Diagnosis and Management of Swallowing Physiology: Standardized Contrast, the MBSImP™, & the IDDSI Framework
Images

This article confers 1.0 ARRT Category A Continuing Education credit, which will be awarded upon completion of an online post test. The entire text of this supplement, learning objectives, and the posttest are available at appliedradiology.org/aici.
Dysphagia (difficulty swallowing) is a serious physiologic disorder seen in individuals of all ages but is relatively common in the elderly and in those with a number of conditions, including stroke, oropharyngeal and esophageal cancers and cancer treatments, certain neurologic diseases, and gastroesophageal reflux disease.1 In pediatric populations, dysphagia is seen most often in those born prematurely and/or with cardiac, pulmonary, craniofacial, airway, and neurologic impairment, as well as those with developmental disorders.2-6 Importantly, published prevalence rates for dysphagia are likely a gross underestimation, as the primary diagnosis of a patient with dysphagia is often the underlying condition (e.g., stroke), not the dysphagia itself.7 The burden of dysphagia includes association with significantly longer lengths of hospital stay, higher likelihood of discharge to a post-acute care facility, and greater odds of inpatient mortality vs comparable inpatients without dysphagia.8
The modified barium swallow study (MBSS), also known as the videofluoroscopic swallowing study (VFSS), uses barium sulfate radiographic contrast media of different viscosities to assess swallowing physiology and diagnose dysphagia. Research shows that standardization of the procedure and its materials optimizes the ability to capture swallowing impairment9 and minimizes radiation exposure,10 leading to safer videofluoroscopy examinations.
There are three important aspects of MBSS standardization, which relate to (1) the contrast media, (2) the examination protocol, and (3) the match between diagnostic stimuli and dietary consistencies, for people with dysphagia. Varibar® (Bracco Diagnostics; Monroe Township, NJ) is a series of barium sulfate-containing preparations available in 5 standard consistencies: Thin Liquid, Nectar, Thin Honey, Honey, and Pudding.11-15 The Modified Barium Swallow Impairment Profile (MBSImP) is a standard protocol and rating system used to accurately and consistently quantify and communicate MBSS findings in adult populations.16,17 The International Dysphagia Diet Standardisation Initiative (IDDSI) is a framework of new, standardized terminology and testing methods to describe food textures and drink thicknesses, with the goal of developing global standardized terminology and descriptors for dysphagia diets.18
The objective of this article is to assist clinicians and other healthcare providers tasked with diagnosing and managing the patient with dysphagia in understanding the relationships between Varibar, the MBSImP, and the IDDSI framework, and how to apply them in clinical practice. In addition, this article seeks to address considerations specific to pediatric patients and practice-related policies and procedures.
Evaluation of Dysphagia Using the MBSS, Barium Sulfate, and the MBSImP
The MBSS is a barium sulfate-enhanced fluoroscopic motion study typically performed by a speech-language pathologist (SLP) together with a radiologist, assisted by a radiologic technologist, to evaluate anatomy and swallowing physiology simultaneously in real time.19 The goals of the MBSS are: (1) to identify and distinguish the presence, type, and severity of physiologic swallowing impairment; (2) to determine the safety and efficiency of oral intake; (3) to determine the impact and appropriateness of selected interventions (postures, maneuvers, bolus variables) on swallowing physiology, airway protection, and efficiency in real time; and (4) in collaboration with the treating physician and interdisciplinary team, to develop intake and diet texture/nutritional management plans.19 Identification of dysphagia pathophysiology also guides selection of appropriate rehabilitative treatment approaches.
Varibar is the only FDA-approved barium sulfate contrast product line for evaluation of swallowing using the MBSS.11-15 Varibar products are multi-use and vary in consistency from thin to thick, with each consistency defined by a viscosity range: Thin Liquid (<15 centipoise [cps]), Nectar (<150-450 cps), Thin Honey (800-1800 cps), Honey (2500-3500 cps), and Pudding (puree). Varibar was scientifically formulated to evaluate oropharyngeal swallowing physiology under fluoroscopy, and these formulations represent consistencies known to affect swallowing physiology. Unlike other barium sulfate contrast agents formulated to maximize the mucosal coating required for standard gastrointestinal (GI) imaging studies,20 Varibar products are formulated to possess minimal coating properties, in order to facilitate clear visualization of the dynamic swallowing process.21,22 Moreover, the 40% weight/volume (w/v) concentration provides uniform opacification across all consistencies, ensuring optimal image quality.23 Off-label mixing of other barium sulfate products with foods and liquids is not recommended, as it is associated with increased risk if aspirated, and involves risks of contamination from a food safety perspective. In addition, it would be very difficult to replicate the standardized consistencies and barium concentration of Varibar in one’s own clinical practice through implementation of individualized barium-based recipes.
