First Patient Dosed in Combined Targeted Radiation Therapy and DNA Inhibitor Clinical Trial
Images
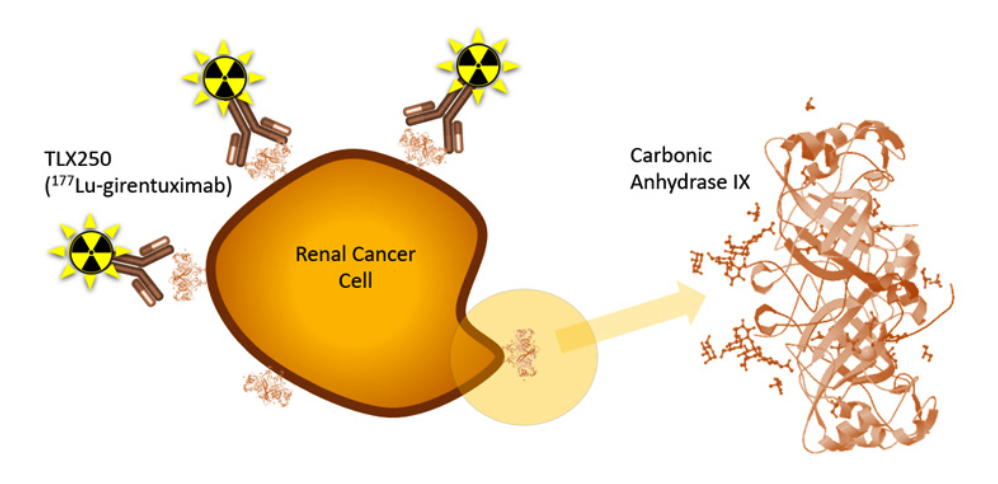
Telix announced the first patient has been dosed in a Phase I study of the Company’s investigational targeted radiation therapy, TLX250, in combination with a Merck KGaA, Darmstadt, Germany (Merck) DNA-dependent protein kinase (DNA PK) inhibitor candidate, peposertib (M3814). The STARSTRUCK Phase Ib, open label, single-arm, multicenter dose escalation and dose expansion study will evaluate the safety profile, dosing and activity of TLX250 (177Lu-DOTA-girentuximab) in combination with the DNA damage response inhibitor (DDRi) peposertib, which is an inhibitor of DNA-PK. The target population is patients with carbonic-anhydrase IX (CAIX) expressing solid tumors that are relapsed or refractory to standard therapies. Up to 80 patients will be assessed in this study in Australian sites.
The clinical hypothesis is that the combination of TLX250 and a DNA-PK inhibitor provides an enhancement in potency through their synergistic action on cancer cells. Targeted radiation effectively induces DNA damage in targeted cancer cells and the DNA-PK inhibitor may act to prevent the cell from repairing this damage, resulting in higher potency at lower doses. This hypothesis was confirmed in preclinical studies, conducted under a strategic research collaboration between Merck and Telix announced in 2019,[1] and provided evidence that the combined effect of Merck's investigational DNA-PK inhibitor peposertib with Telix's targeted radiation candidate has potential to significantly improve efficacy and reduce the required radiation dose for tumour reduction and remission, compared to targeted radiation alone.
Principal Investigator for the STARSTRUCK study, Professor Nat Lenzo, GenesisCare Group Clinical Director Theranostics said, "The treatment of advanced cancer can be extremely challenging. We are pleased to support the commencement of this study with Telix to help determine if there is a potential future clinical benefit regarding the combination of molecular targeted radiation and DNA-PK inhibitors."
Telix Chief Medical Officer, Dr. Colin Hayward stated, "Preclinical data has shown excellent combination response, which has potential to translate to additional response or more tolerable treatment regimens in patients. We would like to thank Prof Lenzo and his clinical team at GenesisCare Murdoch, as well as the patients who will contribute to this important study."
Merck and Telix are continuing the initial scientific collaboration to further explore and optimize the combination of TLX250 or other next-generation targeted radiation therapy candidates with Merck's DDRi compounds in preclinical studies.