IAEA Launches Research Project to Develop New Technetium-99m Radiopharmaceuticals
Images
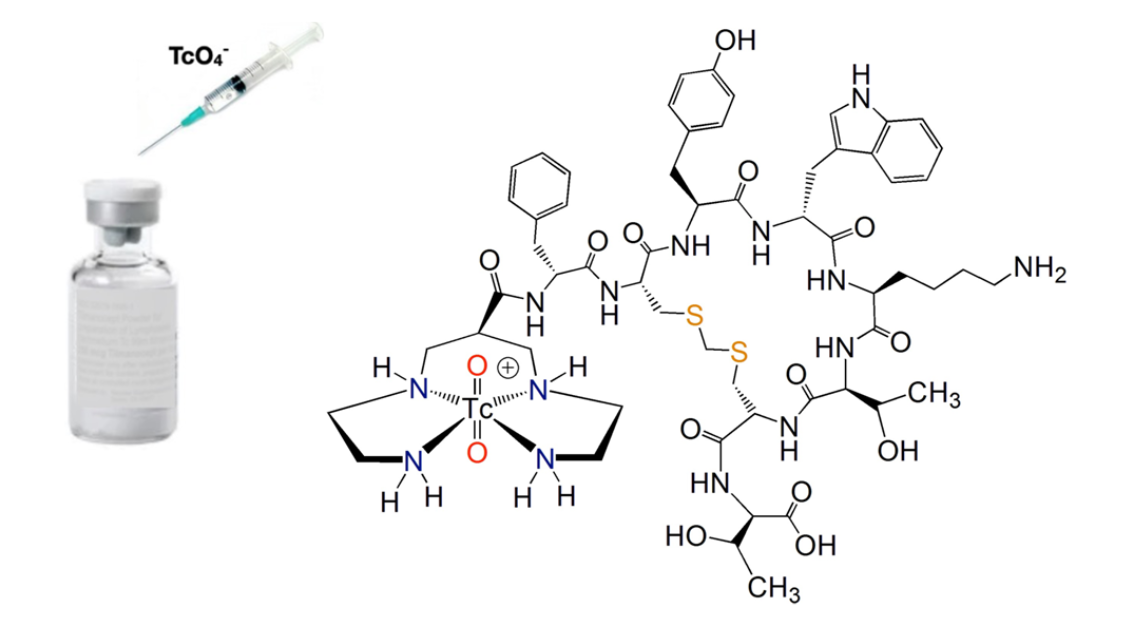
The International Atomic Energy Agency (IAEA) announced a new coordinated research project (CRP) that will establish a global network of producers and researchers for developing a new generation of Technetium-99m (99mTc) kits and radiopharmaceuticals. The CRP is expected to develop standard procedures and guidelines for the production, quality control and preclinical studies of the novel99mTc radiopharmaceuticals. The CRP will also focus on the most clinically relevant items on demand and application of new radiolabelling strategies.
The CRP will focus on transferring the knowledge acquired to produce a series of99mTc radiopharmaceuticals for imaging various biological substrates of relevant clinical interest using the most efficient methods of99mTc labelling. Several99mTc radiopharmaceuticals will be produced for imaging the following biological substrates:
- Research and development on99mTc PSMA receptors ligands.
- Research and development on99mTc based peptides or small molecules targeting receptors expressed by cancerous lesions classified as poor-prognostic solid tumors such as breast cancer.
- Tumor microenvironment studies using newly developed ligands.
- Round robins comprised of labelling kit formulations previously developed for the preparation of99mTc radiopharmaceuticals used in myocardial perfusion, bone imaging, tumor imaging, infection processes and sentinel lymph node detection.
- Identification of suitable and efficient quality control procedures and pre-clinical assays for assessing the overall quality of99mTc radiopharmaceuticals prepared during the CRP.
- Enhancement of capacity building for applying freeze-drying methods and technologies for the development of lyophilized kit formulations of99mTc radiopharmaceuticals investigated during the CRP.
- Non-clinical studies including in vitro cell studies and in vivo animal studies for evaluating stability, dosimetry, therapeutic efficacy, and possible side effects.
- Preparation and formulation of appropriate protocols and guidelines accessible for all IAEA Member States.
Related Articles
Citation
. IAEA Launches Research Project to Develop New Technetium-99m Radiopharmaceuticals. Appl Radiol.
December 1, 2023