FDA Clears Viz.ai Algorithm for Quantifying Intracerebral Hemorrhage
Images
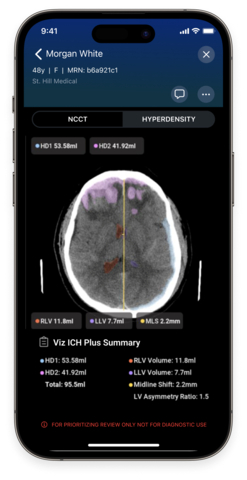
Viz.ai has received US FDA 510(k) clearance for its algorithm, Viz ICH Plus, intended to automate the process of identifying, labeling, and quantifying the volume of segmentable brain structures on non-contrast computed tomography (NCCT) images. The Viz ICH Plus software is indicated for analyzing intracranial hyperdensities, lateral ventricles and midline shift, providing volume measurements of brain bleeds for timely and informed treatment decisions.
"The ability and mobility to obtain accurate and quantifiable measurements of intracerebral hemorrhages through Viz ICH Plus significantly enhances our decision-making process,” said Peter Kan, MD, MPH, FRCSC, FAANS, professor and chair of the Department of Neurosurgery at the University of Texas Medical Branch. “This technology, which marries precision with AI, is poised to transform how we approach intracerebral hemorrhage cases."
Intracerebral hemorrhage accounts for up to 15% of all strokes and has high morbidity and mortality rates, demanding swift response. Accurate volume measurements of brain bleeds are crucial for assessing the severity of cases, monitoring progression, and planning treatment strategies. Radiologists, neurologists and neurosurgeons can incorporate Viz ICH Plus seamlessly into their workflows and automate the manual process of measurement of brain bleeds. The Viz ICH Plus software is available on the AI-powered Viz.ai One™ solution, an enterprise platform that is clinically validated, saves time, improves patient outcomes and increases access to life-saving treatments across more than 1,500 hospitals in the United States.
"At Viz.ai, our mission is rooted in advancing healthcare through innovation. Viz ICH Plus exemplifies our dedication to enhancing patient care by leveraging technology," stated Jayme Strauss, chief clinical officer at Viz.ai. "We are excited to introduce a product that bridges the gap between AI capabilities and improved patient outcomes."