Doppler ultrasound evaluation of renal transplants
Images









Dr. Piyasena is an Assistant Professor, Department of Radiology and Radiological Science, Johns Hopkins University, School of Medicine, Baltimore, MD; and Dr. Hamper is a Professor of Radiology, Urology and Pathology, Director, Division of Ultrasound, Department of Radiology and Radiological Science, Johns Hopkins University, School of Medicine, Baltimore, MD.
Ultrasound (US) is often the modality of choice for primary evaluation of renal transplants in the perioperative period and in follow-up. In this article, a general approach is introduced to assist in performing and interpreting a thorough, yet focused US examination of a renal transplant. The basic post surgical anatomy is presented, as well as the sonographic appearance of several of the complications that can often be detected by US. Early recognition of these complications can be essential for graft and patient survival.
Prolonged graft survival due to improvements in surgical techniques and immunosuppressive drugs has led to an increased number of organ transplantations. Data from the Organ Procurement and Transplant Network shows that 16,520 kidney transplants and 837 combined kidney/pancreas transplants were performed in the United States in 2008 alone. Nevertheless, the number of candidates on the wait list for organs continues to grow. As of August 2010, there were approximately 108,000 candidates on the wait list for kidney transplantation.1
With this increasing number of renal transplants comes the need for fast, reliable diagnosis of the multitude of possible complications that inevitably occur after such complex procedures. Ultrasound is often the initial diagnostic modality as it is noninvasive, relatively inexpensive, does not require intravenous contrast, can be obtained at the bedside, and can often rapidly and accurately depict many of the common complications—most notably, vascular complications. Computed tomography (CT), magnetic resonance imaging (MRI), and angiography are often reserved for confirmation of US findings, when US is inconclusive or for cases where the extent of a finding cannot be fully appreciated by US alone. It should also be noted, however, that some complications from renal transplantation, such as acute or chronic graft rejection, do not create specific sonographic findings. In such cases, although the clinical and laboratory data are often helpful in limiting the differential diagnosis, biopsy may be necessary for a definitive diagnosis.
In order to perform a thorough and accurate US evaluation of a renal transplant, an understanding of the exact post surgical anatomy is imperative. A systematic approach can then be utilized to ensure that the vast majority of the possible complications are detected; particularly those that may require further diagnostic workup or surgical/radiologic intervention. This approach can be subdivided into evaluation of the transplant perinephric space, the renal parenchyma, the urinary collecting system and the renal vasculature.
Anatomy
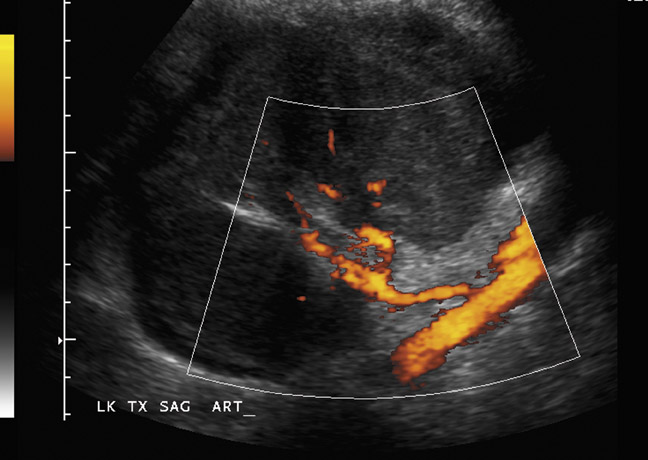
The transplanted kidney is usually placed in an extraperitoneal location in the right or left iliac fossa (Figure 1). The superficial location makes it ideal for US evaluation. The donor left kidney is preferred over the right kidney due to its longer renal vein. The donor organ may be cadaveric (cadaveric renal transplant, CRT), from a living relative (LRT) or a living nonrelated donor (LNRT). Often with cadaveric donors, a Carrel patch (small portion of surrounding aorta) is acquired and anastomosed to the recipient external iliac artery in an end-to-side fashion. Transplants obtained from living donors often involve an end-to-side anastomosis of the donor renal artery to the recipient external iliac artery or an end-to-end anastomosis of the donor renal artery to the recipient internal iliac artery. Variant anatomy with multiple renal arteries can be anastomosed separately to the external iliac artery or with a Carrel patch encompassing all of the origins. Alternatively, the individual arteries can be anastomosed to the largest such that there is essentially a single donor vessel supplying the entire graft. The renal vein is attached in an end-to-side anastomosis to the external iliac vein. Ureteral drainage is restored preferably by means of ureteroneocystostomy. Complex cases may necessitate using older techniques that utilize the recipient’s native ureter, such as a ureteroureterostomy or pyeloureteostomy. There is considerable variation in technique including intraperitoneal transplant placement, anastomosis to internal iliac vasculature and the use of a bowel conduit for urinary drainage. Another procedure sometimes performed for kidneys obtained from donors <5 years of age involves the transplantation of both donor kidneys into a single recipient and using the donor aorta and vena cava for vascular anastomosis (“en bloc” pediatric transplant).2 Knowledge of the exact renal transplant procedure performed is essential for accurate interpretation of both normal and abnormal findings. Particularly important is knowledge of the vascular anatomy, so that all vessels and anastomoses can be evaluated for patency, stenosis or other complications.
