Hereditary Hemorrhagic Telangiectasia
Images
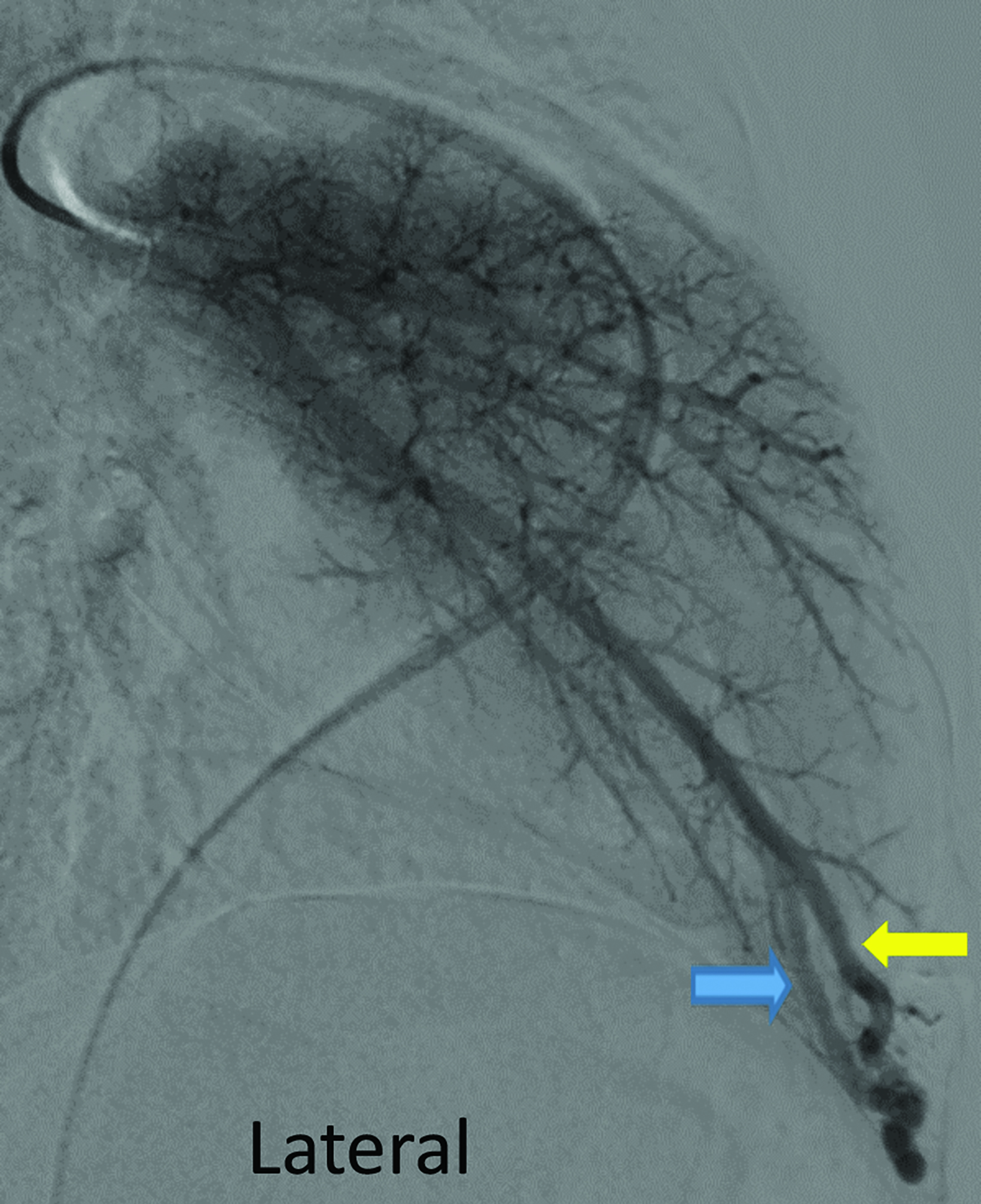
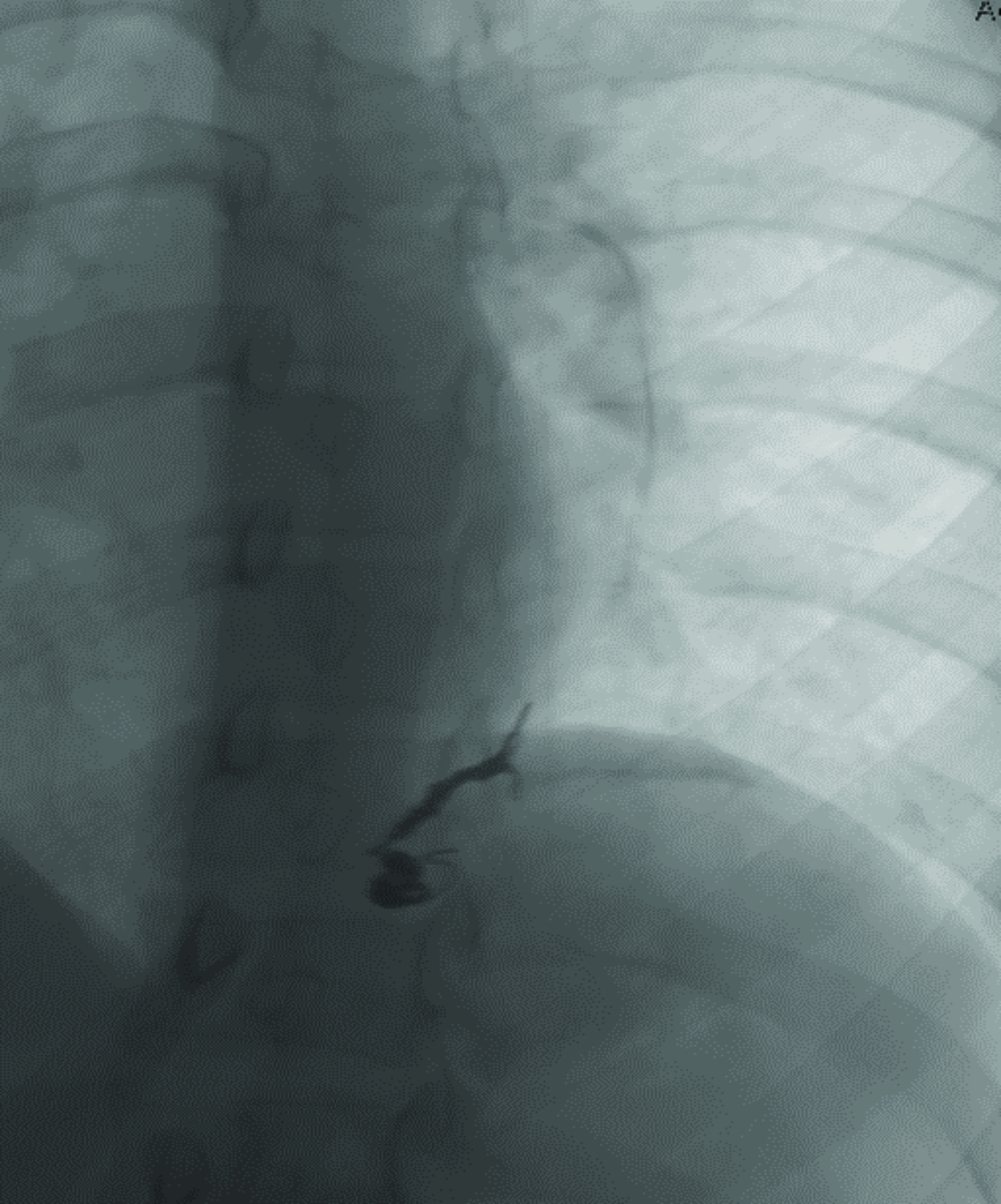
Case Summary
A teenager had a history of a right temporal lobe stroke. Subsequent bubble echocardiogram was suspicious for a pulmonary arteriovenous malformation (AVM). The patient’s parent and parent’s sibling had a history of recurrent epistaxis; the parent also had a prior ruptured brain AVM. The history and ultrasound results raised the concern for hereditary hemorrhagic telangiectasia (HHT), prompting chest CT.
