Fibrolamellar Hepatocellular Carcinoma
Images
Case Summary
A teenager with no significant medical history presented to the emergency department with a 24-hour history of worsening right upper quadrant (RUQ) abdominal pain radiating to their back, associated with vomiting. On examination, the patient was mildly hypertensive (blood pressure = 137/76) and tachypneic (respiratory rate = 20). Vital signs were otherwise unremarkable.
Laboratory values were within normal limits except for mild leukocytosis (13.4×109/L). The patient was admitted for further evaluation upon detection of a palpable epigastric mass. Initial imaging workup suggested a probable benign liver lesion, and the patient was discharged pending further outpatient workup and management. However, the patient returned to the emergency department several days later with worsening abdominal pain.
Imaging Findings
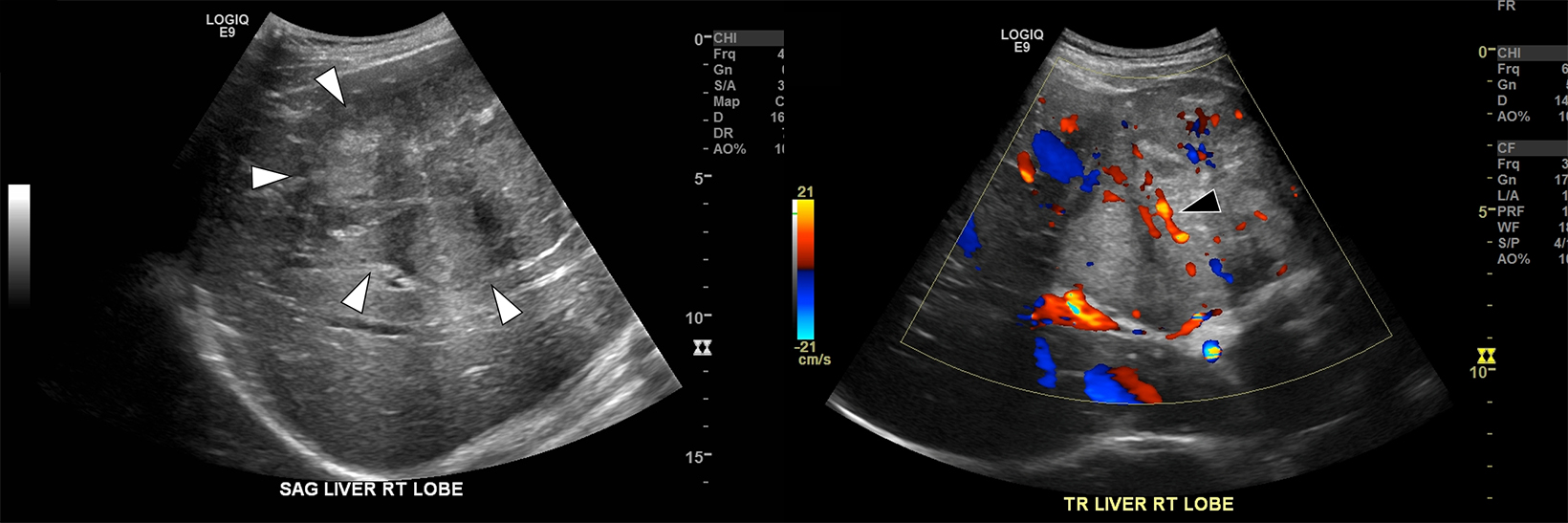
Ultrasound (US) examination demonstrated a 10 cm mass within the central portion of the liver (Figure 1), mildly hyperechoic to background liver, with well-defined margins and internal vascular flow. A differential diagnosis of focal nodular hyperplasia (FNH) versus adenoma was proposed.
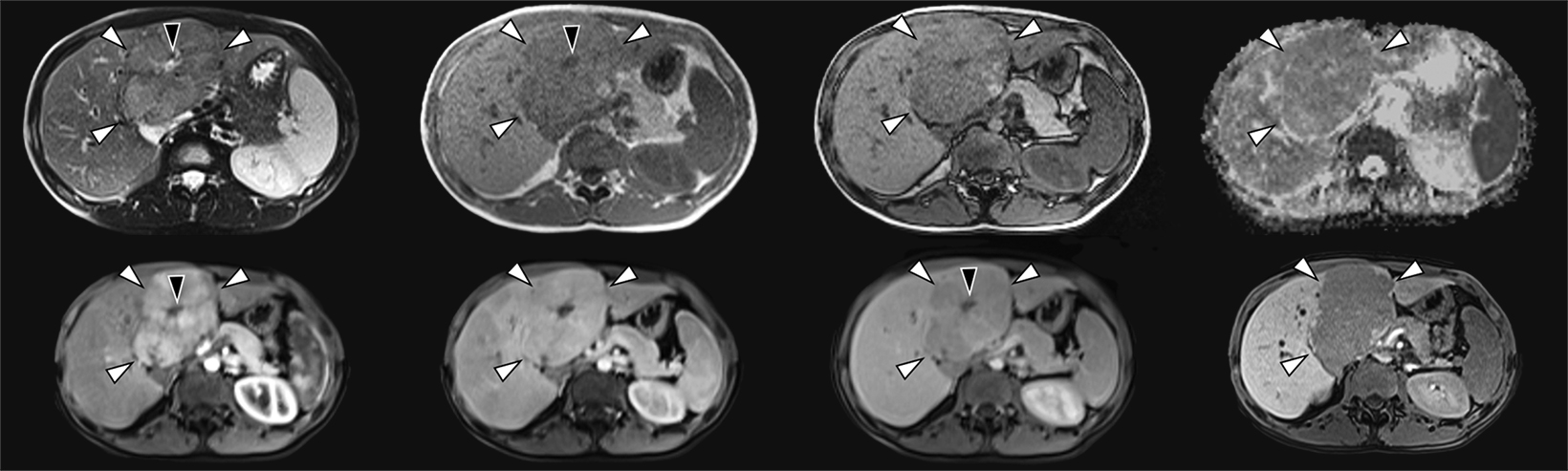
Contrast-enhanced magnetic resonance imaging (MRI) demonstrated the mass centered in the left medial segment with mass effect at the porta hepatis and biliary tree (Figure 2). The mass demonstrated mildly hyperintense signal on T2 imaging and mildly hypointense signal on T1 imaging, with a T2 hyperintense central scar. On contrast-enhanced images the mass showed avid arterial hyperenhancement and washout on the equilibrium phase. The central scar remained nonenhancing throughout. No contrast was retained on the lesion on the 20-minute hepatobiliary phase.