The Modified Barium Swallow Impairment Profile (MBSImP) involves assessment of 17 components of the swallowing mechanism in adults, and includes a scoring metric to objectively profile physiologic impairment of swallowing function.24 (Table 1) The MBS- ImP provides a standardized protocol to interpret and communicate MBSS results in an evidence-based manner that is consistent, specific, accurate, and objective.19 Although research shows that certain swallowing tasks have high probabilities for identifying specific extreme impairment (e.g., large-volume, thin-liquid swallowing for oral containment and airway protection, or cookie swallowing for physiological components of oral clearance), it is recommended that multiple swallowing tasks be performed during an MBSS.9 The MBSImP protocol was and continues to be validated using Varibar,9,16,23 and MBSImP reports contain reference to the Varibar consistencies used for each swallowing task to evaluate primary components of swallowing physiology. Many resources, including training courses and a large case registry, are available on the website https://www.mbsimp.com/.
The IDDSI Framework
Historically, a number of countries have attempted to develop dysphagia diet standards; however, not surprisingly, these standards have used different terminology, labels, values, and levels, creating potential confusion for health professionals and researchers, as well as individuals and caregivers.18 Without international standards, individuals may find that their modified texture diet is called something completely different as they move among hospitals, rehabilitation facilities, and countries. In 2002, the American Dietetic Association (now the Academy of Nutrition and Dietetics) created the National Dysphagia Diet (NDD).25 The NDD specified different diets containing items that were appropriate for patients with swallowing disorders. Although the NDD was based on consensus among dietitians, SLPs, and food scientists, the NDD classified food and drinks based on a subjective comparison of their textural properties to certain “anchor” foods and, therefore, each patient, caregiver, or healthcare provider could potentially interpret the levels differently.
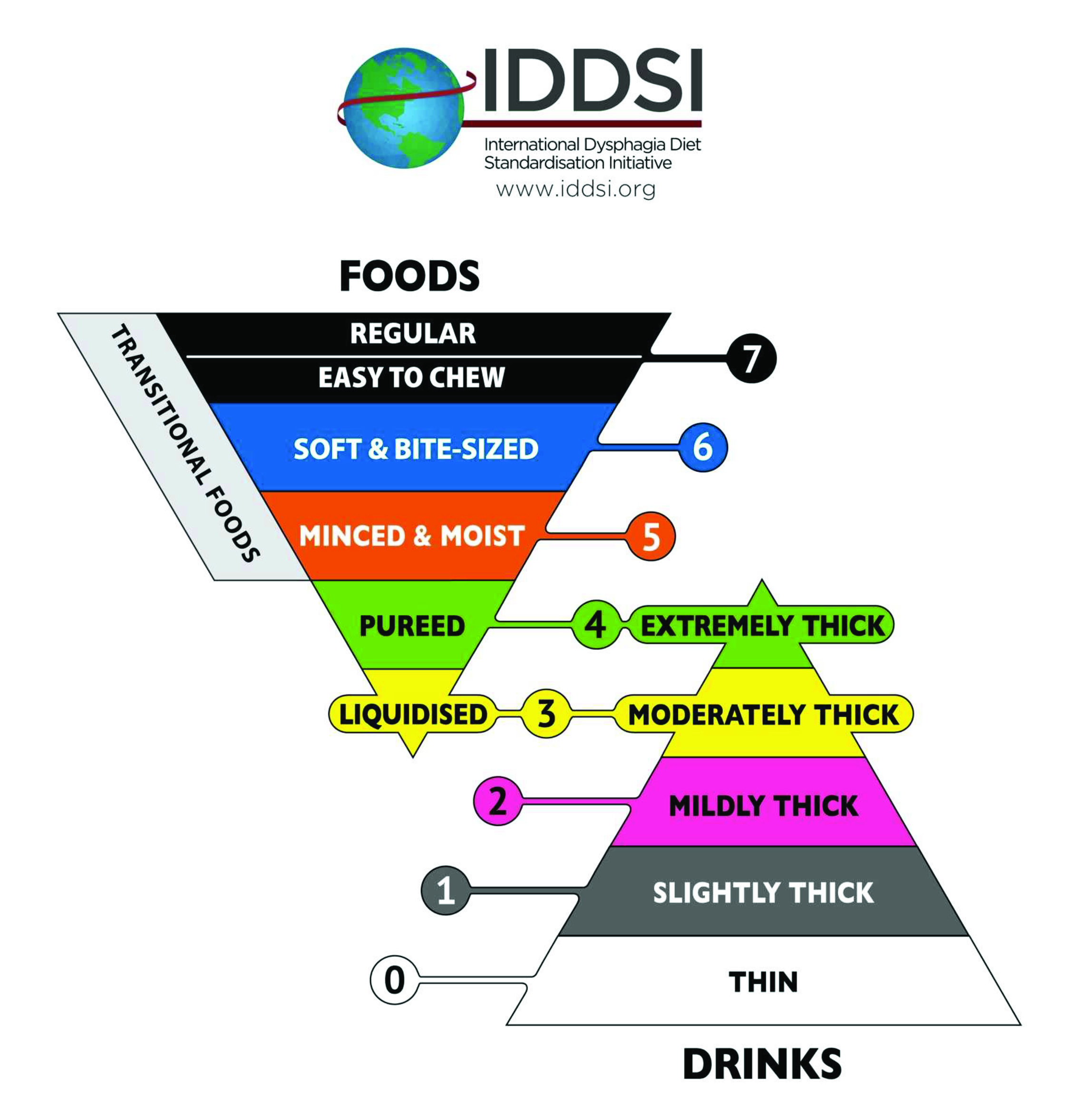
In 2013, the IDDSI Task Force convened with the goal of developing international standardized terminology, descriptors, and testing methods for foods and liquids. The outcome was the creation of the IDDSI Framework.18 (Figure 1) This framework consists of 8 levels: drinks span Levels 0–4, while foods span Levels 3–7. Armed with an understanding of the swallowing physiology, clinicians can use this framework to build a diet that meets patients’ needs across all ages, care settings, and cultures.