Imaging Findings
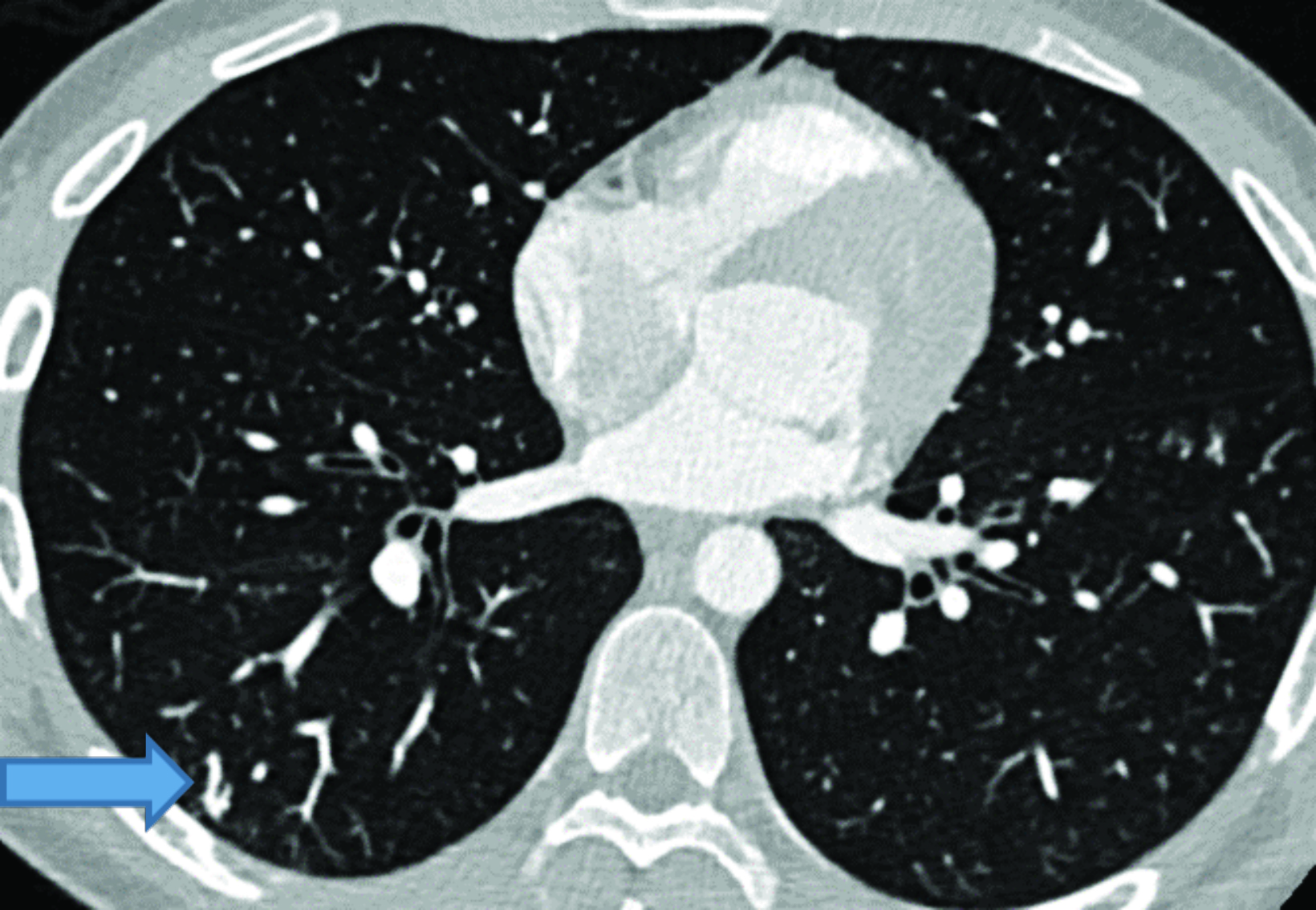
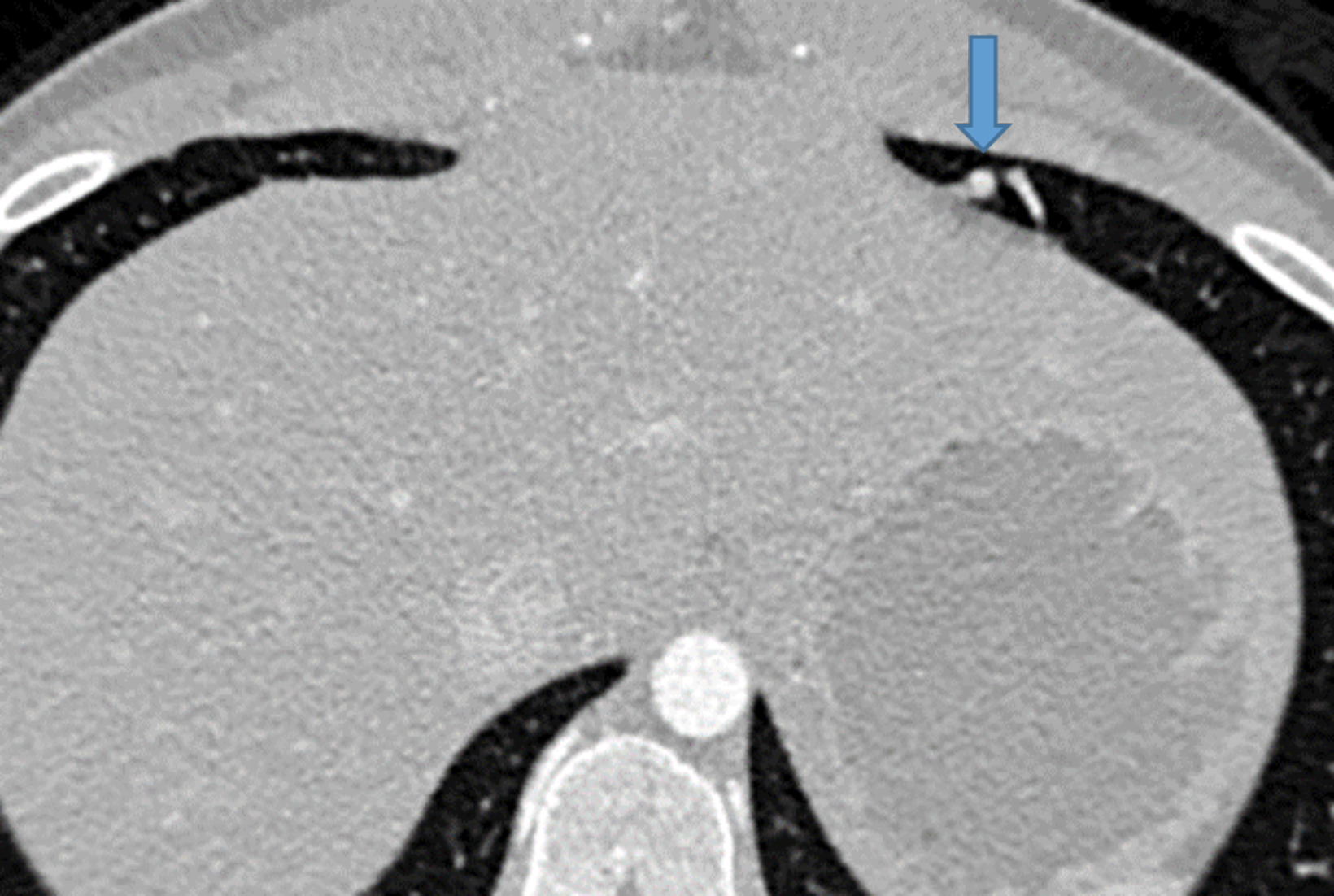
