GE to Acquire Maker of CT Navigation System, IMACTIS
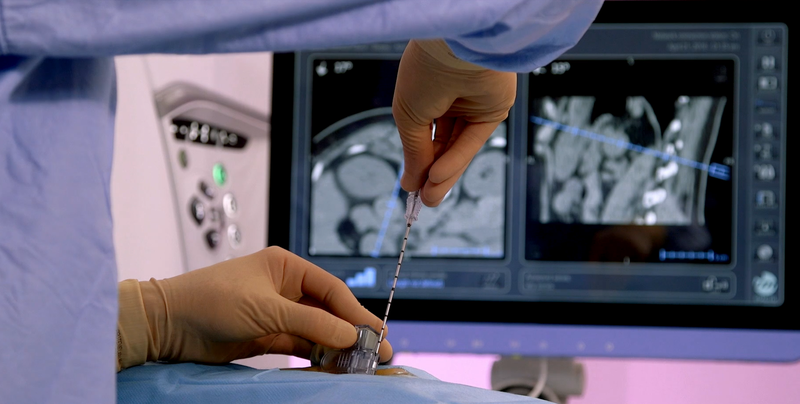 GE HealthCare has entered into an agreement to acquire IMACTIS, a France-based company that created CT-Navigation, an ergonomic universal solution that provides stereotactic needle guidance, enabling intuitive pre-planning and continuous control throughout a wide range of procedures, from diagnosis to treatment.
GE HealthCare has entered into an agreement to acquire IMACTIS, a France-based company that created CT-Navigation, an ergonomic universal solution that provides stereotactic needle guidance, enabling intuitive pre-planning and continuous control throughout a wide range of procedures, from diagnosis to treatment.
“We’re thrilled to take this step in strengthening our interventional guidance offering for patients and customers,” said Jan Makela, President and CEO of Imaging, GE HealthCare. “The IMACTIS CT-Navigation system is designed to improve workflow for interventional radiologists and hospitals by increasing procedural accuracy, while helping to reduce procedure time and radiation dose for patients and physicians. It is an innovative navigation solution for image-guided percutaneous procedures that aims for better patient outcomes, by reducing variability for simple and complex procedures and improving reproducibility.”
“The timing to join GE HealthCare is perfect,” said Pierre Olivier, President and CEO of IMACTIS. “Our solution, which is already deployed in leading healthcare systems in Europe and the U.S., is ready to scale and become a standard of care, thanks to GE HealthCare’s market access. Our product development team also sees significant opportunities to integrate our hardware and software into GE HealthCare solutions and make the workflow of the interventional radiologists and oncologists even simpler and faster.”
Interventional CT capabilities are considered a top purchase driver in the performance and premium CT segments , making it a high opportunity growth driver in the 2022-2026 period. The global Interventional Radiology market includes CT interventional guidance, and the IMACTIS acquisition provides GE HealthCare with access to this growing opportunity. While this innovation currently focuses on CT, GE HealthCare plans to expand the technology to its image guided therapy (IGT) business to drive further growth. Additionally, interventional guidance is increasing due to the growing worldwide need across an array of care areas including oncology, cardiology, urology, nephrology, and gastroenterology.
The IMACTIS CT-Navigation, which includes an integrated workstation, guidance software, and disposable procedure kit, is currently approved under the European Union’s Medical Devices Regulation (MDR) and has FDA clearance for use within the US. GE HealthCare is a leader in CT with a large install base and global scale, which provides significant opportunities for the IMACTIS CT-Navigation system at existing client sites.
The consummation of the transaction is subject to customary closing conditions, including review by the relevant governmental authorities in France. Financial details of the transaction have not been disclosed publicly. GE HealthCare intends to fund this transaction with cash on hand.