In addition to the IDDSI framework, the committee developed simple, objective, reliable, and accessible methods to test and classify any food or drink: the IDDSI Flow Test, the IDDSI Fork Drip Test, the IDDSI Spoon Tilt Test, the IDDSI Fork Test, the IDDSI Fork Pressure Test, and the IDDSI Fork Separation Test.26,27 Depending on the expected framework level, several tests may be required to confirm the properties of a particular food or drink; indeed, several IDDSI testing methods are mandatory for some levels. 26,27
The IDDSI level of drinks is assessed using the IDDSI Flow Test, in which 10 mL of liquid is placed in a standard 10 mL syringe with the clinician’s finger placed over the bottom of the syringe. The finger is then removed and the liquid permitted to flow out the bottom for 10 seconds, timed using a stopwatch. Based on the mL remaining in the syringe, the liquid is assigned to a particular IDDSI level.26 Note that syringes may vary in length, and it is essential to use a syringe of specific dimensions in order to obtain a correct result. Currently, the only recommended syringe for North America is a BD model 303134.
The IDDSI level of foods is assessed using one of four methods: (1) The IDDSI Fork Drip Test for foods/liquids estimated to be at Level 3-5; (2) the IDDSI Fork Test to evaluate food particle size in Levels 5-7; (3) the IDDSI Spoon Tilt Test to assess adhesiveness and cohesiveness; and (4), the IDDSI Fork Pressure Test to assess food hardness in Levels 4-7.27 Videos demonstrating all of these tests are available on the IDDSI Website (https://iddsi.org/),26,27 along with a poster28 and reference cards29 detailing which test(s) are appropriate for each level.
Appropriate Use of the IDDSI Framework
In contrast to Varibar, which is a standardized set of barium-based stimuli to evaluate swallowing physiology during an MBSS, and to the MBSImP, which is a swallowing assessment protocol for use with the MBSS, the IDDSI framework is used to classify the consistency of foods and liquids.
A Description, Not a Prescription
IDDSI is “a description, not a prescription.” This is different from the NDD, which provided preconceived lists of foods and drinks that fit into a specific “diet.” Moreover, as the IDDSI framework is being adopted and implemented worldwide, food manufacturers are increasingly using IDDSI terminology on their labels; however, it is not sufficient to rely on those labels, as consistency can vary with temperature, among batches, in fresh vs stale products, etc. The only truly reliable measure of the IDDSI level of a food/liquid is the result of the appropriate test(s) done at the point of serving. This is particularly true if a patient is having difficulty swallowing a food/liquid that would be expected to be acceptable based on the results of the MBSS or the food package labeling. In that case, an IDDSI Test is recommended to confirm that the product is in fact at the presumed consistency level at the time of consumption.