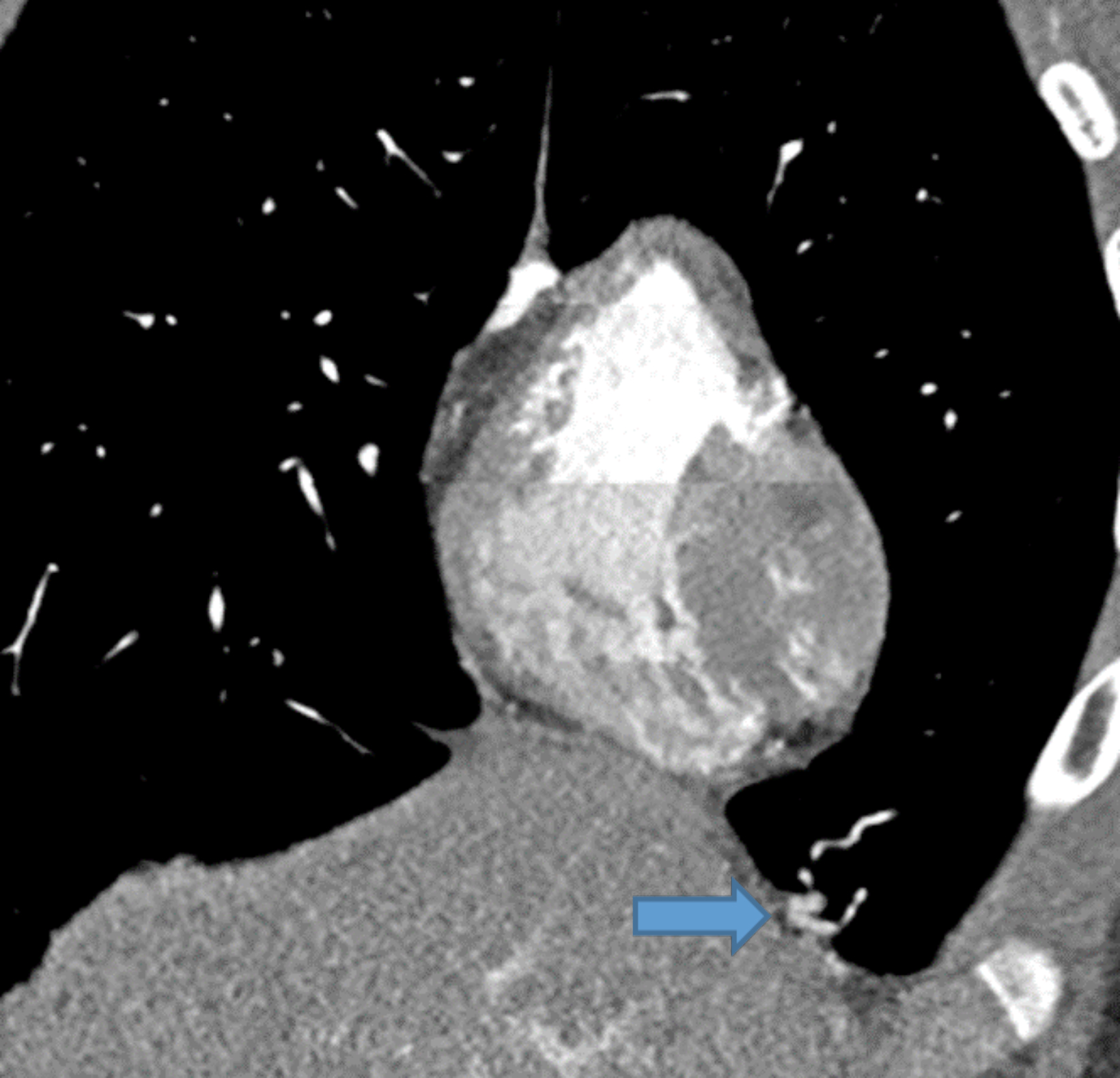
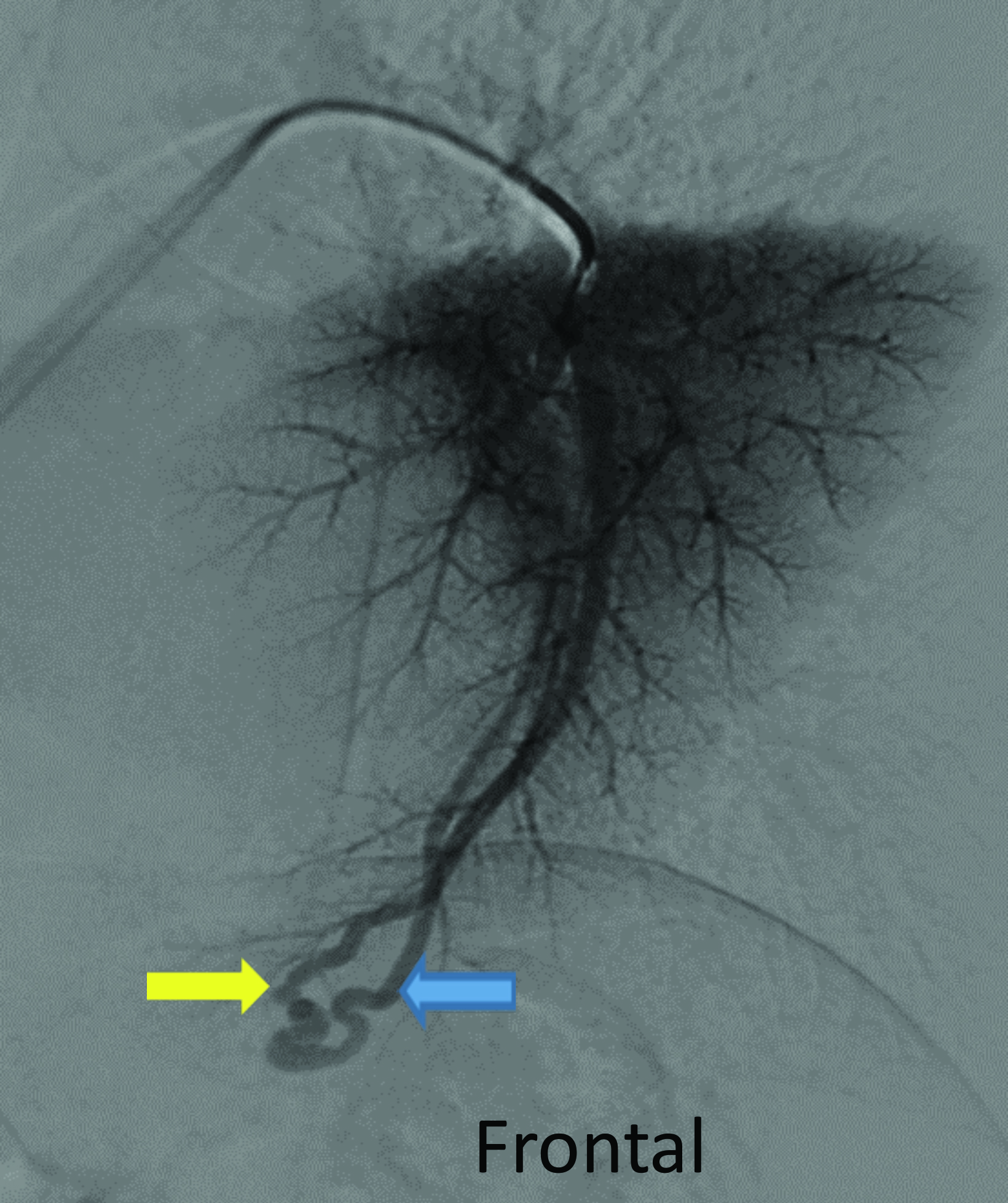
Contrast-enhanced CT of the chest demonstrated small pulmonary AVMs located in the lingula and right lower lobe (Figure 1). The pulmonary AVMs were successfully treated with embolization using coils (Figure 2).
