Development and Use of Elucirem™ (gadopiclenol) Injection, a Macrocyclic, High-relaxivity GBCA for CE-MRI
Images

Please see additional safety information below.
CE credits are available for this supplement here.
Introduction
The use of gadolinium-based contrast agents (GBCAs) in magnetic resonance imaging (MRI) has been extensively researched, and substantial evidence of their efficacy and safety has accumulated over the past several decades.1 Clinical trials and radiology practices have demonstrated that GBCAs enhance the sensitivity, specificity, and diagnostic confidence of MRI, as well as improve reliability and shorten examination times, leading to increased usage and dosing for various neuro and cardiovascular applications in both adult and pediatric patients. However, there has been growing concern among the radiology community regarding the less stable, linear GBCAs, which bind less tightly to the Gd ion.2 In light of the value of contrast for MRI, the Food and Drug Administration (FDA) and the American College of Radiology (ACR) now recommend limiting patient exposure to Gd where possible.1,3 One solution to reduce exposure is the use of high-relaxivity GBCAs, which can be administered at lower doses without affecting image quality. Until recently, the highest-relaxivity GBCA was a linear agent,4 leading to the recognition that it would be preferable for a GBCA to be both macrocyclic in structure and high in relaxivity, thus reducing the amount of Gd injected and exposure to patients.
A novel macrocyclic and high-relaxivity GBCA, Elucirem™ (gadopiclenol) injection, has been developed and received FDA approval in September 2022. An expert panel of radiologists with extensive CE-MRI experience convened to evaluate the development and utilization of this new GBCA. This monograph represents a summary of the expert panel forum.
Gadolinium-based Contrast Agents
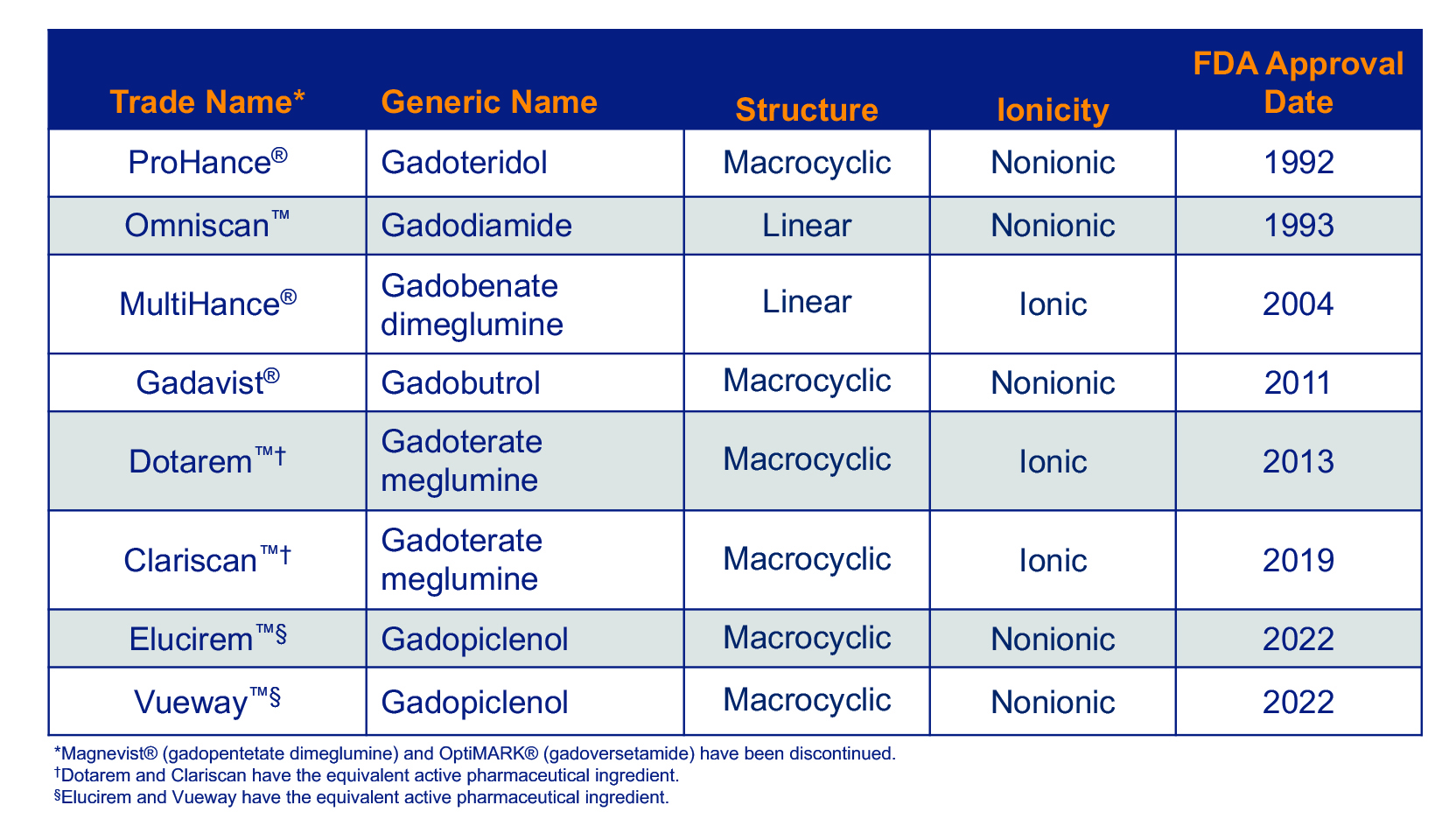
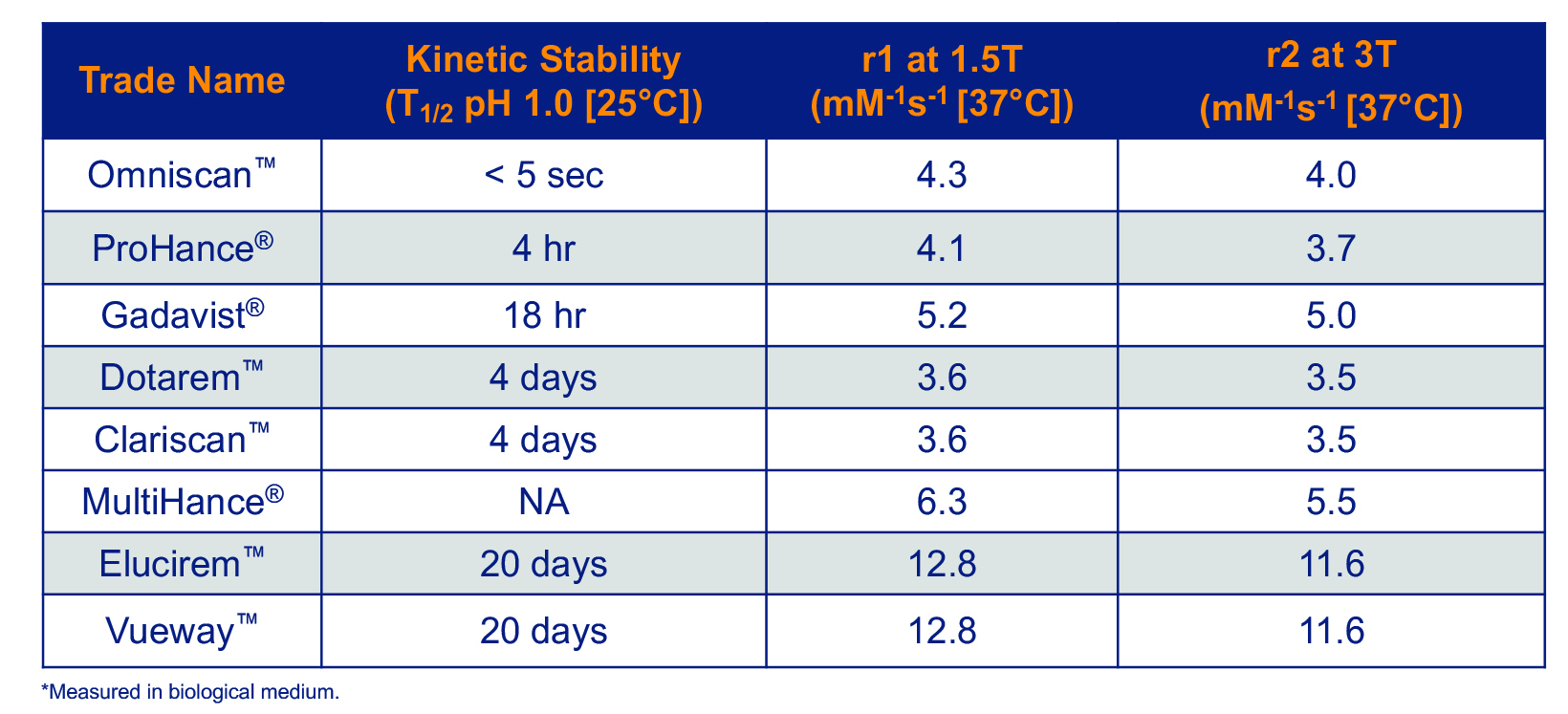
Several GBCAs are approved for use in the United States. 5-12 (Table 1) The oldest of these were approved in the early 1990s, and the most recent were approved in 2022. All consist of a Gd ion chelated to a ligand, and it is the molecular structure of this ligand that determines the unique efficacy and safety profile of each agent.