IDDSI vs Varibar Terminology
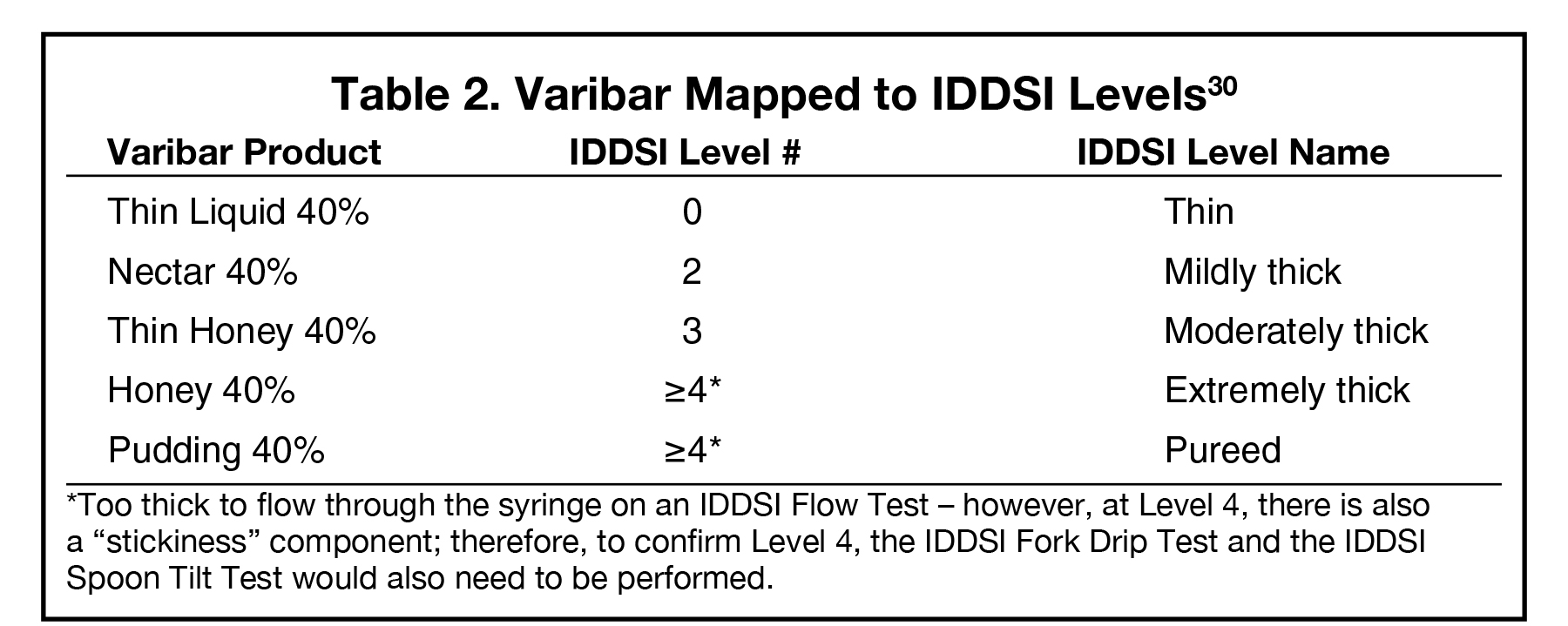
The IDDSI terminology (e.g., Slightly Thick, Mildly Thick, etc.) is not used on Varibar product labels. Rather, the consistency labels that were in use at the time of Varibar product development and FDA approval are used (e.g., Thin Liquid, Nectar, etc.). That said, Varibar products have been mapped to the IDDSI consistency levels (Table 2); SLPs can translate the IDDSI level nomenclature to the caregiver and patient once the assessment is complete.30 Performing an IDDSI Flow Test to confirm food/liquid consistency at the point of use is simple. Such testing is not necessary with Varibar, as the consistencies of these products are factory-controlled and stable; however, flow testing can be used to confirm the similarity between Varibar products and specific foods or liquids that are being considered for inclusion in a patient’s diet.
Role of IDDSI levels in MBSS Stimuli
When performing an MBSS, it is not necessary or appropriate to test a food/liquid from every IDDSI level. Doing so would contradict the principal goal of the MBSS — to use representative, discrete, standardized stimuli and tasks that have been specifically developed to reveal impairments in swallowing physiology. Furthermore, if liquids/foods representative of each IDDSI level were administered, the exam would be too lengthy, exposing the patient to unnecessary radiation. Rather, exploring swallowing ability through the full range of foods/liquids as part of a non-radiological clinical examination outside of the fluoroscopy suite is more appropriate.
It is helpful to think of foods in terms of categories; it is unnecessary to test every item in a category. For example, if the MBSImP protocol is followed, the solid task (Lorna Doone cookie coated with pudding barium) is meant to assess the patient’s ability to break down the solid bolus (IDDSI Level 7). It may then be possible to infer from the cookie task how the patient will perform with IDDSI Levels 5 and 6. If the patient presents with a score of “(1) slow and prolonged chewing and mashing, but complete recollection or formation of the bolus is achieved,” foods with an IDDSI Level 6 consistency (Soft & Bite-Sized) might be suitable. On the other hand, if the patient earns a score of “(2), disorganized chewing and mashing, with solid pieces left unchewed,” food with an IDDSI Level 5 (Minced & Moist) may be more suitable. The foods themselves do not have to be tested in the fluoro suite; rather, evidence has shown that the presence and nature of swallowing impairment are best identified with a standardized set of barium consistencies used in conjunction with the MBSImP protocol.9
Pediatric Considerations
Regardless of the patient’s age, the purpose of the MBSS is to diagnose swallowing pathophysiology and to determine effective compensatory and treatment strategies. As in adults, a standardized protocol permits collection of baseline data and comparison between repeat exams and among patients. However, the MBSImP has not been validated in infants or children. Work is underway to create and validate the BaByVFSSImP© Impairment Profile to quantify swallowing observations made from videofluoroscopic swallowing studies in bottle-fed patients.31 Until the BaBYVFSS is made widely available, clinicians evaluating pediatric dysphagia are advised of the following: (1) a standard protocol should be attempted with all patients and, if there is non-compliance, the protocol can then be modified; (2) protocols must be developmentally appropriate with regard to viscosity of materials presented, presentation of barium material (bottle/nipple, open cup, straw, etc.), and position (semi-upright, upright, etc.).6,19,32,33 Additionally, it is well documented that infant swallowing function changes to a greater degree over time, so the MBSS protocol must allow for evaluation across multiple swallows throughout the first few minutes of swallowing. This may be accomplished by intermittently viewing the infant drinking from the bottle at discrete points where continuous fluoroscopy can be applied to help the clinician and radiologist evaluate for significant changes in swallowing function.33,34