Perinephric space
Perinephric fluid collections are a common occurrence, and are found in ≤50% of renal transplants.3 These collections may contain urine, blood, lymph or pus. Although US is sensitive for their detection, the sonographic appearance of these collections can overlap and the diagnosis ultimately may require fluid aspiration. Minimal perinephric fluid representing small hematomas or seromas are an expected postoperative finding. The clinical relevance of a fluid collection depends on its composition, size, location and whether or not it is exerting mass effect on the transplant kidney, ureter or other adjacent structures.
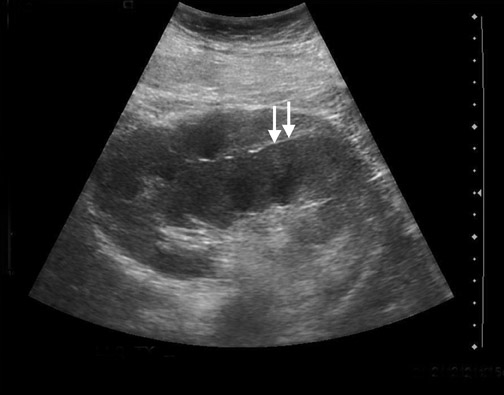
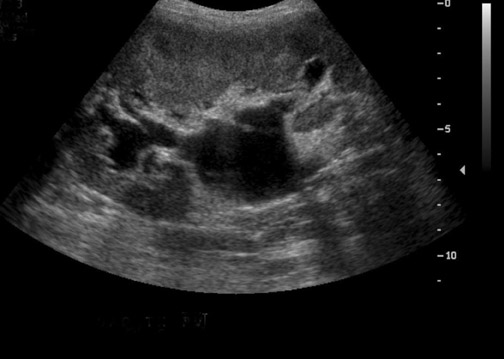
Some generalities can be made based on the time of presentation. Urinomas and hematomas often present in the immediate postoperative period ≤2 weeks after surgery. Lymphoceles are a more delayed complication, occurring 4 to 8 weeks after surgery. Urinomas appear as well-defined anechoic collections without septations, unless infected or mixed with blood. Urinomas can rapidly increase in size. Acute hematomas are echoic and decrease in echogenicity with time, often developing septations. Lymphoceles result from the disruption of the lymphatic channels during the perivascular dissection or disrupted hilar lymphatics. Mass effect from perinephric fluid can result in hydronephrosis; or edema of the leg, abdominal wall, labia or scrotum (Figure 2).4 Perinephric abscesses are uncommon, but can occur in the early postoperative period due to pyelonephritis or bacterial seeding of a urinoma, hematoma or lymphocele. Hematomas can also result as a complication of transplant biopsy (Figure 3).
