Giant Sigmoid Diverticulum: Imaging Features and Management
Images
Diverticular disorders of the colon are common among the aging population of industrial countries. About 50% of individuals older than 60 years of age develop diverticulosis predominantly involving the sigmoid, and 10-20% of them will experience complications during their lifetime, such as diverticulitis, hemorrhage, perforation and fistula to the adjacent organs.1,2 However, a giant sigmoid diverticulum (GSD) is a rare complication and may present a diagnostic and therapeutic challenge for those who are unfamiliar with the condition.3-6
The majority of publications about GSD have been isolated case reports in the surgical literature. This article reviews the imaging and management of GSD.
Radiographic Findings
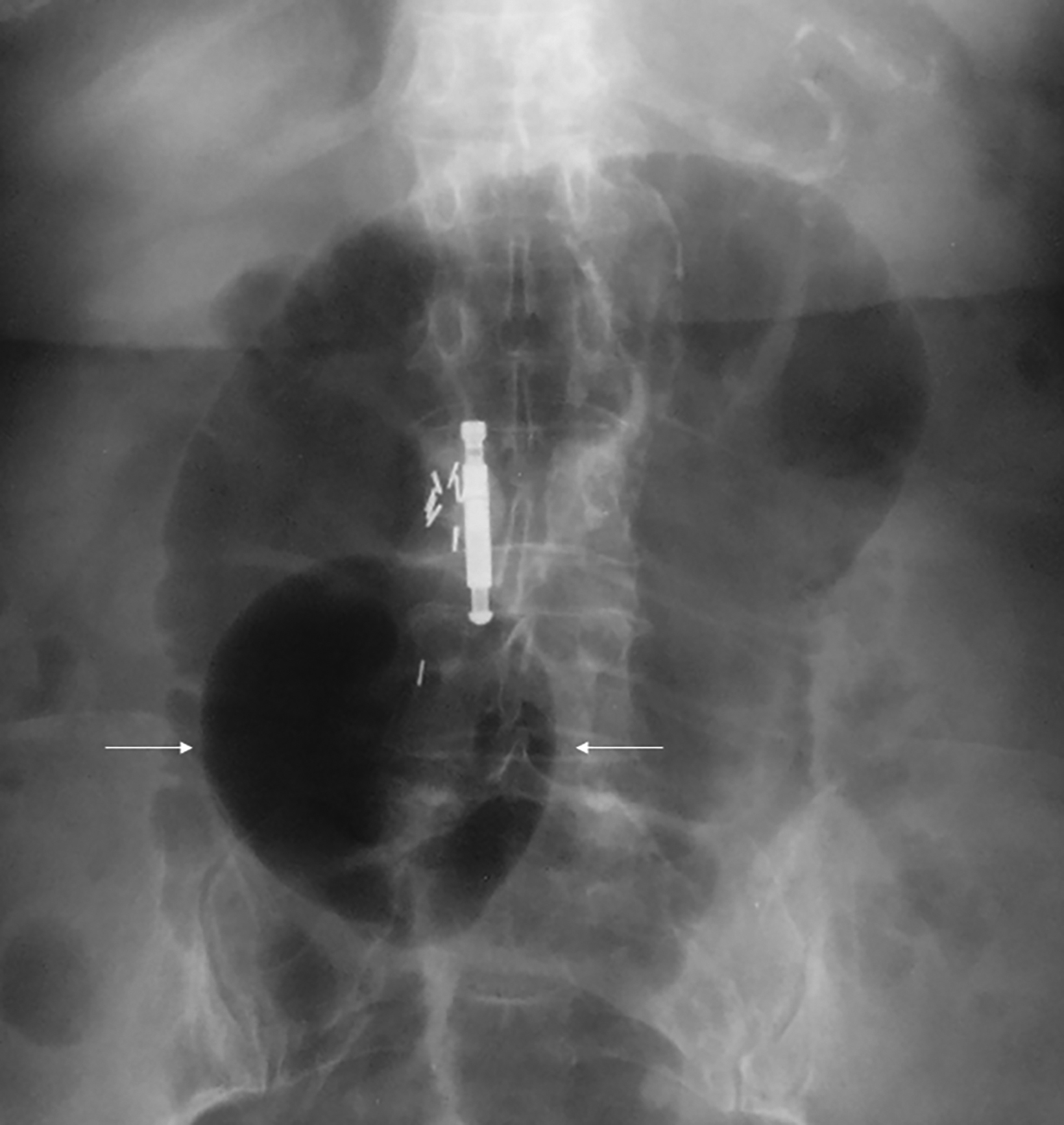
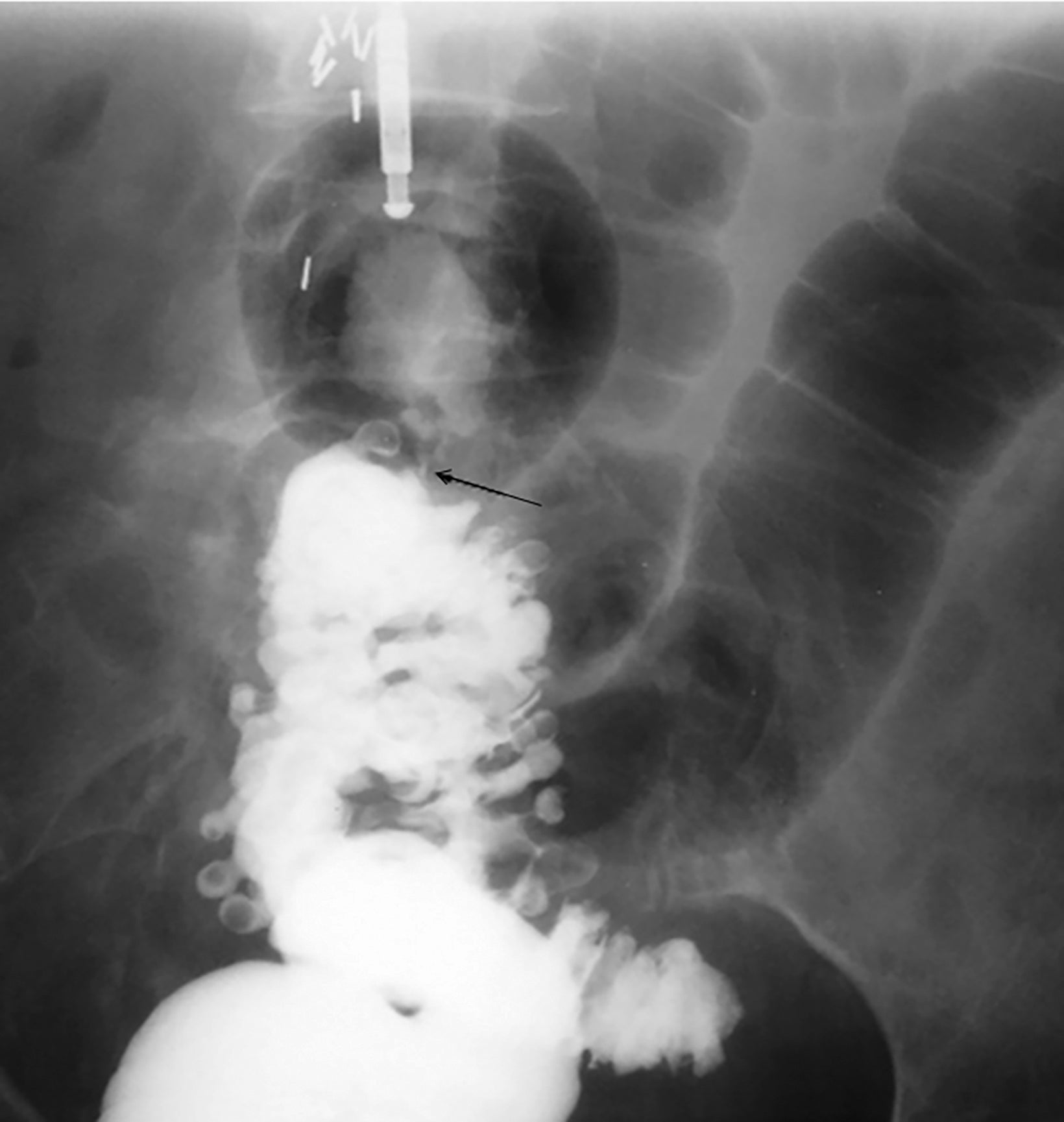
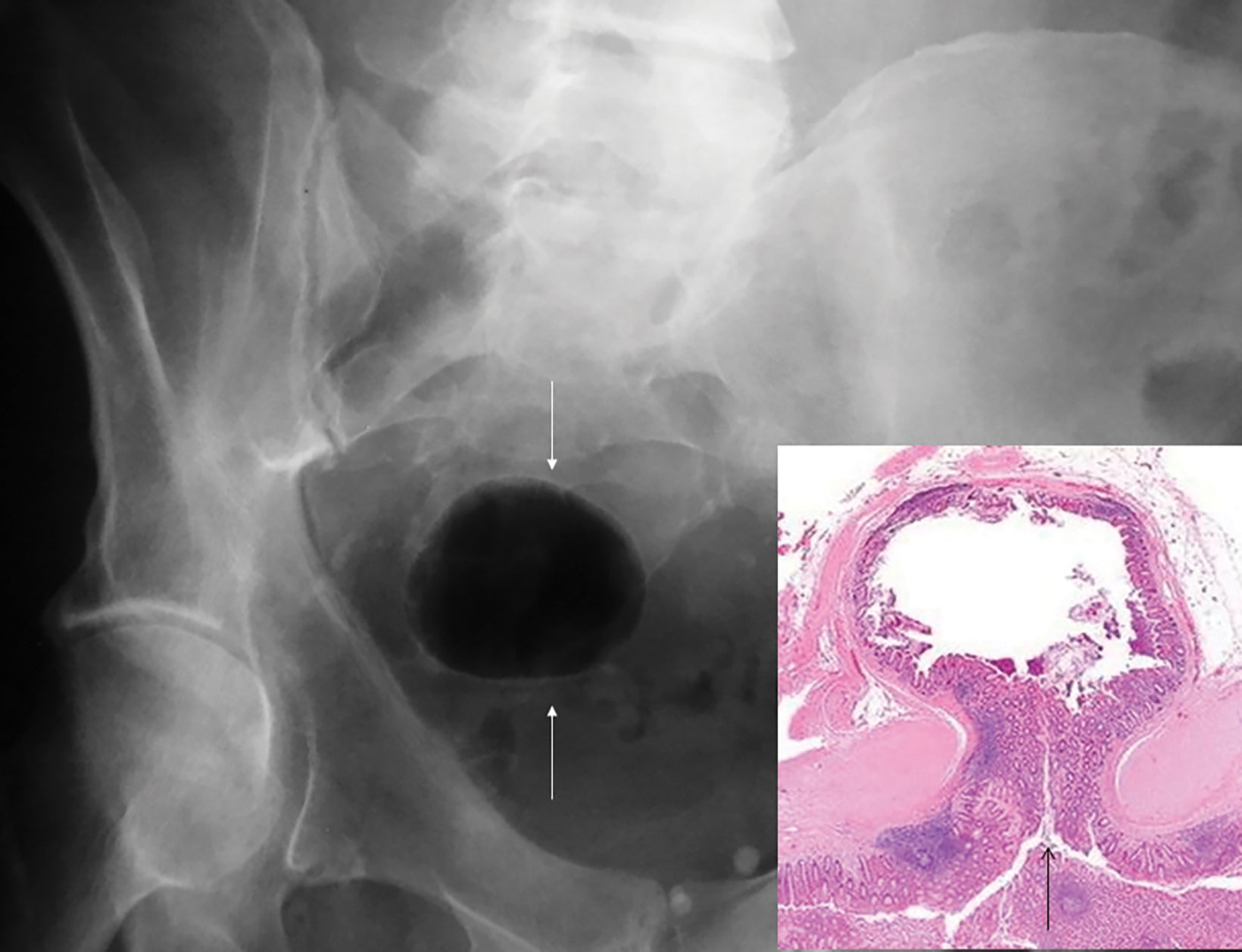