Diagnosis
Hereditary hemorrhagic telangiectasia
Discussion
Hereditary hemorrhagic telangiectasia (HHT), also called Osler-Weber-Rendu syndrome, is an inherited disease of vasculature dysplasia characterized by AVMs and telangiectasias throughout the body.1 In 1896, Rendu was the first to write about the combination of familial epistaxis and telangiectasias separate from hemophilia.2 In the next 10 years multiple case reports were published, including by Osler and Weber, giving rise to the name of the condition.2
HHT is a heterogeneous, autosomal dominant condition where mutations in two genes, ENG (Type 1) and ACVRL1 (ALK1) (Type 2), are implicated in 85% of cases. However, five different genes have been identified.1 Patients can present with telangiectasias, which are blanchable, mucocutaneous, red/pink lesions located on the lips, tongue, face, fingers, and/or the nasal, buccal, and gastrointestinal (GI) mucosa.1 These telangiectasias can sometimes rupture, leading to mild to severe bleeding.3 Complications of telangiectasias include recurrent epistaxis, which can result in iron deficiency anemia.4
AVMs are vascular abnormalities defined as direct communication(s) of arteries to veins, that are often located in the liver, lung, and/or brain. These direct connections lead to more serious complications such as venous emboli entering the arterial circulation, hemoptysis, pulmonary hypertension, gastrointestinal bleeding, brain hemorrhage, strokes, brain abscesses. Myelopathy and back pain may be seen with spinal AVMs.1
HHT is diagnosed clinically by identifying multiple telangiectasias or AVMs in various locations throughout the body. The Curacao criteria created a consensus for diagnosis of HHT based on 4 elements: 1) spontaneous recurrent epistaxis, 2) multiple mucocutaneous telangiectasias, 3) visceral AVMs, and 4) a diagnosis in a first-degree relative.4 A definitive diagnosis is made when three or more criteria are present. A diagnosis of possible or suspected HHT is made when two criteria are present. Diagnosis is unlikely when one or zero findings are present.4 Genetic testing can confirm the diagnosis in approximately 75-85% of cases.
The lifetime prevalence and incidence of ruptured pulmonary AVMs are 2.7% and 0.16%, respectively.5 Imaging for hepatic, pulmonary, or central nervous system AVMs is an important part of the workup of patients with suspected or known HHT. Contrast-enhanced echocardiogram (bubble echocardiogram) demonstrating intravenously injected microbubbles within the left atrium can confirm the presence of a pulmonary AVM. Pulmonary CT angiography (PCTA) is then used to identify the location, size, and number of pulmonary AVMs. On PCTA, AVMs will appear as a well-circumscribed vascular mass with one or more enhancing feeding arteries and draining vein(s).3 Pulmonary AVMs can lead to hemorrhage and hemoptysis. Additional complications include paradoxical emboli, septic emboli, hypoxia, and high output cardiac failure.6
Hepatic AVMs occur in as many as 74% of patients with HHT although the majority are asymptomatic.7 When evaluating patients for liver involvement, ultrasound, CT, or MRI can be used. Catheter angiography is typically not the first-line imaging study, but can be valuable if an intervention is contemplated.7 These imaging modalities can reveal AVMs in the liver, arteriovenous or portal venous shunts, hepatomegaly, biliary necrosis, and bile leaks.7 Hepatic AVMs can lead to high-output cardiac failure, portal hypertension, and biliary disease from shunting of the blood away from the peribiliary plexi.7
MRI is the preferred modality to identify central nervous system lesions; it can show telangiectasias and AVMs of the brain or spinal cord and any associated aneurysms.8 Brain and spinal cord involvement can cause headaches, seizures, myelopathy, and hemorrhage.8
The GI tract is often evaluated via endoscopy, colonoscopy, or capsule endoscopy. Imaging may be performed if a patient has acute GI bleeding., CT, nuclear medicine-tagged red blood cell scans and catheter angiography are the tests of choice to evaluate active bleeding and embolization can be performed.9,10These imaging modalities can reveal AVMs or angiodysplasia in the esophagus, stomach, small bowel, or large bowel, which can be the cause of recurrent bleeding.9,10
Conclusion
Imaging is necessary to diagnose and treat HHT. Contrast-enhanced chest CT, liver ultrasound, and abdominal, brain, and spine MRI help to identify AVMs, as well as to plan therapy and predict potential complications. CT and catheter angiography can play a role in AVM diagnosis and treatment, respectively. Embolization has become the treatment of choice for occluding AVMs in any organ.
References
Citation
RB RNT, CM S, AJ T. Hereditary Hemorrhagic Telangiectasia. Appl Radiol. 2023; (5):41-43.
September 12, 2023