GBCA Ligand Structure and Stability
The ligand that binds the potentially toxic Gd ion can be linear or macrocyclic, and ionic or nonionic. These properties largely determine the stability of the Gd-ligand chelate which, in turn, affects the likelihood of the chelate to release free Gd.13 In general, the linear, nonionic GBCAs have lower stability than do the macrocyclic, ionic GBCAs.13,14
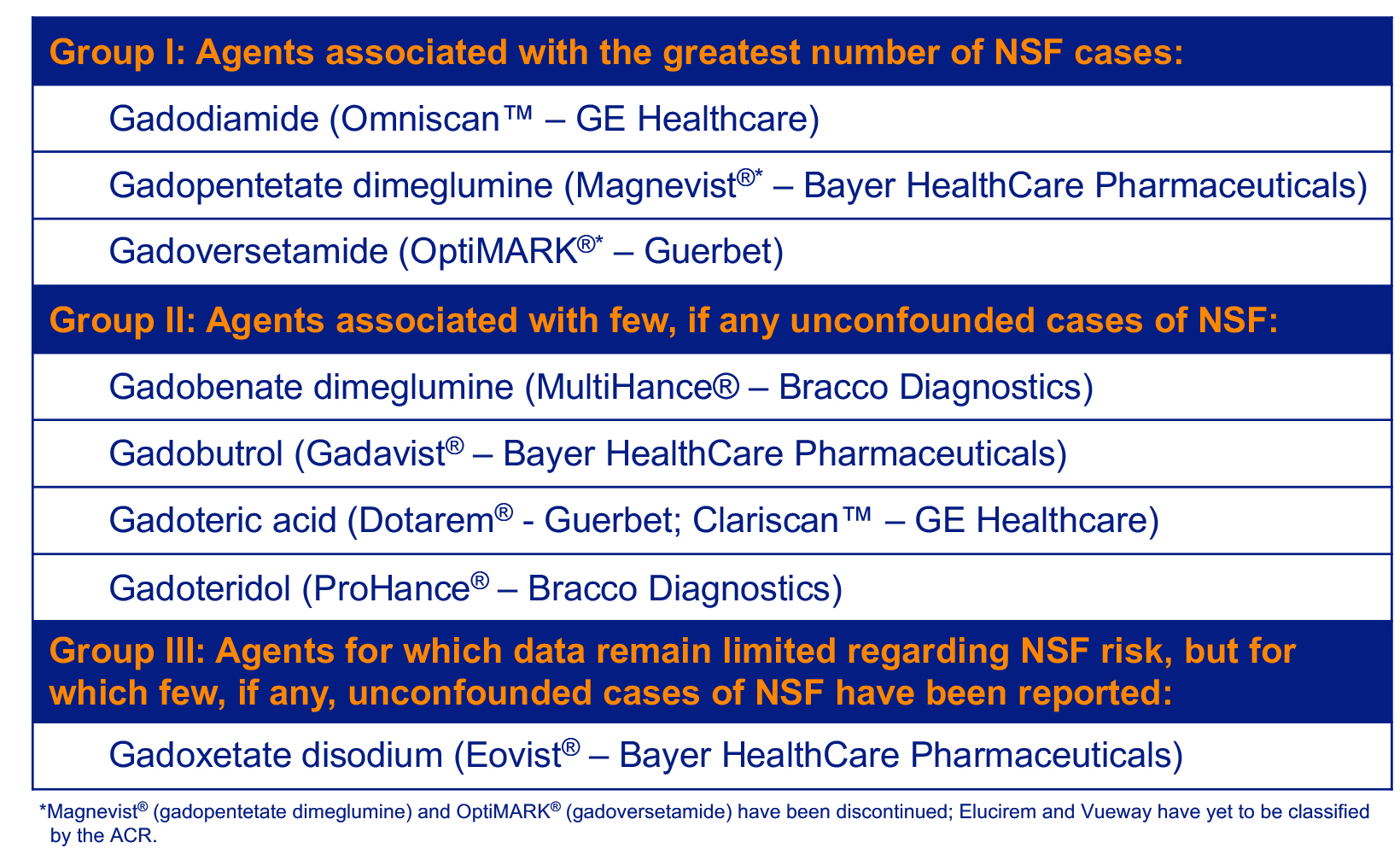
The significance of GBCA stability cannot be overstated. In 2006, lower-stability GBCAs were found to be associated with the development of nephrogenic systemic fibrosis (NSF).15 This sometimes-fatal systemic fibrosing disorder developed almost exclusively in patients with severe renal dysfunction who had received multiple and/or higher doses of Omniscan, OptiMARK® (gadoversetamide), or Magnevist® (gadopentetate dimeglumine); the latter two of which have been discontinued.1 This led the American College of Radiology to classify GBCAs according to their NSF risk. (Table 2)
Although the pathophysiologic mechanism of NSF is not completely understood, the longer half-life of GBCAs in patients unable to eliminate them via renal excretion, coupled with the lower stability of the high-risk GBCAs, is believed to lead to the dermal fibrosis associated with NSF.2 In support of this hypothesis, Gd has been detected in the skin of patients with NSF.16 Changes in practice (eg, screening at-risk patients, using more stable agents) have greatly reduced the risk for NSF, with very few cases still being reported.