Abdominal radiographs reveal the appearance of a GSD as a round or oval, gas-filled structure in middle or left side of the abdomen, usually measuring from 5 to 12 cm in maximal diameter. Colonic examination with contrast enema can demonstrate partial opacification in most patients (Figures 1, 2). In some instances, however, barium may not enter the GSD on either the colon study or peroral administration of contrast material. On rare occasions multiple giant sigmoid diverticula may be encountered in the same patient. GSD is associated with comorbid diverticulosis.
Abdominal Computed Tomography Findings
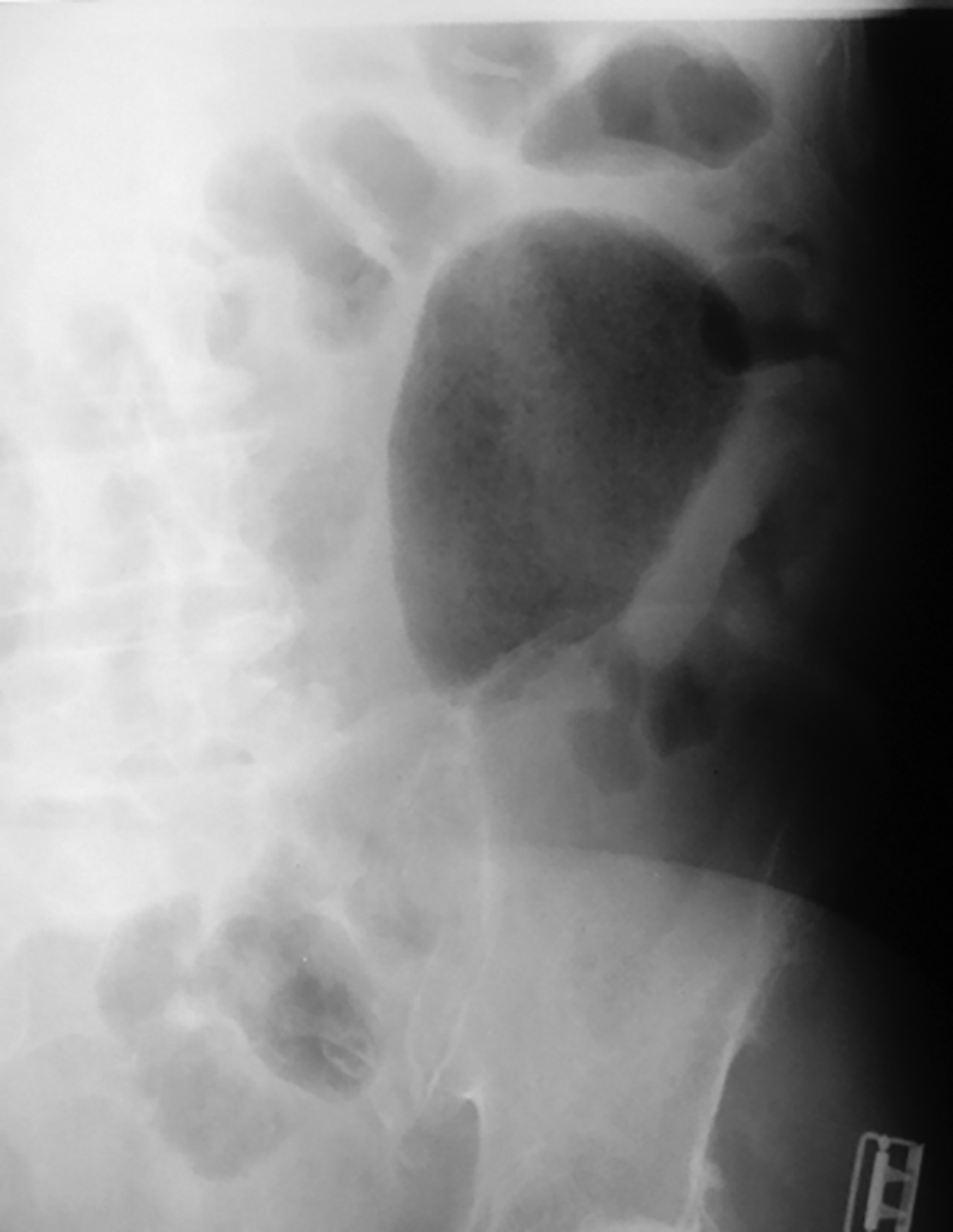
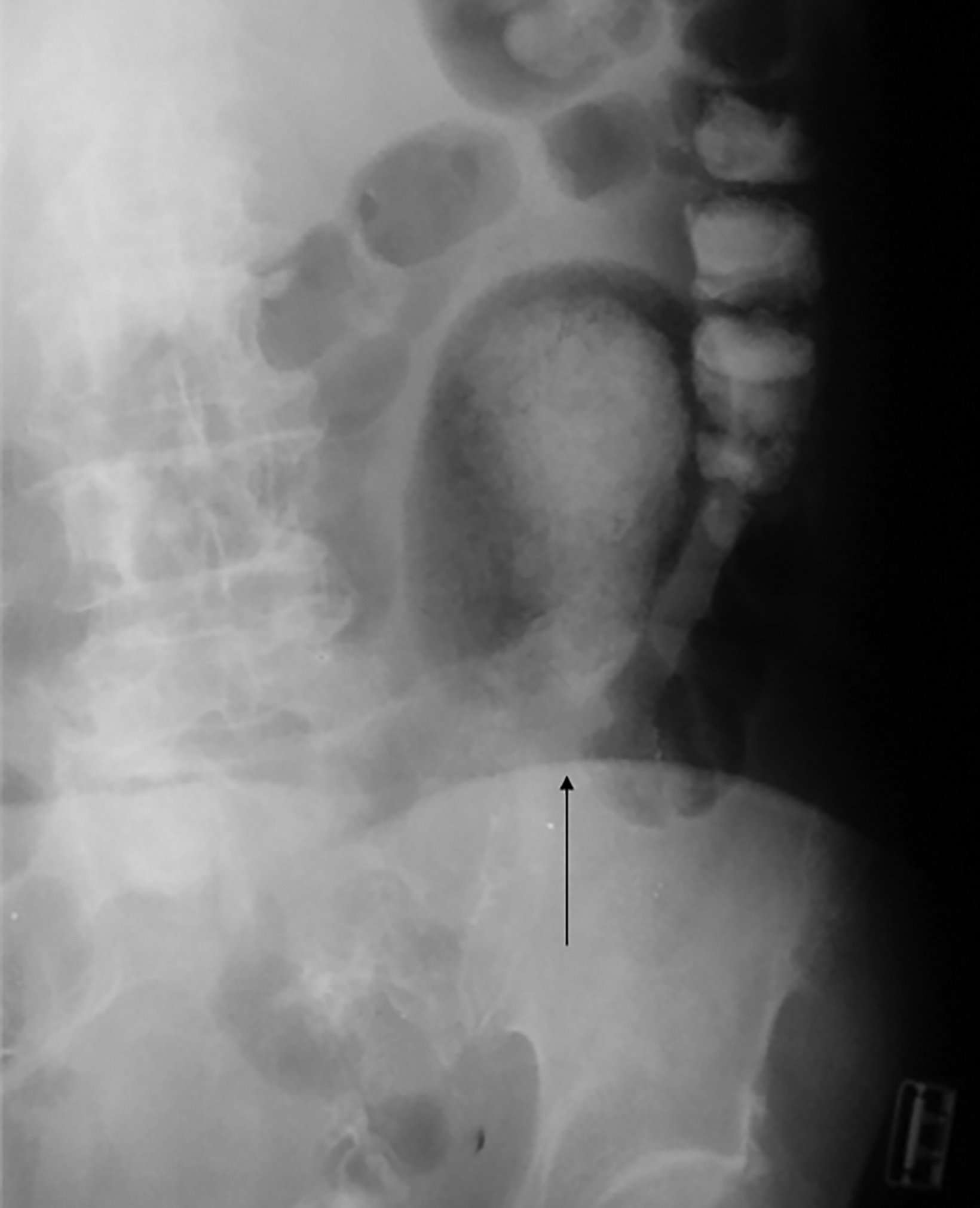
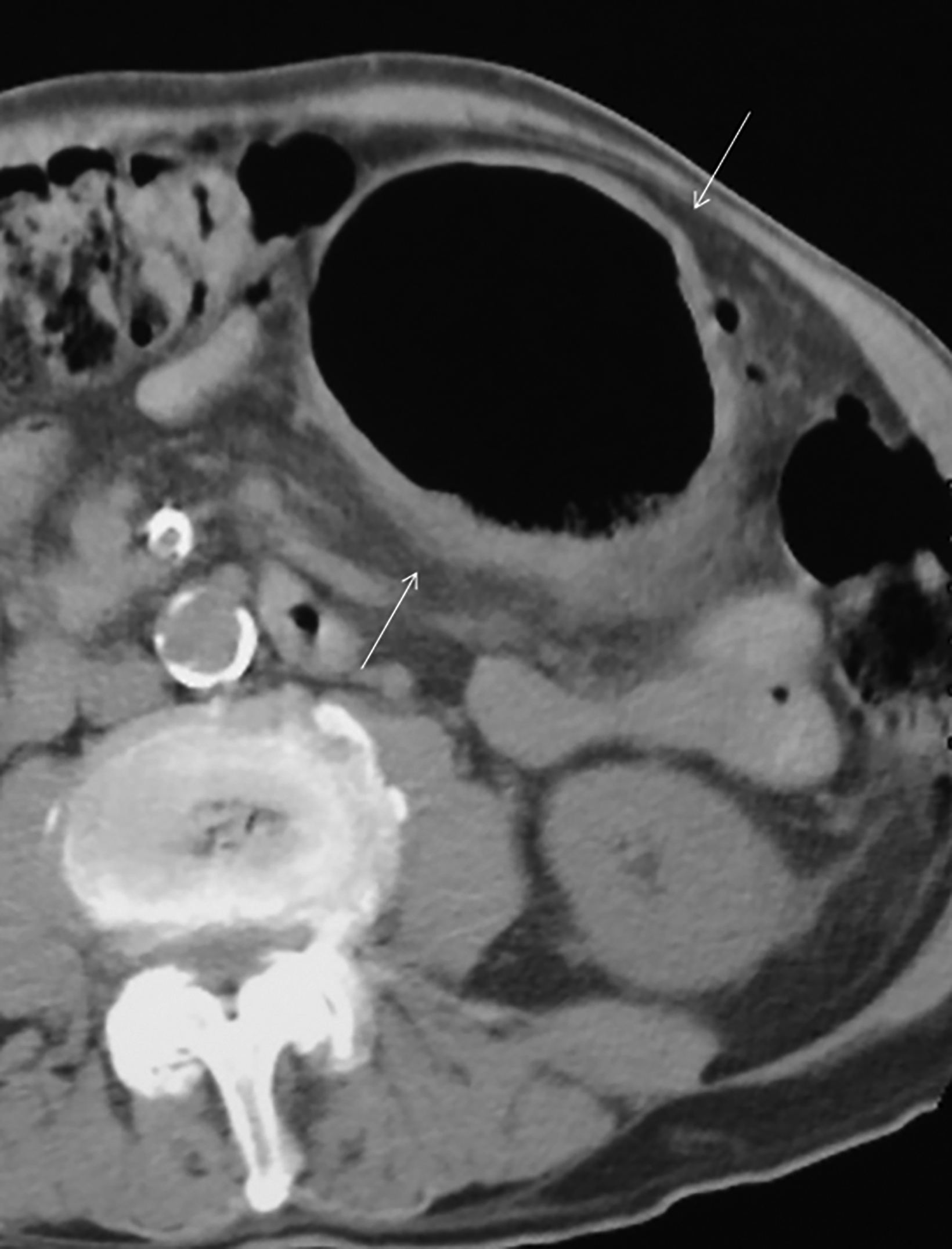
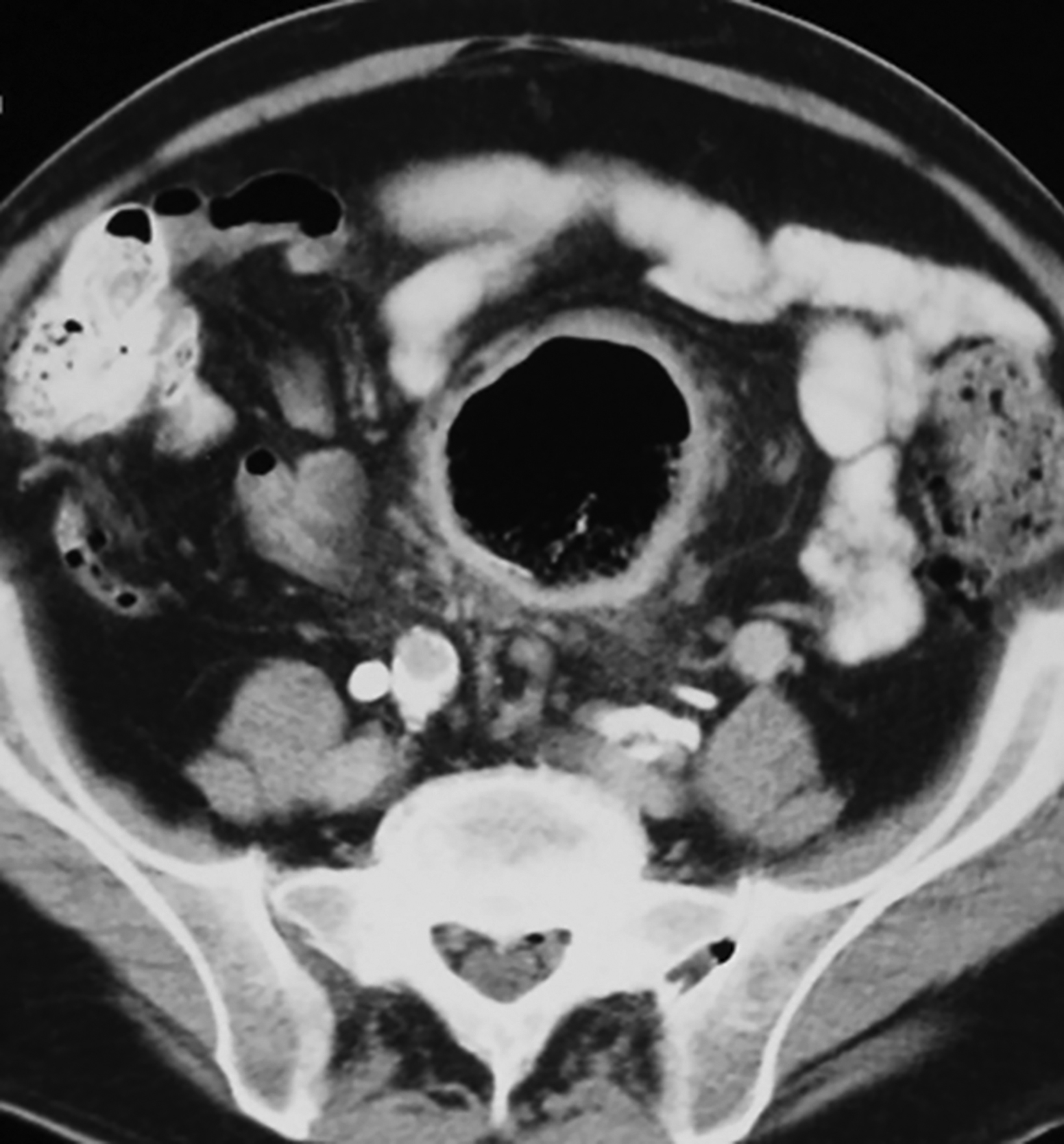
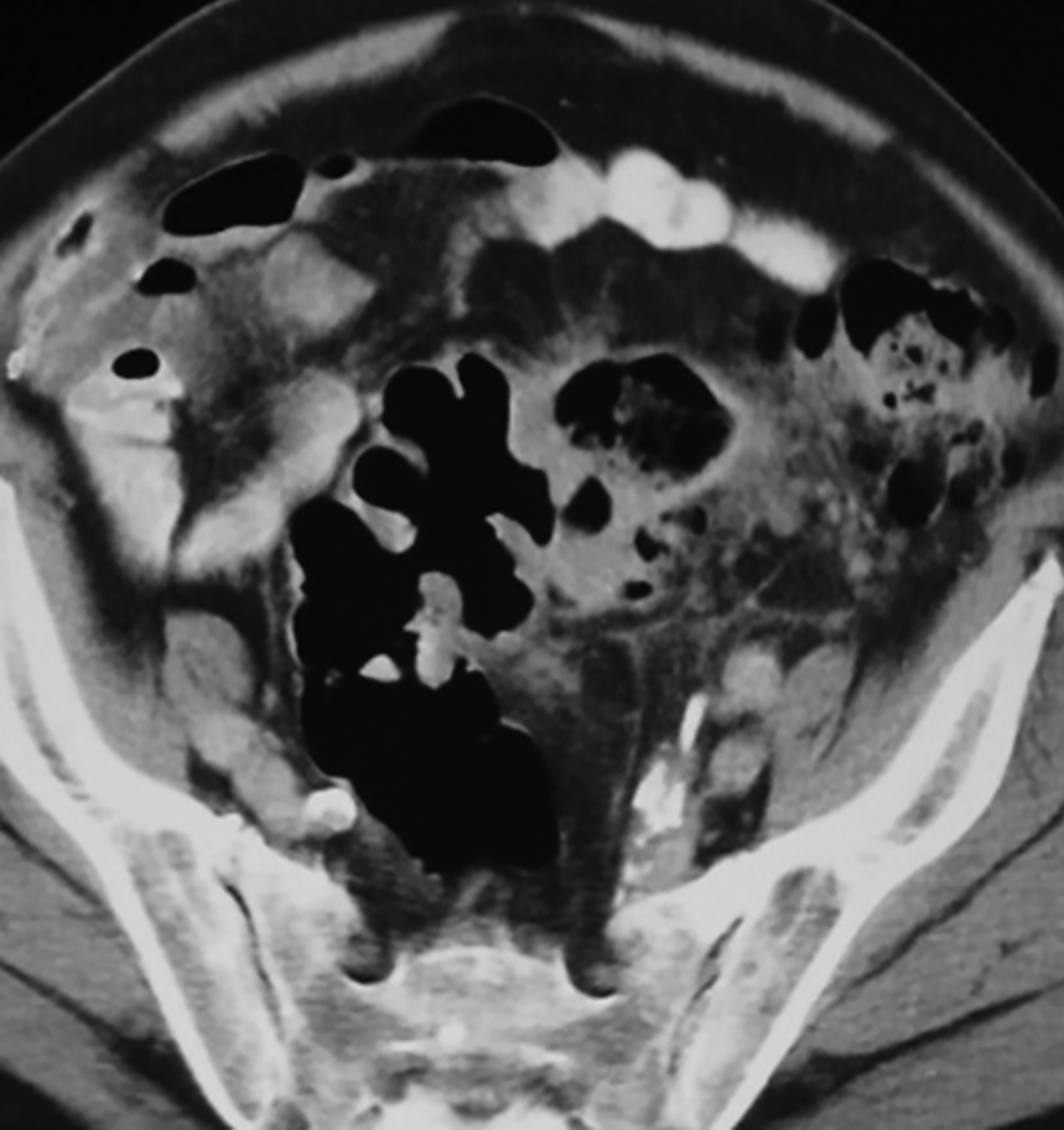
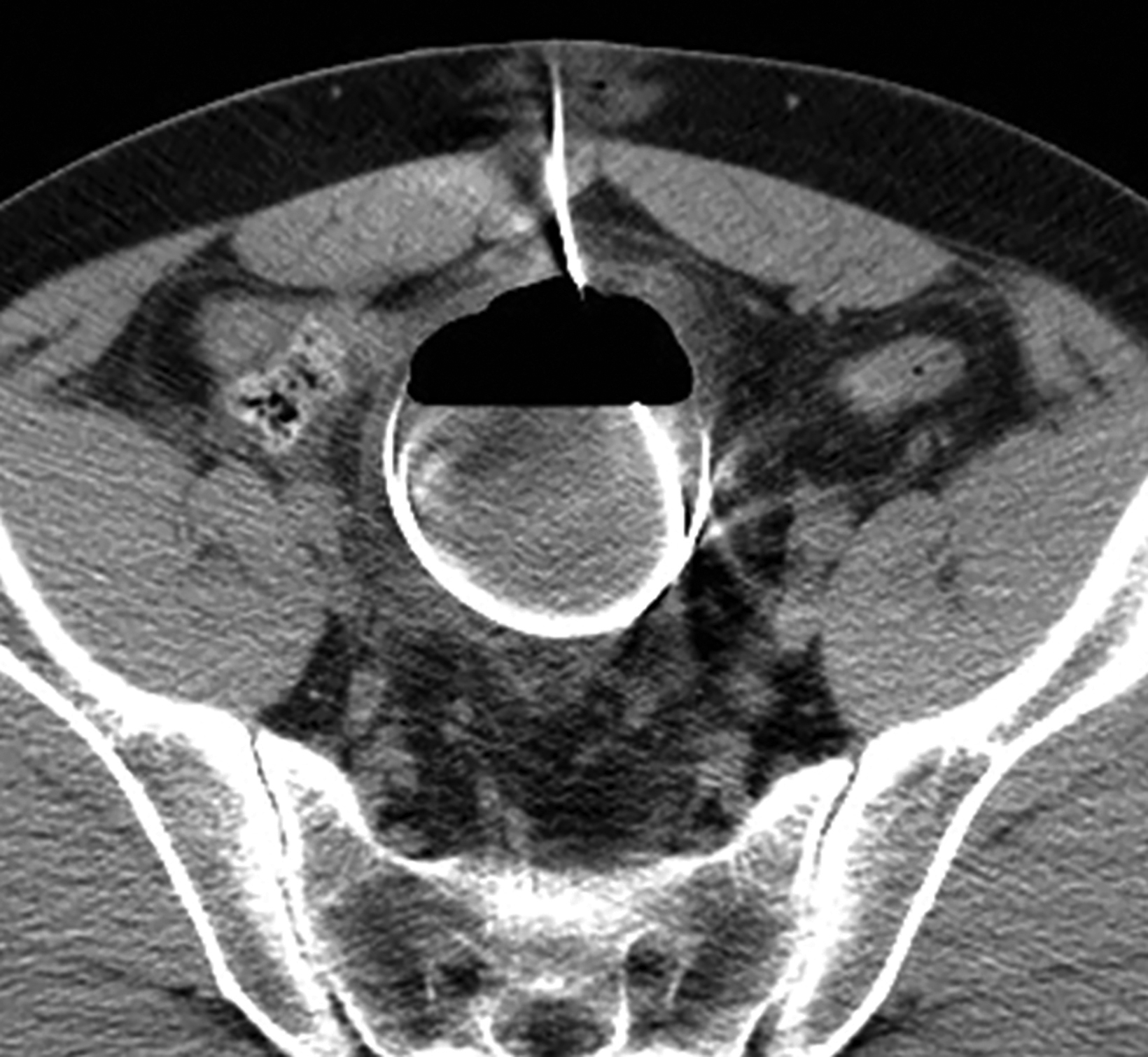
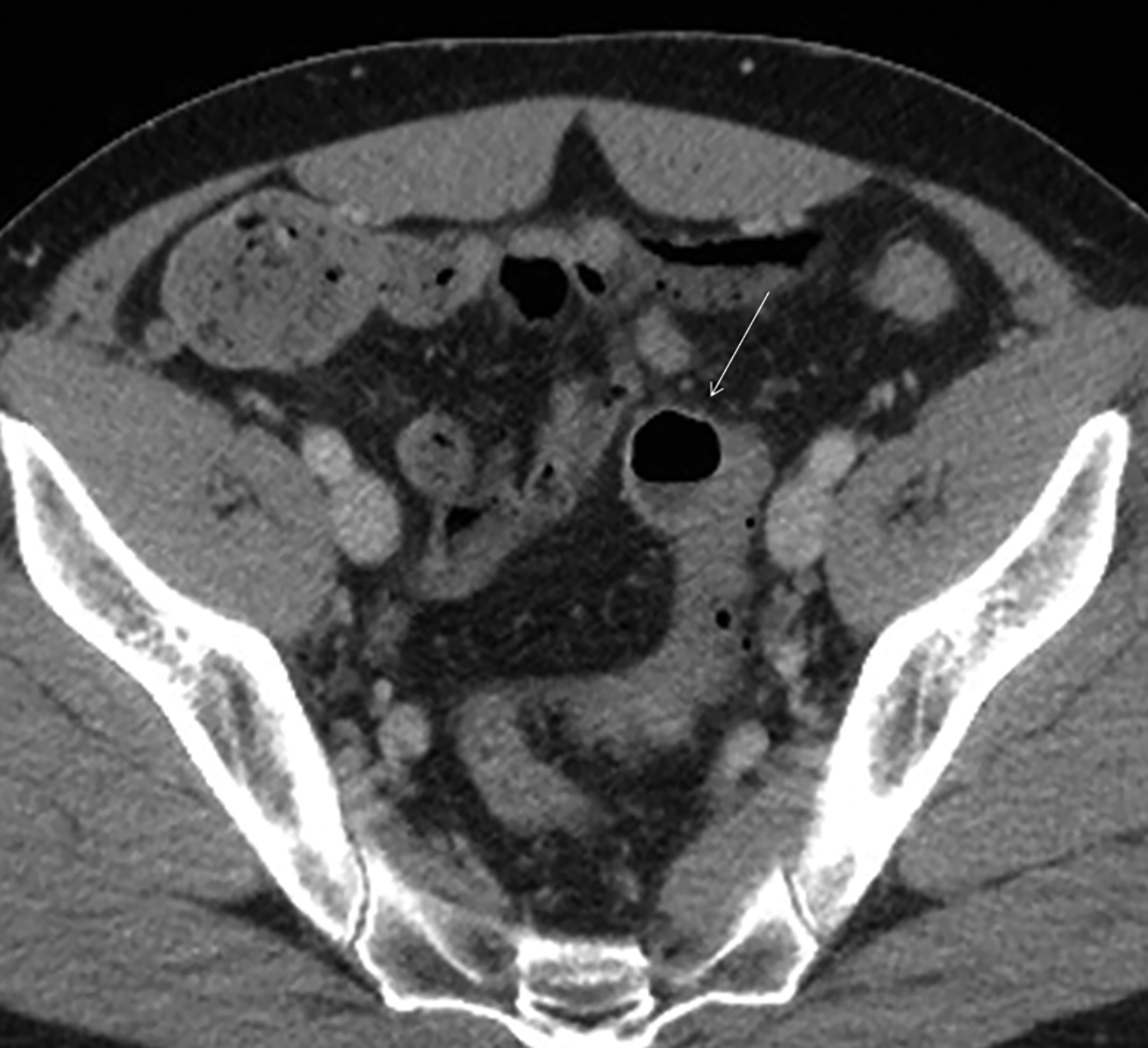
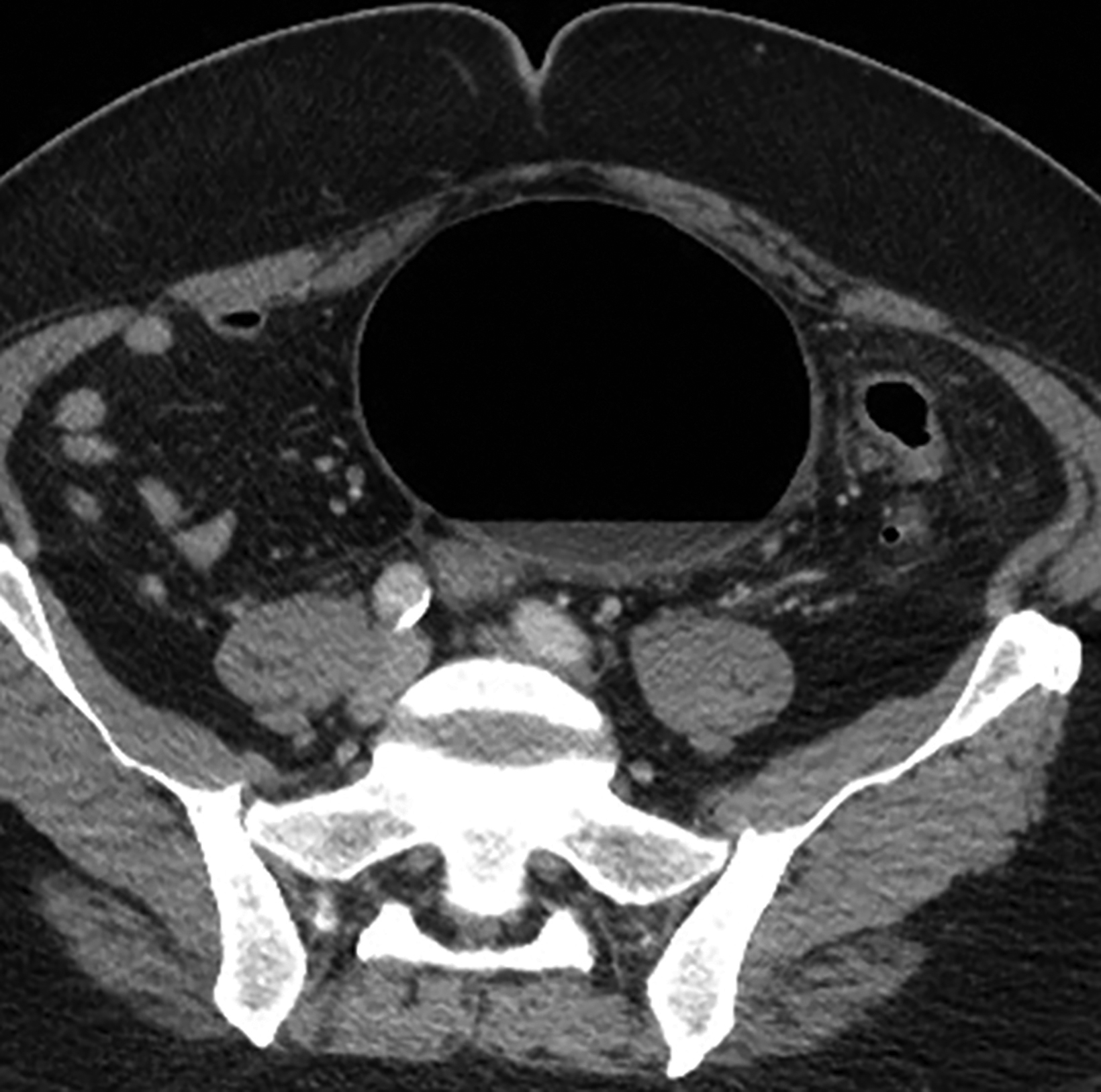