Renal parenchyma
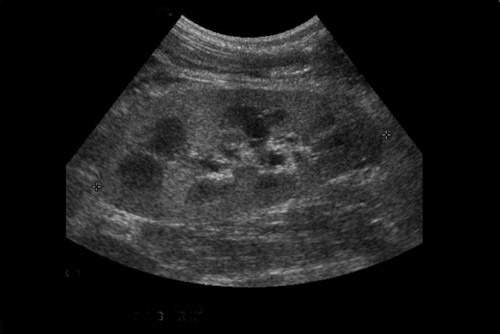
The superficial location of the renal transplant allows for normal corticomedullary differentiation of the renal parenchyma, with the medullary pyramids being relatively hypoechoic to the cortex.
Unfortunately, the sonographic appearance of many diffuse renal parenchymal abnormalities is nonspecific, including acute tubular necrosis (ATN), acute or chronic rejection, and toxicity associated with immunosuppressive calcineurin inhibitors (cyclosporine and tacrolimus). ATN occurs in the immediate post transplant period as a result of ischemia, particularly warm ischemia or prolonged cold ischemia, and is thus more commonly seen in cadaveric transplants. Acute rejection takes several days to develop and peaks at 1 to 3 weeks after transplant.5 Diffuse renal enlargement, cortical thickening, increased or decreased cortical echogenicity, loss of corticomedullary differentiation, prominent pyramids, and thickening of the collecting system can all be seen in the setting of diminished renal function. Ultimately, differentiation between these entities often requires biopsy. Although an elevated resistive index obtained at the arcuate arteries was previously described as an accurate method of detecting acute rejection,6 it has been subsequently shown that increases in the resistive index can also be seen in various other conditions, including ATN, renal vein thrombosis, graft infection, compressive perinephric fluid collections and obstructive hydronephrosis.7 An elevated resistive index (>0.8) is now used as a nonspecific parameter of renal dysfunction.
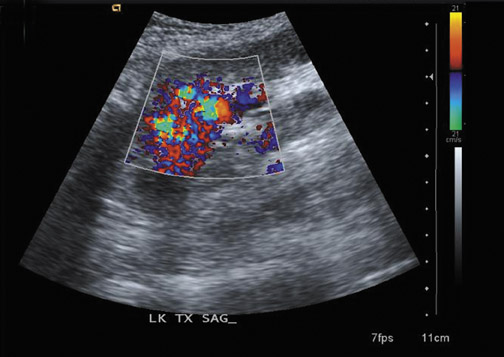
Focal pyelonephritis, infarct or rejection can appear as an area of increased or decreased echogenicity. A segmental infarct can appear as a hypoechoic mass with poorly defined border or a well-defined echogenic wall. Color or power Doppler will show lack of vascularity (Figure 4), although similar findings can be seen in severe pyelonephritis. The presence of an echogenic line with distal reverberation artifact or “dirty shadowing” should raise the suspicion of gas associated with emphysematous pyelonephritis.
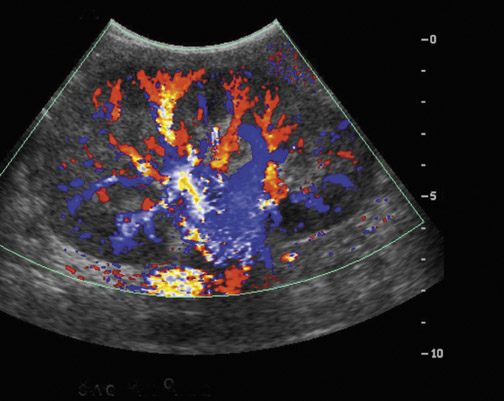
Color and power Doppler are limited in their ability to evaluate perfusion of the renal cortex. However, contrast-enhanced sonography is a technique that shows promise as a possible tool to assess renal perfusion both qualitatively and quantitatively.8 Microbubbles are used as the contrast agent and can pass throughout the capillary bed, improving visualization of renal cortical perfusion. Utilizing a high mechanical index pulse to deliberately burst the bubbles in the field, subsequent imaging can allow vascular kinetic information to be obtained as the kidney shows increasing enhancement over time. Unfortunately, this technique is currently not routinely performed in the United States due to lack of United States Food and Drug Administration approval of US contrast agents for use in radiology.