Varibar Thin Liquid (IDDSI Level 0) is representative of both breastmilk and standard infant formula in terms of thickness.22,35-37 Therefore, it is the preferred first consistency to administer during the MBSS in pediatric patients. Other liquid consistencies (e.g., Varibar Nectar [IDDSI Level 2] and Varibar Thin Honey [IDDSI Level 3]) are administered only if performance indicates potential for improved swallowing safety with their administration. Importantly, many infants show improved safety of oropharyngeal swallowing function under MBSS with increased thickness to IDDSI Level 1 (Slightly Thick). There is currently no Varibar product that meets the clinical characteristics of IDDSI Level 1 (Slightly Thick). IDDSI Level 1 (Slightly Thick) can consistently and accurately be produced by mixing equal parts Varibar Thin Liquid with Varibar Nectar. This ratio has been confirmed to be accurate with the IDDSI Flow Test across multiple trials.38 Then, as for adults, the results of the MBSS should assist the clinician in determining which IDDSI Level (0- Thin, 1- Slightly Thick, 2- Mildly Thick, or 3- Moderately Thick) is most appropriate for the safe and adequate oral intake of liquid/food for pediatric patients.
In infants and young children, considerations for dietary recommendations need to account for the fact that significant nutrition is often consumed via breastmilk, formula, and/or baby food; therefore, it is important to understand how these items fit into the IDDSI framework. Studies show that the viscosity of expressed breastmilk is usually consistent with a thin liquid consistency (IDDSI Level 0).35 Similarly, most standard cow’s milk formulas meet the clinical criteria of a thin liquid consistency (IDDSI Level 0).22,36,37 A notable exception is Enfamil™ A.R. Ready to Feed infant formula (vs the powder version that has to be reconstituted with water), which contains rice starch and has been shown to be equivalent to IDDSI Level 1 (Slightly Thick).38 If IDDSI Level 1 (Slightly Thick) is not sufficient to achieve safe and efficient oral intake, and thicker IDDSI Levels (Mildly Thick IDDSI Level 2 or Moderately Thick IDDSI Level 3) are shown to significantly improve oropharyngeal swallowing function, the clinician must use clinical testing methods, such as the IDDSI Flow Test, to determine the appropriate thickener-to-formula ratio to match the IDDSI Level that was determined to be safest under MBSS. Note that when mixing formula with commercially available thickening agents, a number of factors have been shown to influence the resulting thickness, including: the type of thickening agent used, the base fluid (formula) characteristics, the amount of base fluid, the temperature of the formula at mixing and at serving, the sit time (time from preparation to consumption), and the method of mixing the thickening agent into the fluid (i.e., shaking, stirring, and/or immersion blending).22,37
With respect to baby food, research conducted at The University of Alabama evaluated the relationship between commercially available Stage 1 through 4 baby foods and corresponding IDDSI Levels.39 The findings demonstrated no relationship between the manufacturer’s marketed stage and IDDSI levels. Most baby foods marketed as Stage 1 were classified as IDDSI Level 3 (Moderately Thick), with only one Stage 1 food classified as IDDSI Level 4 (Extremely Thick). Stage 2 baby foods were classified as IDDSI Levels 3 (Moderately Thick) and 4 (Extremely Thick). Stage 3 and 4 baby foods were classified as IDDSI Levels 3 (Moderately Thick), 4 (Extremely Thick), and 5 (Minced and Moist). Therefore, it may be prudent to evaluate each brand/stage of baby food independently using the appropriate test to determine the corresponding safe IDDSI level determined by MBSS.
Clinical Practice Considerations
There are quality, safety, and efficiency benefits to using standardized, on-label, FDA-approved barium sulfate products in MBSS clinical practice. Overall quality is improved by a reduction in variability: the use of standardized products contributes to the ability to adhere to uniform standard operating procedures (SOPs)/protocols, thus reducing study-to-study variability. Comparisons to follow-up studies in the same patient become more valuable, since the specific barium preparation used is no longer a variable. The potential need for repeat studies due to inadequate results may also be reduced. With respect to the stimuli themselves, when a barium sulfate product designed for other GI imaging procedures is added to liquids/foods by hand, there is the potential for variability in the textures of preparations among individual SLPs and different facilities. By standardizing the viscosities and barium concentration, reproducibility in both the results and documentation of results (i.e., professional communication) can be improved.