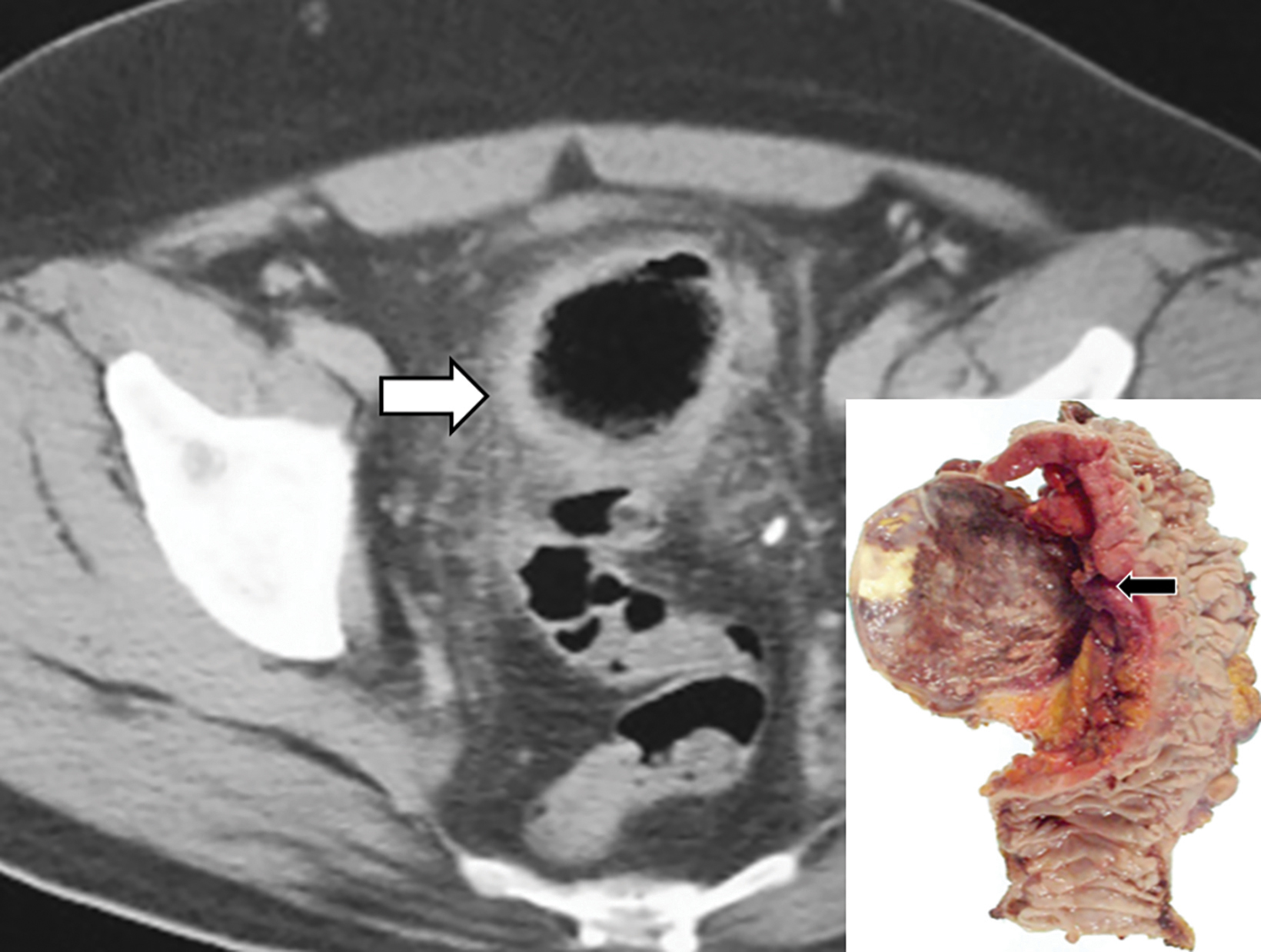
The initial diagnosis of GSD is often made on computed tomography (CT) of patients with a history of diverticular disease and present with recurrent symptoms. GSD appears as a gas-filled mass with thin walls of about 1 mm, or as a thick-walled cavity containing gas and fluid in some other patients (Figures 2-5). The connection with the colonic lumen may not visible unless rectal contrast is administered.