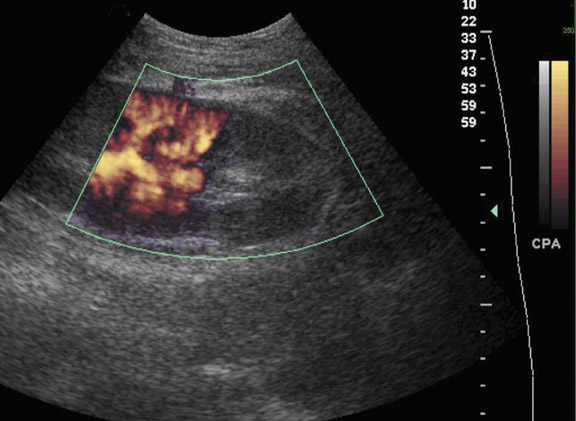
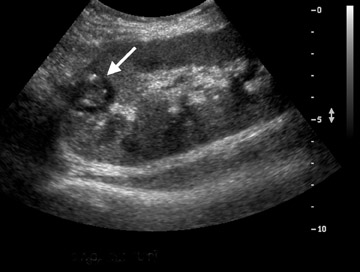
Prolonged immunosuppression increases a transplant patient’s risk of developing cancer, the majority of which are skin cancer and lymphoma.9 The prevalence of renal cell carcinoma may also be increased and 90% of such occurrences are seen in the native kidneys.10 Post transplantation lymphoproliferative disorder (PTLD) complicates approximately 1% of renal transplant patients with a spectrum of disease ranging from mild diffuse polyclonal lymphadenopathy to malignant monoclonal lymphoma.11 Any of the solid organs, hollow viscera, abdominal, retroperitoneal and iliac lymph nodes, retroperitoneal musculature, or peritoneum of the abdomen can be involved in PTLD, with the extranodal disease predominating (Figure 5). Involvement of the transplant kidney appears as single or multiple hypo- or mixed-echogenic masses.
Urinary collecting system
Urologic complications occur in 4% to 8% of patients, including urine leak/urinoma, urinary obstruction, and urinary calculi.12 Urine leaks and urinomas often occur in the early post operative period presenting with pain and swelling at the surgical site and discharge from the wound. Leaks at the uretero-vesicle anastomosis are often due to surgical technique or distal ureteral necrosis, as a result of vascular insufficiency or increased pressure caused by obstruction. Urine leaks at the more proximal ureter, renal pelvis and calices are often caused by ischemia and resultant necrosis. Urinomas appear as nonspecific well-defined anechoic collections without septations.
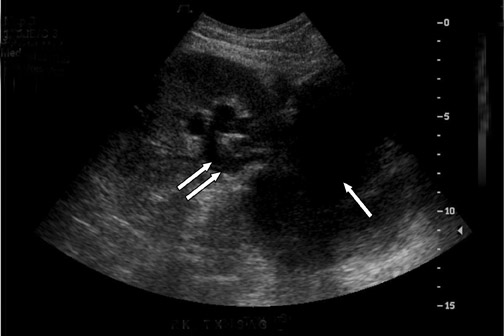
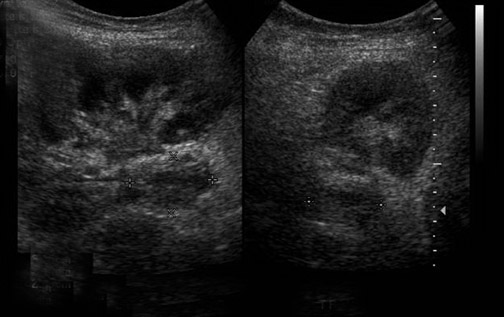
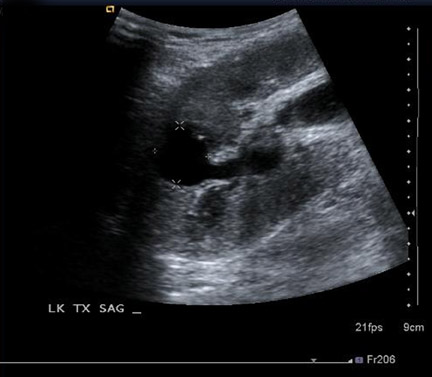
Urinary tract obstruction occurs in approximately 2% of renal transplants, most often during the first 6 postoperative months. More than 90% occur at the distal third of the ureter, most commonly at the ureterovesicle junction.13 Causes of obstruction include edema at the anastomosis; ischemia or rejection leading to fibrosis and stenosis (Figure 6); technical error during the ureteroneocystostomy; and kinking of the ureter. Other less common causes include calculi, papillary necrosis, fungus ball, hematoma and extrinsic compression by perinephric fluid.