Safety is enhanced with fewer infection control risks. Mixing barium sulfate products with commercial liquids or foods increases the potential for the introduction of pathogens, either from the liquids or foods themselves, or from the increased handling by personnel. Therefore, it is less of an infection control risk to use a preformulated, FDA-approved, multi-use product.
Streamlined study preparation and workflow both contribute to improved efficiency and decreased waste. The use of standardized products: (1) decreases the number of items on the tray for each study, leading to reduced handling, better organization, and less discarded surplus (see Figure 2 for optimal tray set up); and (2) eliminates the time and resources needed to procure food and prepare food-barium mixtures. Routine use of the same products for each study also increases SLP familiarity with those MBSS-tailored products and the manufacturer’s recommendations on their handling and use, minimizing the risk of human error. Finally, onboarding and education for new SLPs is streamlined; consistent processes and procedures can help new SLPs be successful in learning the organization’s study protocols and products.
In addition to the above, performing risk assessment in partnership with an organization’s infection prevention leader and safety officer can help address any needed updates in infection prevention and control best practices, as well as review of any SOPs/protocols for compliance with the institution’s policies (see Table 3 for Sample Checklist for Implementation of Update in Best Practices). It is important to promote a culture of safety throughout an organization. Highly reliable organizations have standardized processes to reduce variability and help provide the same outcome when a test is repeated by various staff members; implementation of standardized, FDA-approved barium sulfate products is an example of this. Toward that end, one can also consider the adoption of standardized contrast as a department-wide performance improvement project (PIP). As part of this PIP, one can track how adopting standardized practices increases the ability to compare intra-patient results over time, reduces variability by helping to provide the same outcome when a test is repeated by various staff members, and lessens the frequency of incident reports related to infection, suboptimal outcome, and/or repeat exams over time.
Finally, preparing staff for regulatory and accreditation agency inspections and surveys is important. Surveyors may want to observe a procedure, which includes setting up the room and tray and preparing the patient, as well as cleaning protocols after the procedure. SLPs should be prepared to answer the following questions: How do you know which study to complete for the patient? How do you select which products to use during the study? How/where are supplies/products stored? How/where do you set up your tray for the study? How do you ensure to minimize transmission of infection between studies? How were you trained or oriented to your department protocols? How and how often is competency assessed? Use of standardized barium products (1) simplifies demonstration of tray set up, as well as description of which products are used and how they are stored, and (2) ensures that all queried SLPs will provide similar answers to the surveyor’s questions.
Conclusions
Evaluation of patients with dysphagia across their lifespan often involves an MBSS, a videofluoroscopic examination in which the patient is administered a set of barium sulfate-containing stimuli of different consistencies. The use of Varibar, a standardized barium sulfate product line designed for the MBSS, along with the MBSImP methodology, provides an accurate and reliable assessment of swallowing physiology and the effectiveness of any interventions. Once any swallowing impairment is identified and described, an appropriate diet can be recommended, and the IDDSI framework and testing techniques can be used to ensure the suitability of any food or liquid.
References
- Peterson R. Modified Barium Swallow for Evaluation of Dysphagia. Radiol Technol. 2018;89:257-275.
- Brackett K, Arvedson JC, Manno CJ. Pediatric feeding and swallowing disorders: General assessment and intervention. Perspectives on Swallowing and Swallowing Disorders (Dysphagia). 2006;15:10-14.
- Lefton-Greif MA. Pediatric dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):837-ix.
- Benfer KA, Weir KA, Bell KL, Ware RS, Davies PS, Boyd RN. Oropharyngeal dysphagia in preschool children with cerebral palsy: oral phase impairments. Res Dev Disabil. 2014;35:3469-3481.
- Sharp WG, Berry RC, McCracken C, et al. Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature. J Autism Dev Disord. 2013;43:2159-2173.
- Dodrill P, Gosa MM. Pediatric Dysphagia: Physiology, Assessment, and Management. Ann Nutr Metab. 2015;66(Suppl 5):24-31.
- Altman KW. Dysphagia evaluation and care in the hospital setting: the need for protocolization. Otolaryngol Head Neck Surg. 2011;145:895-898.
- Patel DA, Krishnaswami S, Steger E, et al. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus. 2018;31:1–7.
- Hazelwood RJ, Armeson KE, Hill EG, Bonilha HS, Martin-Harris B. Identification of Swallowing Tasks From a Modified Barium Swallow Study That Optimize the Detection of Physiological Impairment. J Speech Lang Hear Res. 2017;60:1855-1863.
- Bonilha HS, Wilmskoetter J, Tipnis S, Horn J, Martin-Harris B, Huda W. Relationships Between Radiation Exposure Dose, Time, and Projection in Videofluoroscopic Swallowing Studies. Am J Speech Lang Pathol. 2019;28:1053-1059.