Therapeutic Management
Segmental resection of the sigmoid with the attached GSD is usually performed by open laparotomy or laparoscopic surgery in most patients. The pathological specimens show that the GSD protrudes from the anti-mesenteric aspect of the sigmoid, and most have inflamed and thickened walls associated with adjacent chronic diverticulitis. Acute diverticulitis is usually treated conserva- tively rather than by prophylactic sigmoidectomy.
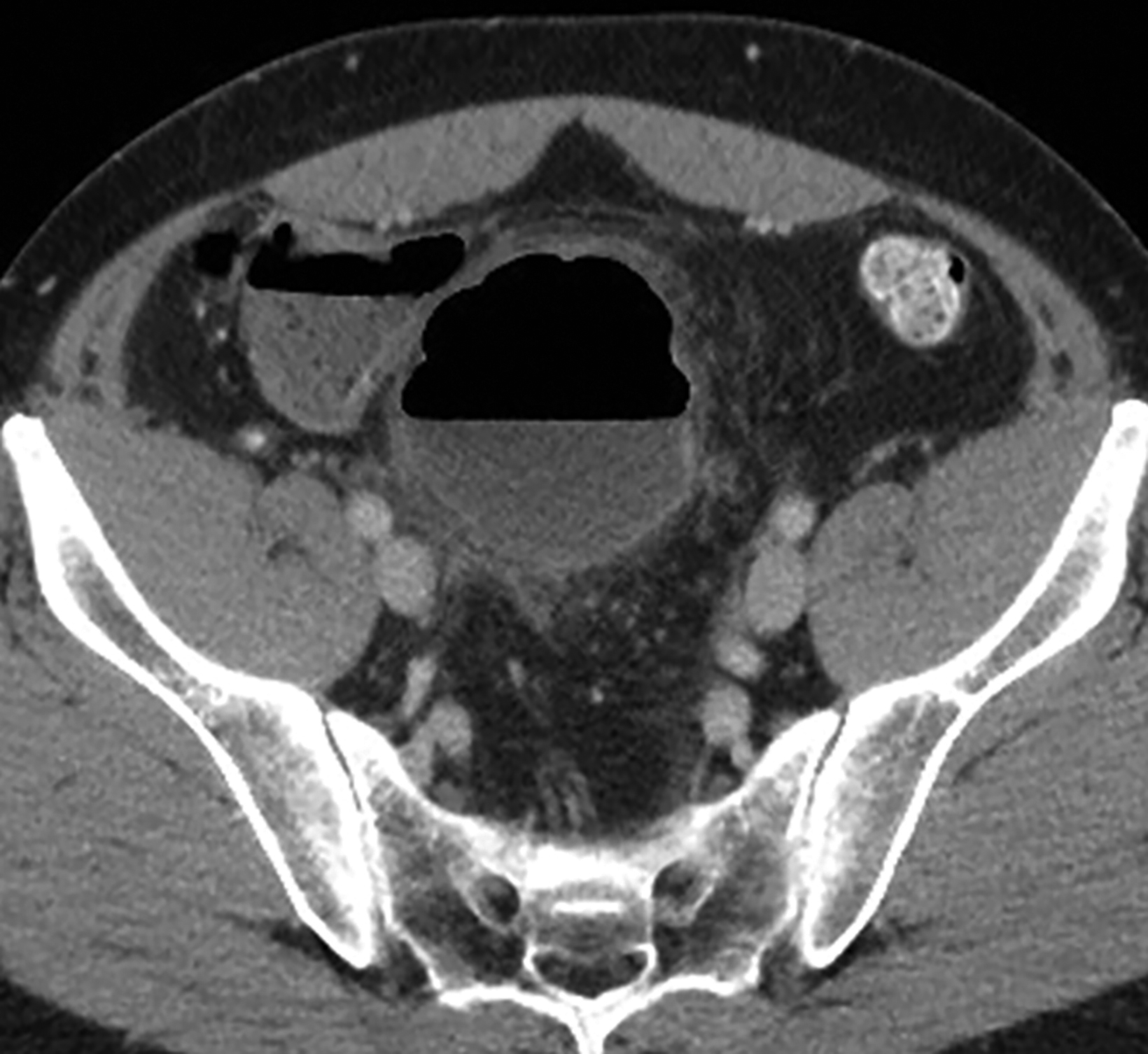
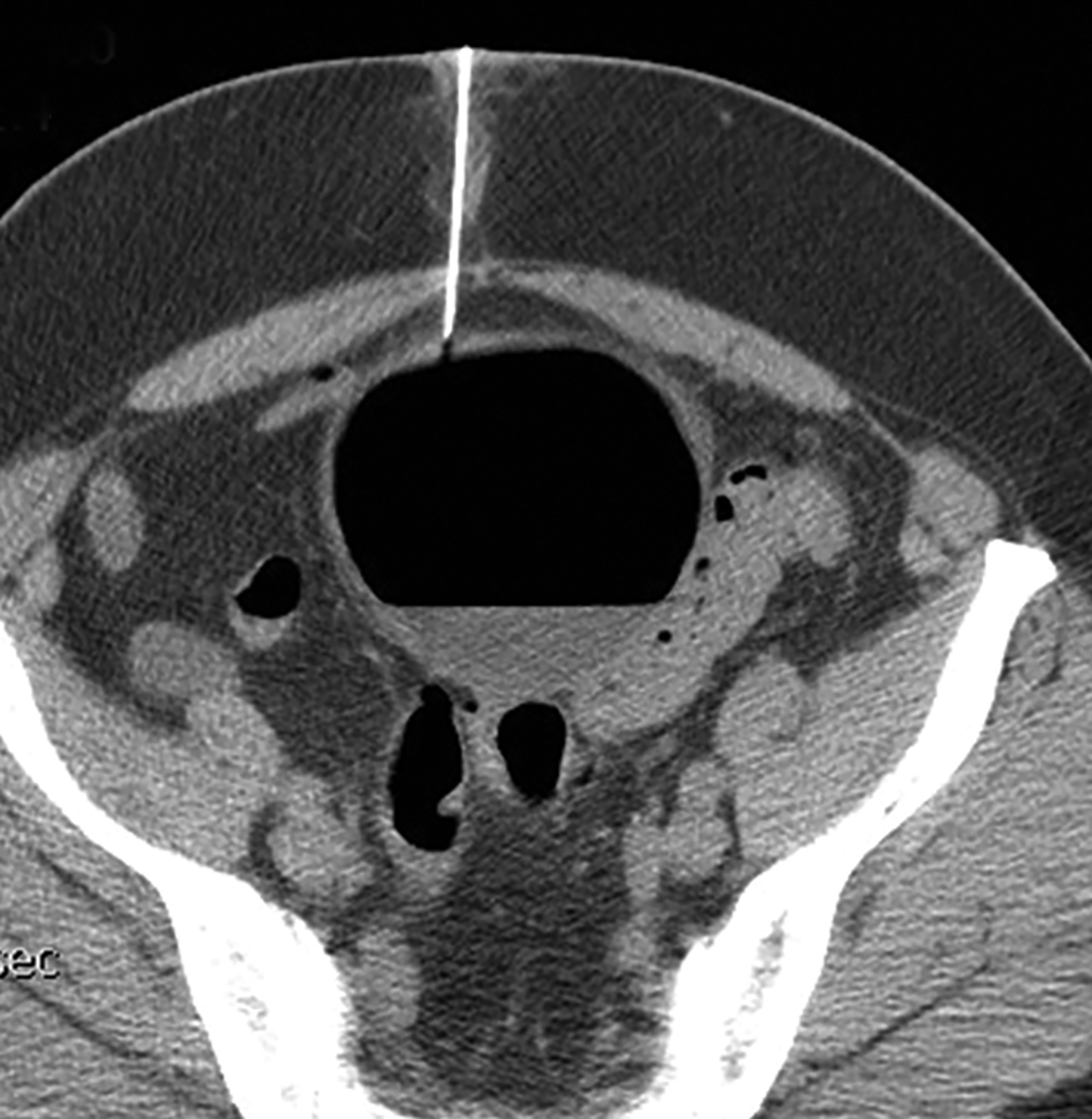
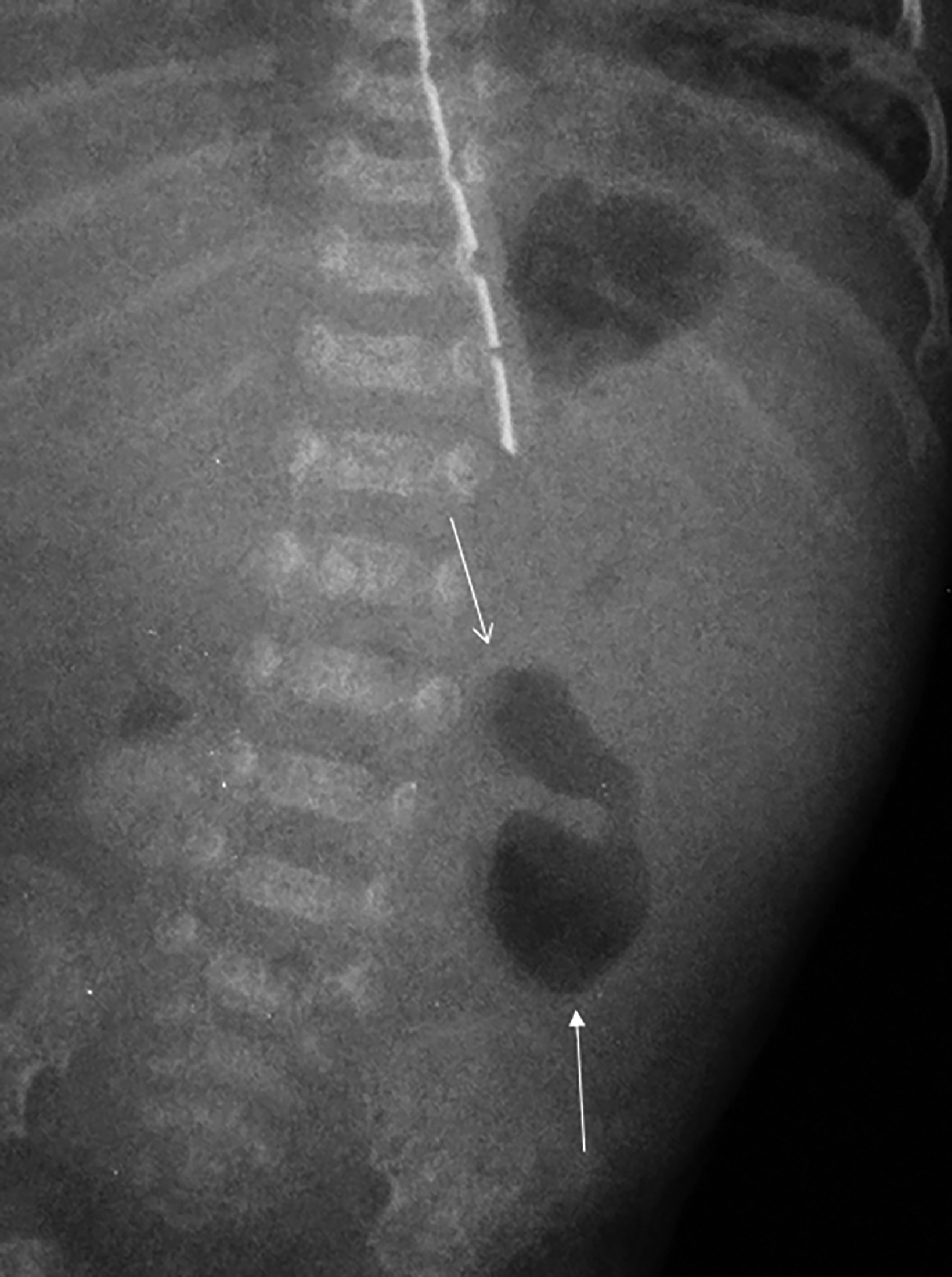
Interventional radiologists treat some patients with GSD via percutaneous drainage, typically under CT guidance. During this procedure, a needle is inserted to suction any fluid content, and then replaced by a pigtail catheter for external bag drainage (Figures 4, 5). Follow-up CT usually shows gradual collapse of the lesion over 4-6 weeks. No recurrence of GSD was detected during 3 years of observation in four cases treated by this technique at our institution.