The patient with urinary obstruction may not complain of typical renal colic as the transplant kidney is denervated. Additionally, this denervation prevents the transplanted kidney from maintaining any intrinsic tone, often resulting in a persistent appearance of mild dilatation. It should also be noted that edema and fibrosis associated with ATN, or rejection, may prevent the normal dilatation seen with hydronephrosis.14 Thus, the clinical setting and the use of further imaging such as diuretic renography may be necessary to determine the functional significance of the appearance of a dilated collecting system.
Renal transplant recipients are at increased risk for developing renal calculi.15 Renal stones typically appear as echogenic, strongly shadowing structures in the collecting system. Fungus balls should be suspected when a highly echogenic mass in the transplanted collecting system exhibits weak shadowing.
Renal vasculature
Vascular complications occur in 1% to 10% of patients.12,16 Renal artery occlusion and renal vein thrombosis are both devastating complications seen in the early post operative period that can rapidly lead to graft loss. Delayed complications include renal artery and renal vein stenosis and post biopsy complications (e.g. arteriovenous fistulas, pseudoaneurysms).
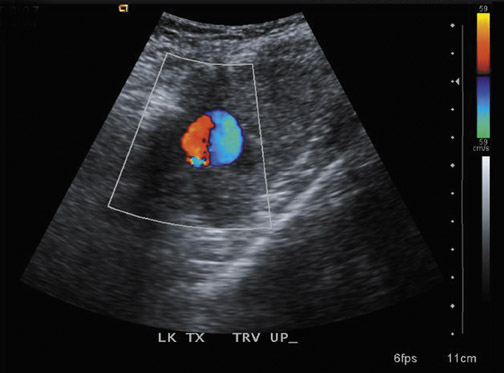
Renal artery thrombosis is a rare complication that can be caused by severe rejection, acute tubular necrosis or faulty surgical technique. Doppler US shows absent intrarenal arterial and venous flow (Figure 7). It should be noted that color Doppler alone may not detect the markedly diminished blood flow that can be seen in severe rejection or ATN,17 thus incorrectly suggesting renal artery or renal vein thrombosis. Use of power Doppler, low-pulse repetition frequency, high gain and low filters can increase the sensitivity for detecting slow blood flow.
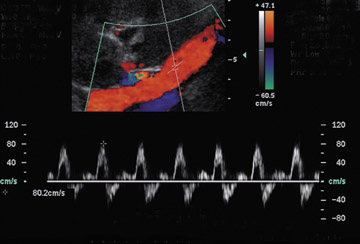
Renal vein thrombosis is more common than renal artery thrombosis and typically occurs between the third and eighth days post transplant (Figure 8).18 Possible etiologies include poor surgical technique, compression of the renal vein by a fluid collection or hypovolemia. The kidney may appear enlarged and hypoechoic with lack of Doppler signal in the renal vein. The renal artery shows increased resistance, often with a reversed plateau of diastolic flow.19 Reversal of diastolic flow can also be seen in the setting of severe rejection or acute tubular necrosis, but the additional finding of absent venous flow is diagnostic for renal vein thrombosis.
