Epica Nets Updated FDA Clearance for Point-of-Care CT System
Images
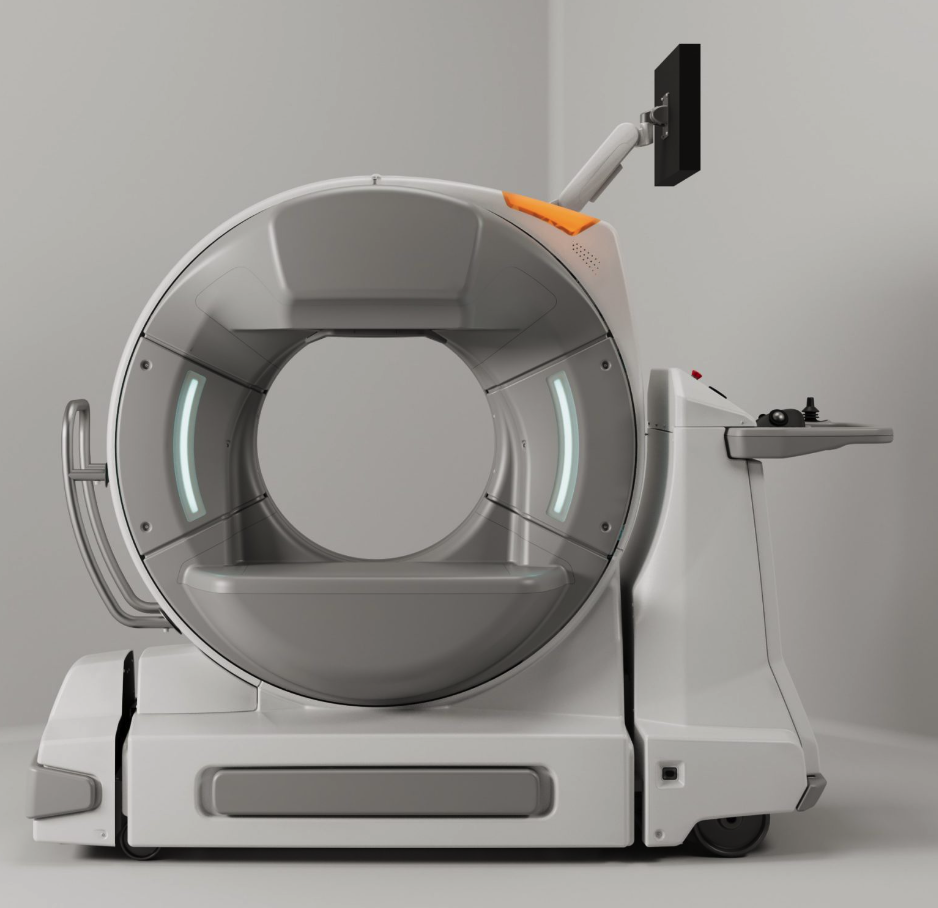
Epica International announced that its See Factor CT3 System has received an updated 510(k) clearance from the US FDA. This achievement underscores Epica International’s commitment to revolutionizing high-resolution CT imaging for human point-of-care applications, particularly in operating rooms. The latest clearance reflects significant enhancements made to the device since its original 2019 release, empowering Epica International to market and distribute the newly upgraded See Factor CT3 across the US.
The See Factor CT3 now boasts mobility and multi-modality capabilities, featuring an innovative indexing gantry designed to deliver high-resolution, orthogonal, and three-dimensional CT images for diverse clinical applications. This imaging technology enables clinicians to conduct CT, fluoroscopy, and digital radiography in any adequately shielded location, eliminating the need to transport patients. With its onboard DICOM viewer, images can be reviewed by the treating physician within minutes, enhancing diagnostic and treatment efficiency.
The See Factor CT3 is a flat-panel cone beam CT imaging system designed for acquiring detailed images of the head, neck, and limbs in adult patients. It also captures images of portions of the thorax, spine, and pelvic bones, focusing on high X-ray attenuation areas such as bony structures. With a 62.5 cm gantry bore and a 30 cm field of view, the device provides 2D and 3D imaging for both intra-operative and clinical use. Additionally, it is capable of acquiring digital radiography and fluoroscopy images.
Key features of the See Factor CT3 system include:
- High-resolution imaging: Exceptional image clarity and detail down to 0.1 mm (100 microns), ensuring precise diagnostics.
- Mobility: Requires no extensive infrastructure to house, cool, or power large, fixed scanning equipment—only adequate lead shielding.
- Multi-modal capabilities: Integrates various imaging modalities into a single, flexible platform.
- User-friendly interface: Intuitive design promotes ease of use for healthcare professionals, reducing training time and boosting efficiency. Onboard viewer allows bedside image review.
- Compact and versatile design: Perfectly suited for a wide range of clinical settings, from large hospitals to small clinics.
Joe Soto, CEO of Epica International, Inc., remarked, “This is another significant achievement by Epica's regulatory team. These additional indications for use further illustrate our technology's expanding capabilities.”