Radiologic-Pathologic Correlation
Based on the appearance of GSD on imaging studies and pathologic specimens, two patterns can be identified.
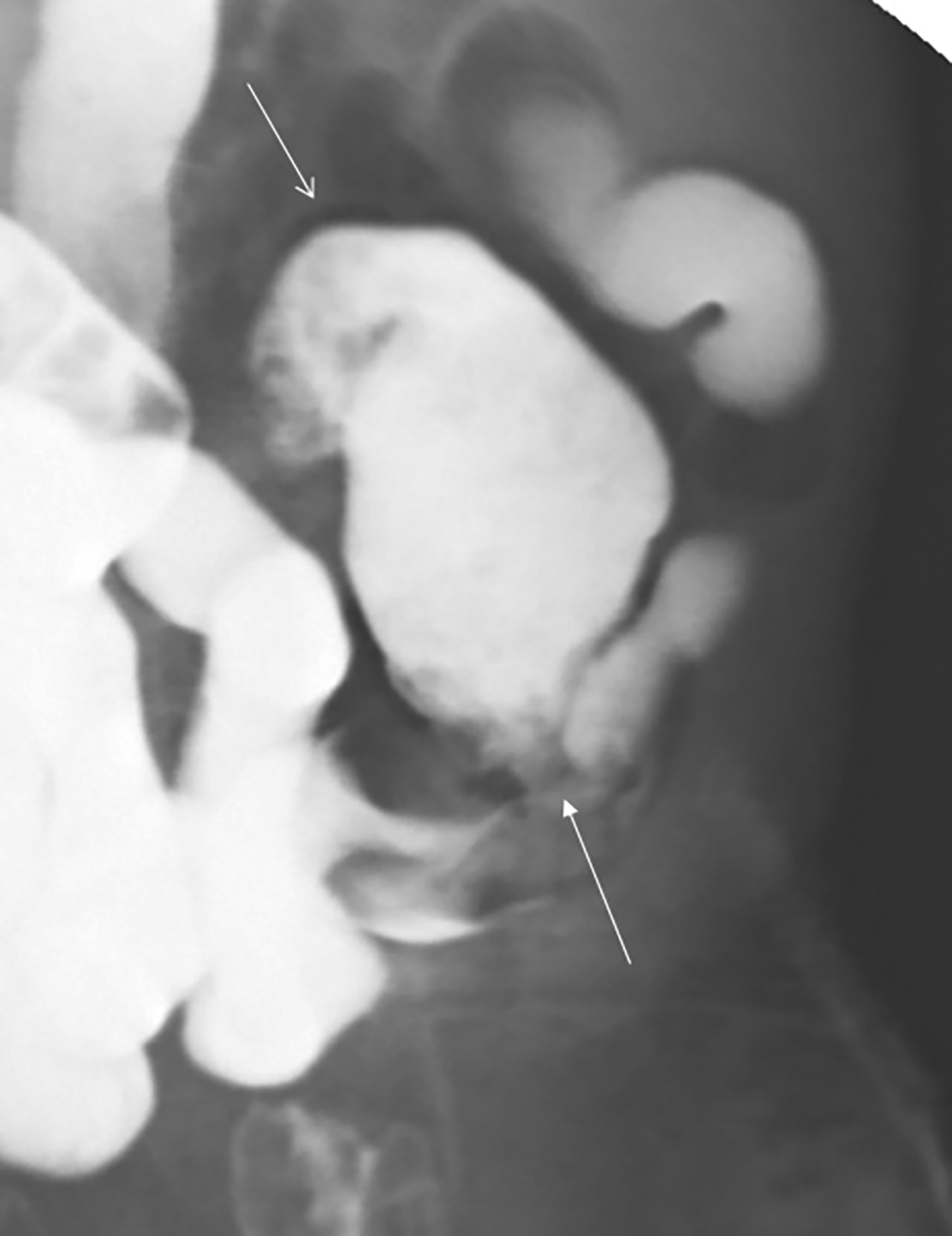
Type 1 includes cases whereby the GSD has thin walls (about 1 mm) on CT, protrudes into the pericolic region beneath the serosal layer, and harbors no inflammatory changes within it or the surrounding tissue. This type results from progressive distention of a pulsion diverticulum, where its narrowed neck serves as a unidirectional valve, thus permitting passage of colonic gas and its entrapment within the diverticular lumen (Figure 6). Such a pulsion diverticulum can result from a focal outpouching of the wall caused by elevated intraluminal pressure. Although the walls of GSD are originally composed of intact colonic mucosa and submucosal layers, they may be later replaced by fibrotic tissue.