- VARIBAR® HONEY (barium sulfate) oral suspension full Prescribing Information. Monroe Twp., NJ: Bracco Diagnostics Inc.; March 2018.
- VARIBAR® NECTAR (barium sulfate) oral suspension full Prescribing Information. Monroe Twp., NJ: Bracco Diagnostics Inc.; February 2018
- VARIBAR® PUDDING (barium sulfate) oral paste full Prescribing Information. Monroe Twp, NJ: Bracco Diagnostics Inc.; Oct. 2016.
- VARIBAR® THIN HONEY oral suspension full Prescribing Information. Monroe Twp., NJ: Bracco Diagnostics Inc.; January 2018.
- VARIBAR® THIN LIQUID (barium sulfate) for oral suspension full Prescribing Information. Monroe Twp., NJ: Bracco Diagnostics Inc.; April 2019.
- Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J. MBS measurement tool for swallow impairment--MBSImp: establishing a standard. Dysphagia. 2008;23:392-405.
- Beall J, Hill EG, Armeson K, Garand KLF, Davidson KH, Martin-Harris B. Classification of Physiologic Swallowing Impairment Severity: A Latent Class Analysis of Modified Barium Swallow Impairment Profile Scores. Am J Speech Lang Pathol. 2020;29:1001-1011.
- IDDSI Website. What is the IDDSI Framework? Available at: https://iddsi.org/framework/. Accessed October 20, 2020.
- Martin-Harris B, Canon CL, Bonilha HS, Murray J, Davidson K, Lefton-Greif MA. Best Practices in Modified Barium Swallow Studies. Am J Speech Lang Pathol. 2020;29:1078-1093.
- Evers K, Kressel HY. Principles of performance and interpretation of double-contrast gastrointestinal studies. Radiol Clin North Am. 1982;20:667-685.
- Steele CM, Molfenter SM, 21. Péladeau-Pigeon M, Stokely S. Challenges in preparing contrast media for videofluoroscopy. Dysphagia. 2013;28:464-467.
- Gosa MM, Dodrill P, Robbins J. Frontline Interventions: Considerations for Modifying Fluids and Foods for Management of Feeding and Swallowing Disorders Across the Life Span. Am J Speech Lang Pathol. 2020;29:934-944.
- Martin-Harris B, Humphries K, Garand KL. The Modified Barium Swallow Impairment Profile (MBSImP™©) – Innovation, Dissemination and Implementation. Perspectives of the ASHA Special Interest Groups. 2017:2:129-138.
- MBSImP. (2020). Retrieved from https://www.northernspeech.com/mbsimp/
- McCullough G, Pelletier C, Steele C. National Dysphagia Diet: What to Swallow? The ASHA Leader. 2003;8(20).
- IDDSI Website. Drink Testing Methods. Available at: https://iddsi.org/framework/drink-testing-methods/. Accessed October 20, 2020.
- IDDSI Website. Food Testing Methods. Available at: https://iddsi.org/framework/food-testing-methods/. Accessed October 20, 2020.
- IDDSI Website. Food & Drinks Classification and Testing). Available at: https://ftp.iddsi.org/Documents/IDDSI_Whole_Framework_A3_Poster_Final.pdf. Accessed October 20, 2020.
- IDDSI Website. Testing Reference Card. Available at: https://ftp.iddsi.org/Documents/IDDSI_Reference_Card_Folded_DL_Sponsors_May162020.pdf. Accessed October 20, 2020.
- Steele C. Mapping Bracco’s Varibar® barium products to the IDDSI Framework. June, 2017. Available at: https://iddsi.org/wp-content/uploads/2017/07/Mapping-Varibar_Short-version.pdf. Accessed August 12, 2020.
- Martin-Harris B, Carson KA, Pinto JM, Lefton-Greif MA. BaByVFSSImP© A Novel Measurement Tool for Videofluoroscopic Assessment of Swallowing Impairment in Bottle-Fed Babies: Establishing a Standard. Dysphagia. 2020; 35:90-98.
- Newman LA. Optimal care patterns in pediatric patients with dysphagia. Semin Speech Lang. 2000;21:281-291.
- Newman LA. Videofluoroscopy in Infant and Pediatric Populations. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia). 2007;16:11-15.
- McGrattan KE, McGhee HC, McKelvey KL, et al. Capturing infant swallow impairment on videofluoroscopy: timing matters. Pediatr Radiol. 2020;50:199-206.
- Frazier J, Chestnut AH, Jackson A, Barbon CE, Steele CM, Pickler L. Understanding the Viscosity of Liquids used in Infant Dysphagia Management. Dysphagia. 2016;31:672-679.
- Gosa MM, Dodrill P. Effect of Time and Temperature on Thickened Infant Formula. Nutr Clin Pract. 2017;32:238-244.
- Rush OM, Bolland AC, Gosa MM. Effect of mixing method on resulting thickness of infant formula. J Texture Stud. 2020. Epub ahead of print.