Diagnosis
Fibrolamellar variant of hepatocellular carcinoma (HCC). Differential diagnoses: FNH, hepatic adenoma, metastases.
Discussion
Fibrolamellar HCC is a rare malignancy of young adults, with unimodal distribution between the ages of 24.8 ± 13 years and without predilection based on sex.1 Histopathologically, fibrolamellar HCC is characterized by laminated fibrous layers between malignant cells.2 Unlike other forms of HCC, the fibrolamellar variant occurs independently of chronic liver disease (cirrhosis, alcohol use, and hepatitis B and C infection), and background features of hepatic fibrosis/cirrhosis should be absent at pathology.3 Clinical presentation is often nonspecific; symptoms include abdominal pain, fatigue, and weight loss.4 These tumors are often large at presentation, resulting in hepatomegaly or a palpable abdominal mass on physical examination, as in our case.1 Less commonly, patients may exhibit gynecomastia due to elevated estrone levels.1
At imaging, fibrolamellar HCC typically appears as a large, solitary tumor with central scar and lobulated margins, occasionally with centrally located calcifications.5 At MRI, lesions are typically iso- to hypointense relative to background liver at T1 imaging and iso- to hyperintense on T2 imaging.6 At contrast-enhanced computed tomography (CT) or MRI, lesions typically demonstrate arterial phase hyperenhancement, with iso- (“fading”) or hypoenhancement (“wash-out”) to background liver on more delayed images.6
When present, the central scar typically shows low signal intensity on all MRI sequences, distinguishing it from FNH, which shows high signal intensity on T2 images.6 However, the central scar may occasionally demonstrate high T2 signal intensity, as in our case, mimicking FNH.7 In both cases, the central scar may demonstrate delayed progressive enhancement owing to its fibrotic nature.2 While many imaging features overlap those of FNH (relative isointensity to background liver, “fading” on delayed-phase contrast-enhanced images, and central scar), fibrolamellar HCC is characteristically hypointense to background liver at hepatobiliary phase imaging, making hepatocyte-specific gadolinium agents useful in distinguishing these two etiologies.8
Additional differential diagnoses may include hepatic adenoma, conventional HCC, metastases, and other pediatric liver tumors. Distinguishing fibrolamellar HCC from hepatic adenoma may be challenging at imaging alone, given the widely variable appearance of hepatic adenomas and overlapping patient demographics. While both inflammatory and HNF-1α-mutated adenomas also occur in younger patients, there is a strong female predilection (associated with oral contraceptives), and the mean age at presentation is slightly older, often in the fourth decade.9
Inflammatory adenomas may be characterized by the “atoll” sign (concentric T2 signal hyperintensity) and persistent hyperenhancement on delayed phase imaging, whereas HNF-1α-mutated adenomas will typically show signal drop on opposed-phase T1 imaging due to intralesional lipid.9 However, imaging features, particularly for the β-catenin-mutated subtype, may be indistinguishable from fibrolamellar HCC, including delayed phase “wash-out” and central scar.9
Clinical features will be most helpful in the differentiation of the fibrolamellar variant of HCC from conventional HCC, with the latter occurring in older patients with underlying chronic liver disease.1 Hepatic metastases typically will be multiple, particularly if achieving the size demonstrated in our case, and in many cases the patient will already have an established diagnosis of known primary malignancy.8 Other pediatric liver tumors such as hepatoblastoma and mesenchymal hamartoma are lesions of early childhood, with hepatoblastoma presenting as a solid mass, usually in children under 4 years, and mesenchymal hamartoma presenting as a mixed solid/cystic mass, usually in children under 2 years.10
In our case, fibrolamellar HCC was confirmed by image-guided core needle biopsy. The patient was subsequently treated for cure with liver transplant.
Conclusion
Fibrolamellar HCC is a rare malignancy that occurs in young adults without underlying liver disease. Clinical presentation is typically nonspecific but may include a palpable abdominal mass in many cases. Appropriate imaging, particularly hepatobiliary phase MRI, may help to narrow the differential diagnosis, as in our case.
References
Citation
N S, AD C. Fibrolamellar Hepatocellular Carcinoma. Appl Radiol. 2023;(2):48A-48C.
March 7, 2023