FDA clears GE Healthcare’s Critical Care Suite mobile DR AI algorithm
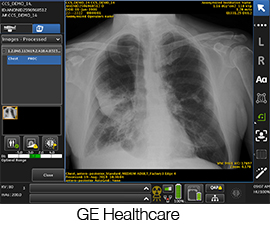 The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to GE Healthcare’s Critical Care Suite, artificial intelligence (AI) algorithms that are embedded on the company’s mobile X-ray devices. GE Healthcare stated that the AI algorithms are designed to help reduce the turnaround time for radiologists to review cases of suspected pneumothorax.
The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to GE Healthcare’s Critical Care Suite, artificial intelligence (AI) algorithms that are embedded on the company’s mobile X-ray devices. GE Healthcare stated that the AI algorithms are designed to help reduce the turnaround time for radiologists to review cases of suspected pneumothorax.
When a pneumothorax is detected through automatic analysis of chest images, the Critical Care Suite will automatically generate a priority alert and forward the chest radiographs to a designated picture archive and communications (PACS) diagnostic workstation. Quality-focused AI algorithms, developed using GE Healthcare’s Edison platform, simultaneously analyze and flag protocol and field of view errors as well as autorotate the images.
GE Healthcare stated in a press release that the system’s ability to generate AI findings within seconds of image acquisition without any dependency on connectivity or transfer speeds to produce AI results decreases processing speed. Automatic quality checks run on the mobile X-ray device are integrated into a radiologic technologists standard workstation, enabling the technologist to reprocess them or reject them without delay.