- Dodrill P, Gosa MM. Evidence into Practice: The evolution of feeding services in the NICU. 2018. ASHA Convention.
- Summerford MS. All purees are not created equal: thickness, adhesiveness, and cohesiveness of commercially available first foods. 2019. Theses and Dissertations - Department of Communicative Disorders. The University of Alabama. Available at: https://ir.ua.edu/handle/123456789/93.
Frequently Asked Questions
Does Varibar “comply” with IDDSI?
IDDSI is not a regulation or guidance to comply with; instead, it provides a method of classifying the wide array of existing food and beverages based on consistency. IDDSI can also be used to describe Varibar products, the standardized set of stimuli used for the MBSS, based on their consistencies. Therefore, “IDDSI compliant” is not an appropriate term to describe the Varibar products, and IDDSI is not asking manufacturers to change or rename their products.
Does the MBSImP “comply” with IDDSI?
The MBSImP is a protocol used during the MBSS that focuses on the physiologic components of the swallow, while the IDDSI framework presents a standardized system for classifying liquid and food by consistency, to guide dietary preparations once the physiologic problem is identified. It is important to recognize that the MBSS is not a “feeding test” – it should not attempt to test every possible consistency level of food and liquid. Rather, the MBSS uses a standardized set (reproducible across clinicians, clinics, and hospitals) of representative consistencies known to affect swallowing physiology, in order to evaluate the nature and severity of swallowing impairment and airway invasion. A treatment plan indicating safe consistency levels (including the IDDSI levels) can then be formulated based on the MBSS results. Moreover, recommended IDDSI levels can be included within the MBSImP standardized reporting template as part of the plan of care.
Will Varibar products be renamed to match IDDSI terminology?
Varibar products have been formulated specifically for healthcare providers to use during the MBSS based on consistencies known to affect swallowing physiology. At the conclusion of the MBSS, the SLP should be able to identify safe consistency levels, and translate that to the IDDSI dietary framework for patients and caregivers. Patients and caregivers do not handle/manage barium sulfate products and, therefore, the renaming of FDA-approved barium sulfate products to match IDDSI terminology is unnecessary and medically inappropriate.
Is Varibar a multi-use product? What if my institution has a single-use only policy?
Varibar is a multi-use product.11-15 Multi-use containers are allowable by accrediting bodies provided that the organization has a policy supporting safe use, and that those safety standards are followed. Therefore, if your institution does not currently have a multi-use policy, it can be created together with relevant stakeholders, including SLPs, department safety officers, etc. Other than the MBSImP, there are no specific protocols as to how much of each Varibar product preparation is used with any one patient; it will depend on the individual healthcare practitioner and patient requirements. All unopened, pre-mixed Varibar products have a shelf life of 24 months from the date of manufacture; the Thin Liquid powder has an unopened shelf life of 18 months. Once opened, Varibar products can be stored up to 21 days at controlled room temperature, with the exception of Varibar Thin Liquid, which is supplied as a powder and reconstituted and, following reconstitution, can be stored for up to 72 hours under refrigerated conditions. Note that every Varibar product bottle has a “dating field,” where pertinent information can be recorded. As long as proper storage and labeling SOPs are in place and adhered to, approved and standardized multi-use products are preferable to unapproved and/or non-standardized single-use products.
What if there is no refrigerator to store reconstituted Varibar Thin Liquid?
Varibar is the only FDA-approved barium sulfate product for use with the MBSS. In addition, Varibar Thin Liquid is both scientifically as close in viscosity to water as possible, and has been shown to be a good indicator of oral containment and airway protection.9 Therefore, it is important to work with your department to identify a suitable/convenient refrigerator to store reconstituted Varibar Thin Liquid. It is likely that such a refrigerator is available within the Radiology Department or its vicinity.
Should Varibar products be manipulated (e.g., thinned down) to match IDDSI levels? For example, should Varibar Thin Honey be thinned down so that it lands in the midrange on the IDDSI Flow Test?
This is not necessary or recommended. Each IDDSI consistency level is a range, and this reflects the wide variability in dietary pre-thickened beverages. A “nectar thick” beverage from manufacturer A may differ in consistency from a “nectar thick” beverage from manufacturer B, and a “nectar thick” orange juice from one manufacturer may not be the same consistency as a “nectar thick” cranberry juice from that same manufacturer. That is the purpose of IDDSI – to be able to classify dietary liquids objectively, based on the IDDSI Flow Test, into one of the IDDSI levels. In contrast, Varibar products are tightly controlled for consistency, reliably mapping to specific IDDSI levels based on reproducible flow results. The only exception to the above is for the creation of an IDDSI Level 1 Slightly Thick consistency. This consistency is most often used in pediatric dysphagia practice. There is currently no Varibar product that meets the clinical characteristics of IDDSI Level 1 (Slightly Thick), but it can consistently and accurately be produced by mixing equal parts Varibar Thin Liquid with Varibar Nectar, and is recommended for testing during infant MBSS.