:Webb WR, Higgins CB, eds. Thoracic Imaging pulmonary and cardiovascular radiology. 1st ed. Philadelphia: Lippincott Williams and Wilkins; 2005:381-383.
- Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin N Am. 2003;17:41-57.
- Arsura EL, Kilgore WB. Miliary coccidioidomycosis in the immunocompetent. Chest. 2000;117:404-409.
- Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45:26-30.
- Williams PL. Coccidioidal meningitis. Ann NY Acad Sci. 2007; 1111:377-384.
- Davis LE, Porter BS. Central nervous system: Coccidioides immitis infections. Curr Treat Options Neurol. 2005;7:157-165.
- Blair JE. Coccidioidal meningitis: Update on epidemiology, clinical features, diagnosis, and management. Curr Infect Dis Rep. 2009;11: 289-295.
- Batra P. Pulmonary coccidioidomycosis. J Thorac Imaging. 1992;7:29-38.
- Capone D, Marchiori E, Wanke B, et al. Acute pulmonary coccidioidomycosis: CT findings from 15 patients. Br J Radiol. 2008;81:721-724.
- Tonelli AR, Khalife WT, Cao M, Young VB. Spherules, hyphae, and air-crescent sign. Am J Med Sci. 2008;335:504-506.
- Jain KK, Mittal SK, Kumar S, Gupta RK. Imaging features of central nervous system fungal infections. Neurol India. 2007;55:241-250.
- Winston DJ, Kurtz TO, Fleischmann J, et al. Successful treatment of spinal arachnoiditis due to coccidioidomycosis. J Neurosurg. 1983;59: 328-331.
- Drake KW, Adam RD. Coccidioidal meningitis and brain abscesses: analysis of 71 cases at a referral center. Neurology. 2009;73:1780-1786.
- Erly WK, Labadie E, Williams PL, et al. Disseminated coccidioidomycosis complicated by vasculitis: a cause of fatal subarachnoid hemorrhage in two cases. AJNR Am J Neuroradiol. 1999;20:1605-1608.
- Scanarini M, Rotilio A, Rigobello L, et al. Primary intrasellar coccidioidomycosis simulating a pituitary adenoma. Neurosurgery. 1991;28: 748-751.
- Arsura EL, Johnson R, Penrose J, et al. Neuroimaging as a guide to predict outcomes for patients with coccidioidal meningitis. Clin Infect Dis. 2005;40:624-627.
- Erly WK, Bellon RJ, Seeger JF, Carmody RF. MR imaging of acute coccidioidal meningitis. AJNR Am J Neuroradiol. 1999;20:509-514.
- Wrobel CJ, Meyer S, Johnson RH, Hesselink JR. MR findings in acute and chronic coccidioidomycosis. AJNR Am J Neuroradiol. 1992;13: 1241-1245.
- Romeo JH, Rice LB, McQuarrie IG. Hydrocephalus in coccidioidal meningitis: case report and review of the literature. Neurosurgery. 2000;47:773-777.
- Wrobel CJ, Chappell ET, Taylor W. Clinical presentation, radiological findings, and treatment results of coccidioidomycosis involving the spine: report on 23 cases. J Neurosurg. 2001;95:33-39.
- Zeppa MA, Laorr A, Greenspan A, et al. Skeletal coccidioidomycosis: imaging findings in 19 patients. Skeletal Radiol. 1996;25:337-343.
- Garvin GJ, Peterfy CG. Soft tissue coccidioidomycosis on MRI. J Comput Assist Tomogr. 1995;19:612-614.
- Olson EM, Duberg AC, Herron LD, et al. Coccidioidal spondylitis: MR findings in 15 patients. AJR Am J Roentgenol. 1998;171:785-789.
- Sheldon PJ, Forrester DM, Learch TJ. Imaging of intraarticular masses. Radiographics. 2005;25:105-119.
- Cohen AJ, Braunstein P, Pais MJ. The role of gallium and bone scintigraphy in disseminated coccidioidomycosis. Clin Nucl Med. 1984;9: 538-539.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann NY Acad Sci. 2007;111:411-421.
- Carpenter JB, Feldman JS, Leyva WH, DiCaudo DJ. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
- Biller JA, Scheuller MC, Eisele DW. Coccidioidomycosis causing massive cervical lymphadenopathy. Laryngoscope. 2004;114:1892-1894.
- Dudley JE. Coccidioidomycosis and neck mass “single lesion” disseminated disease. Arch Otolaryngol Head Neck Surg. 1987;113:553-555.
- Adam RD, Elliott SP, Taljanovic MS. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med. 2009;122:770-777.
- Dooley DP, Reddy RK, Smith CE. Coccidioidomycosis presenting as an omental mass. Clin Infect Dis. 1994;19:802-803.
- Eyer BA, Qayyum A, Westphalen AC, et al. Peritoneal coccidioidomycosis: a potential CT mimic of peritoneal malignancy. Abdom Imaging. 2004;29:505-506.
- Ellis MW, Dooley DP, Sundborg MJ, et al. Coccidioidomycosis mimicking ovarian cancer. Obstet Gynecol. 2004;104:1177-1179.
- Saw EC, Smale LE, Einstein H, Huntington RW. Female genital coccidioidomycosis. Obstet Gynecol. 1975;45:199-202.
- Sohail MR, Andrews PE, Blair JE. Coccidioidomycosis of the male genital tract. J Urol. 2005;173:1978-1982.
Citation
Imaging manifestations of primary and disseminated coccidioidomycosis. Appl Radiol.
February 12, 2015