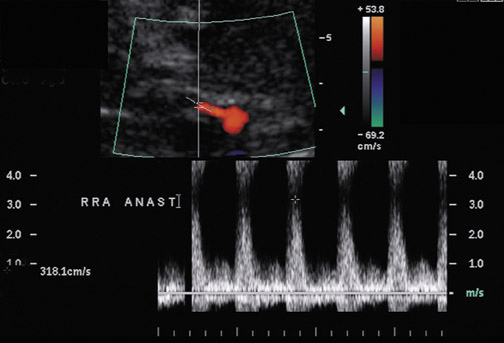
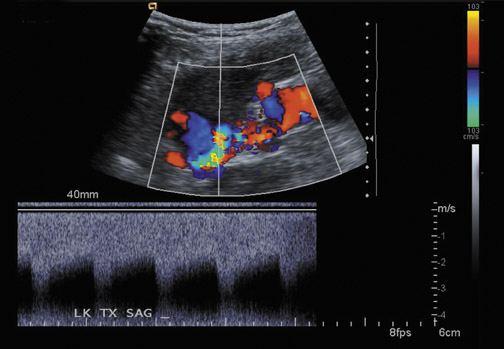
Renal artery stenosis usually occurs during the first 3 years after surgery and is the most common vascular complication after renal transplantation, occurring in ≤10% of patients (Figure 9).20 Approximately half of stenoses occur at the anastomosis due to perfusion cannula injury, faulty surgical technique or reaction to suture material, with end-to-end anastomoses having a higher risk of stenosis. Stenosis can occur proximal to the anastomosis, often due to atherosclerotic disease. Stenosis distal to the anastomosis may be secondary to rejection, or turbulent flow. Patients often present with severe hypertension, audible bruit over the graft and graft dysfunction. Doppler US will show a focal area of color aliasing with peak systolic velocities >2 m/sec, a velocity gradient between the stenotic and prestenotic segment of more than 2:1, and post stenotic spectral broadening. A tardus-parvus waveform may be appreciated in the arcuate and interlobar arteries of the renal parenchyma.21 It is important to realize that transplant renal arteries are often more tortuous than native renal arteries as a result of the surgical procedure and the need for longer vessels in order to perform the vascular anastomosis. Care should be taken when measuring peak velocities, as there is normal acceleration of blood flowing around a tight curve or kink.22
Renal vein stenosis is less common and usually results from extrinsic compression by fluid collections or perivascular fibrosis. Doppler US shows focal aliasing with a three- to fourfold increase in velocity indicating a significant stenosis.23
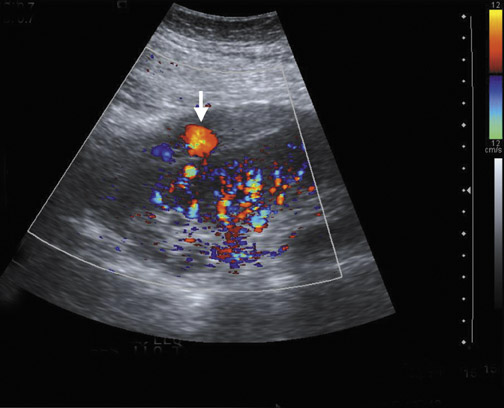
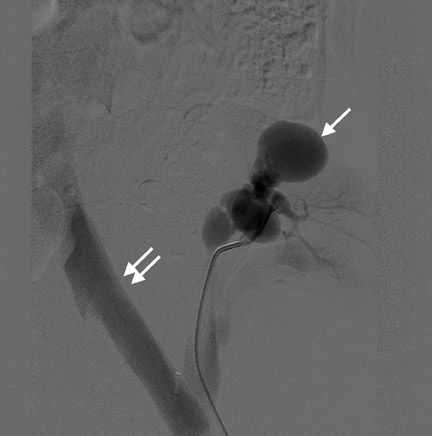
Arteriovenous fistulas and pseudoaneurysms are possible complications from percutaneous biopsy of a transplant kidney. They can often be seen in combination (Figure 10). The majority of these lesions are small and clinically insignificant. However, large shunts may lead to renal ischemia, and rupture of large arteriovenous fistulas and pseudoaneurysms can cause hematuria or perigraft hemorrhage. Grayscale US may show findings similar to a simple or complex renal cyst, but with color Doppler, intense, disorganized flow is identified. Larger arteriovenous fistulas may show a focal flurry of disorganized color beyond the vessel lumen thought to be due to vibration of the tissue surrounding the fistula. The feeding artery will demonstrate a high-velocity, low-resistance waveform and the draining vein may show pulsatile, arterialized flow.24 Pseudoaneurysms with a narrow neck show a “to-and-fro” waveform (forward and reverse flow) when the spectral gate is placed on the neck. Pseudoaneurysms can occur at vascular anastomoses, in the renal parenchyma at biopsy sites or in association with infection.