Type 2 consists of GSD cases with irregular and thickened walls, associated with inflammation both in the diverticulum and the attached segment of sigmoid colon (Figure 6). The underlying pathogenesis is a perforated diverticulitis, causing a pericolic abscess cavity with fistulous connection to the bowel lumen. The development of anaerobic bacterial infection within the abscess likely contributes to the gas- distended appearance of GSD.
Clinical Implications
Despite the high incidence of diverticular disease among the aging population of the West, GSD are a rather rare complication.2-5 This pathologic process was first described in the French medical literature in 1946 by Bonvin and Bonte.7 Another description ap- peared 6 years later in an English surgical article.8 A comprehensive review of the world literature was conducted in 2015 by Nigri, et al.6 Their investigation revealed a total of 166 cases of GSD in 138 publications, with the majority being isolated case reports or small case series. Our experience from a series of 12 cases will bring that total closer to 180. However, it is likely that the GSD will be recog- nized more frequently in the future owing to the increasing prevalence of diverticular disorders of the colon affecting adult patients.1-3
A giant diverticulum may very seldom occur in other segments of the large bowel, such as the transverse colon.9-11 Furthermore, some of these giant diverticula have been previously called a “solitary air cyst” or “sigmoid pneumatocele.”8, 12, 13
Three distinct categories of GSD were classified by McNull, et al:14
- Type 1 is formed by a diverticulum with thin walls and a narrow neck that allows its inflation with the colonic gas (Figures 1, 2, 5, 6A).
- Type 2 GSD represents a thick-walled abscess cavity resulting from perforated sigmoid diverticulitis (Figures 3, 4, 6B).
- Type 3 is the manifestation of a congenital anomaly, a communicating duplication cyst with all the colonic wall layers. This particular lesion usually manifests in early childhood15 (Figure 7). Patients with type 1 GSD may be asymptomatic or have only vague abdominal discomfort. Therefore, the lesion may be detected incidentally during radiological examinations for unrelated abdominal conditions. In some instances, a fluctuant mass may be palpable and enlarge with straining or defecation.2 This lesion may also deflate intermittently and be considered a phantom tumor.16,17 In contrast, type 2 GSD is usually associated with clinical symptoms of diverticulitis; thus, these patients will present with abdominal pain and tenderness, fever, and leukocytosis.
The preferred method of treating a GSD is segmental resection with primary colorectal anastomosis. This may be performed through either open laparotomy or laparoscopy.4, 6, 18 A simple diverticulectomy is rarely done but may be considered in the absence of concomitant colonic diverticular disease.6
Patients with type 1 GSD can be treated by CT-guided interventional drain (Figures 5, 6). This method has become a viable alternative to surgery in recent years, and it may be considered for uncomplicated GSD or in patients who are not good candidates for surgery.19,20
Some authors have recommended that all patients with GSD undergo prompt treatment to prevent complications such as torsion, volvulus, rupture into the peritoneal space, and malignant transformation.5, 21
Differential Diagnosis
The imaging features of GSD are unique, but other pathological processes should also be considered in the differential diagnosis. These include a gas-containing Meckel diverticulum, intestinal duplication cyst, infected pancreatic pseudocyst, necrotic tumor, and tubo-ovarian or intra-abdominal abscess.2, 22
Conclusion
Giant sigmoid diverticulum is a rare complication of diverticulosis of the colon in adults beyond their fourth decade of life and is a rarely seen congenital finding in children. The lesion may be asymptomatic and incidental on imaging studies. However, most patients present with symptoms of inflammation. Treatment may benefit from a multidisciplinary surgical and interventional radiology approach.
We may expect GSD to be encountered more frequently in the West, owing to an aging population. Therefore, radiologists, surgeons, and gastroenterologists should be familiar with the imaging features of GSD and its management.
References
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009; 22: 141-146.
- Thakrar KH, Gore RM, Yaghmai Y, Balthazar EJ. Diverticular disease of the colon. In: Gore RM, Levin MS, editors. Textbook of Gastrointestinal Radiology. Philadelphia, PA: Elsevier Saunders Company, 2015. p. 934-954.
- Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999; 3: 543-548.
- Steenvoorde P, Vogelaar FJ, Oskam J etal. Giant colonic diverticula. Review of diagnostic and therapeutic options. Digest Surg. 2004; 21: 1-6.
- Zeina AR, Nachtigal A, Matter I et al. Giant colon diverticulum: clinical and imaging findings in 17 patients with emphasis on CT criteria. Clinical Imaging. 2013; 37: 704-710.
- Nigri G, Petrucciani N, Giannini G et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015; 21: 360-368.
- Bonvin MMP, Bonte G. Diverticules geante du sigmoide. Arch Mal Dig. 1946; 35: 353-355.
- Hughes WL, Greene RC. Solitary air cyst of peritoneal cavity. Arch Surg. 1953: 67: 931-936.
- Sagar S. Giant solitary diverticulum of the transverse colon with diverticulosis. Br J Clin Pract. 1973; 27: 145-146.
- Olakowski M, Jablonska B, Lekstan A et al. Gastrointestinal image: a true giant transverse colon diverticulum. J Gastrointest Surg. 2011; 15: 1289-1291.
- Yoon SE, Lee YH, Yoon KH et al. Complicated giant diverticulum of the transverse colon accompanied by right inguinal hernia of the greater omentum. Br J Radiol .2007; 80: 201-204.
- Barratt JG. Giant cyst of the sigmoid colon. Australas Radiol. 1971; 15: 38-40.
- Ritchie AJ, Carson JG, Humphreys WG. Encysted pneumatocele: a complication of diverticular disease. Br J Surg. 1991;78:683.
- McNutt R, Schmitt D, Schulte W. Giant colonic diverticula-three distinct entities. Report of a case. Dis Colon Rectum. 1988; 31:624-628.
- Barlow B, Goodhue WW, Schullinger JN. Giant congenital diverticulum of the sigmoid colon. J Pediatr Surg. 1975: 10:549-550.
- Abdelrazeq AS, Owais AE, Aldoori MI, Botterill ID. A giant colonic diverticulum presenting as a “phantom mass”: a case report. J Med Case Rep. 2009; 3:29.
- Scott DA, Glancy S. Spontaneous resolution of a giant sigmoid diverticulum. Clin Radiol. 2008; 63:833-835.
- Collin JE, Atwal GSS, Dunn WK, Acheson AG. Laparoscopic-assisted resection of a giant colonic diverticulum: a case report. J Med Case Rep. 2009; 3:1-6. e7075.
- Singh AK, Raman S, Brooks CPA et al. Giant colonic diverticulum. Percutaneous computed tomography-guided treatment. J Comput Assist Tomogr. 2008; 32:204-206.
- De Filippo M, Puglisi S, D’Amuri F et al. CT-guided percutaneous drainage of abdominopelvic collections: a pictorial essay. Radiol Med. 2021; 126: 1561-1570.
- Abou-Nukta F, Bakhos C, Ikekpeazu N, Ciardiello K. Ruptured giant colonic diverticulum. Arch Surg. 2005; 71: 1073-1074.
- Telepak RJ, Huggins TJ, Bova JG. Tuboovarian abscess simulating giant colonic diverticulum. Gastrointest Radiol .1984; 9:369-371.
Citation
GG G. Giant Sigmoid Diverticulum: Imaging Features and Management. Appl Radiol. 2023;(1):17-21.
February 1, 2023