Conclusion
Several of the major complications after renal transplantation can be detected by US imaging, often making US the first-line diagnostic tool. However, there are several limitations to US including its inability to assess renal function or differentiate the causes of parenchymal abnormalities such as acute rejection, ATN and immunosuppressive drug toxicity. Recognition of these complications and understanding the limitations of the examination are essential so that proper management, including further diagnostic imaging, biopsy or other surgical/radiologic intervention, can ensue.
REFERENCES
- United Network for Organ Sharing and Scientific Registry data. Data from the Organ Procurement and Transplantation Network. Available online: http://www.unos.org. Accessed August 24, 2010.
- Mahdavi R, Arab D, Taghavi R, et al. En bloc kidney transplantation from pediatric cadaveric donors to adult recipients. Urol J. 2006;3:82–86.
- Silver T, Campbell D, Wicks J, et al. Peri-transplant fluid collections. Radiology. 1981;138:145–151.
- Odland M. Surgical technique/post-transplant surgical complications. Surg Clin North Am. 1998;78:55–60.
- Cosgrove D, Chan K. Renal transplants: What ultrasound can and cannot do. Ultrasound Q. 2008;24:77–87.
- Rifkin M, Needlernan L, Pasto M. Evaluation of renal transplant rejection by duplex Doppler examination: Value of the resistive index. AJR Am J Roentgenol. 1987;148:759–762.
- Finlay D, Letourneau J, Longley D. Assessment of vascular complications of renal, hepatic, and pancreatic transplantation. RadioGraphics. 1992;12:981–996.
- Jimenez C, Lopez M, Gonzalez E, Selgas R. Ultrasonography in kidney transplantation: Values and new developments. Transplant Rev (Orlando). 2009;23:209–213.
- Penn I, Brunson M. Cancers after cyclosporine therapy. Transplant Proc. 1988;20(suppl 3):885–892.
- Scott M, Sells R. Primary adenocarcinoma in a transplanted cadaveric kidney. Transplantation. 1988; 46:157–158.
- Vrachliotis T, Vaswani K, Davies E, et al. CT findings in posttransplantation lymphoproliferative disorder of renal transplants. AJR Am J Roentgenol. 2000;175:183–188.
- Kocak T, Nane I, Ander H, et al. Urological and surgical complications in 362 consecutive living related donor kidney transplantations. Urol Int. 2004; 72:252–256.
- Bennett L, Voegeli D, Crummy A, et al. Urologic complications following renal transplantation: Role of interventional radiologic procedures. Radiology. 1986;160:531–536.
- Akbar S, Jafri S, Amendola M, et al. Complications of renal transplantation. RadioGraphics. 2005;25:1335–1356.
- Cho DK, Zackson DA, Cheigh J, et al. Urinary calculi in renal transplant recipients. Transplantation. 1988;45:899–902.
- Jordan ML, Cook GT, Cardella CJ. Ten years of experience with vascular complications in renal transplantation. J Urol. 1982;128:689–692.
- Grenier N, Douws C, Morel D, et al. Detection of vascular complications in renal allografts with color Doppler flow imaging. Radiology. 1991;178: 217–223.
- Singh AK, Sahani DV. Imaging of the renal donor and transplant recipient. Radiol Clin North Am. 2008;46:79–93.
- Kaveggia LP, Parrella RR, Grant EG, et al. Duplex Doppler sonography in renal allografts: the significance of reversed flow in diastole. AJR Am J Roentgenol. 1990;155:295–298.
- Gray DWR. Graft renal artery stenosis in the transplanted kidney. Transplant Rev. 1994;8:15–21.
- Dodd G, Tublin M, Shah A, et al. Imaging of vascular complications associated with renal transplantation. AJR Am J Roentgenol. 1991;157:449–459.
- Friedewald S, Molmenti E, Friedewald J, et al. Vascular and nonvascular complications of renal transplants: Sonographic evaluation and correlation with other imaging modalities,surgery, and pathology. J Clin Ultrasound. 2005;33:127–139.
- Tublin ME, Dodd GD 3rd. Sonography of renal transplantation. Radiol Clin North Am. 1995;33:447–459.
- Middleton WD, Kellman GM, Melson GL, Madrazo BL. Postbiopsy renal transplant arteriovenous fistulas: Color doppler US characteristics. Radiology. 1989;171:253–257.