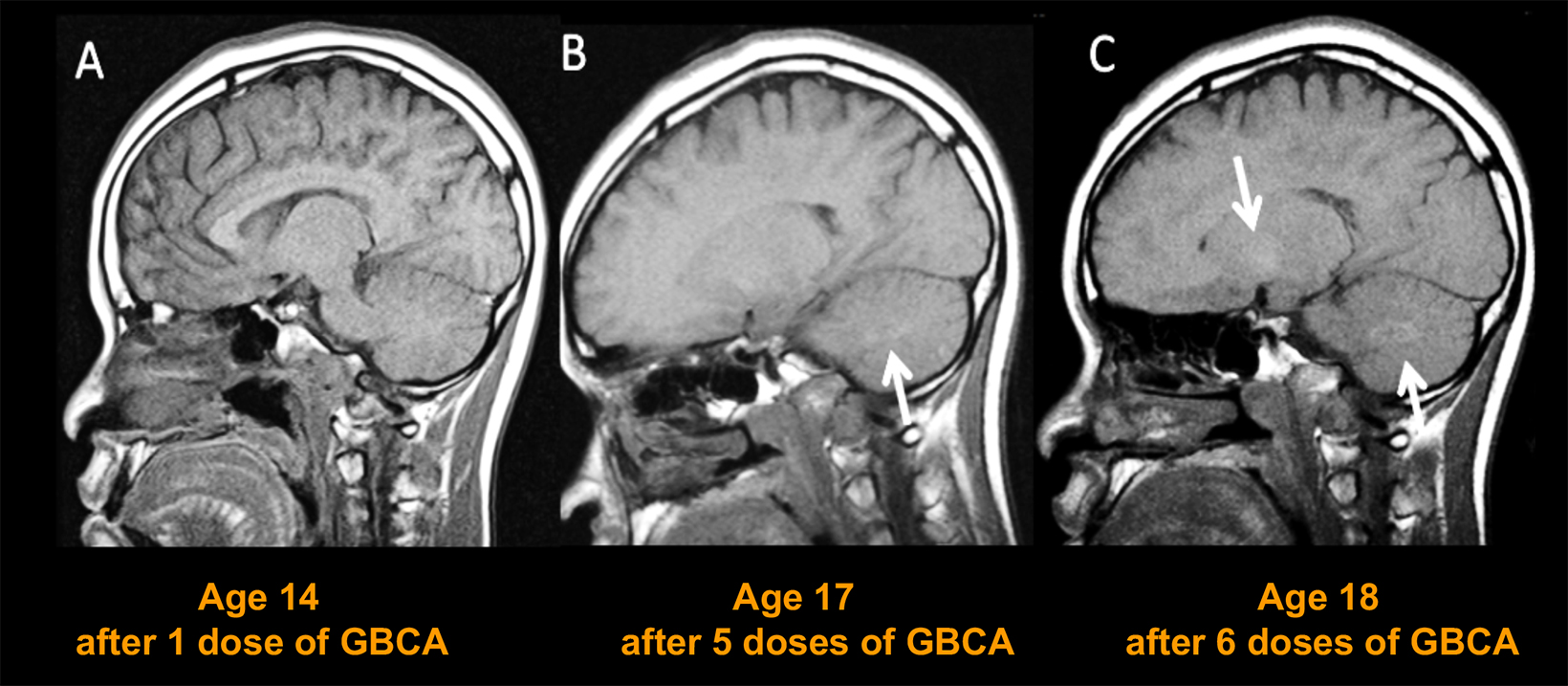
Even before concerns were raised over NSF, Gd was known to accumulate in the bones and organs of GBCA recipients.17,18 Years later, many publications demonstrated contrast enhancement in noncontrast scans of the brain in patients who had previously received a GBCA.19-23 Although this enhancement was seen primarily in patients who had received a linear GBCA, very sensitive analysis revealed Gd in brain tissue following administration of all GBCAs, regardless of whether they were linear or macrocyclic.24 Consequences of gadolinium retention in the brain have not been established; however, the potential for Gd to accumulate in the body is clearly not desirable, particularly in young patients and/or those who require frequent surveillance MRI exams with contrast.
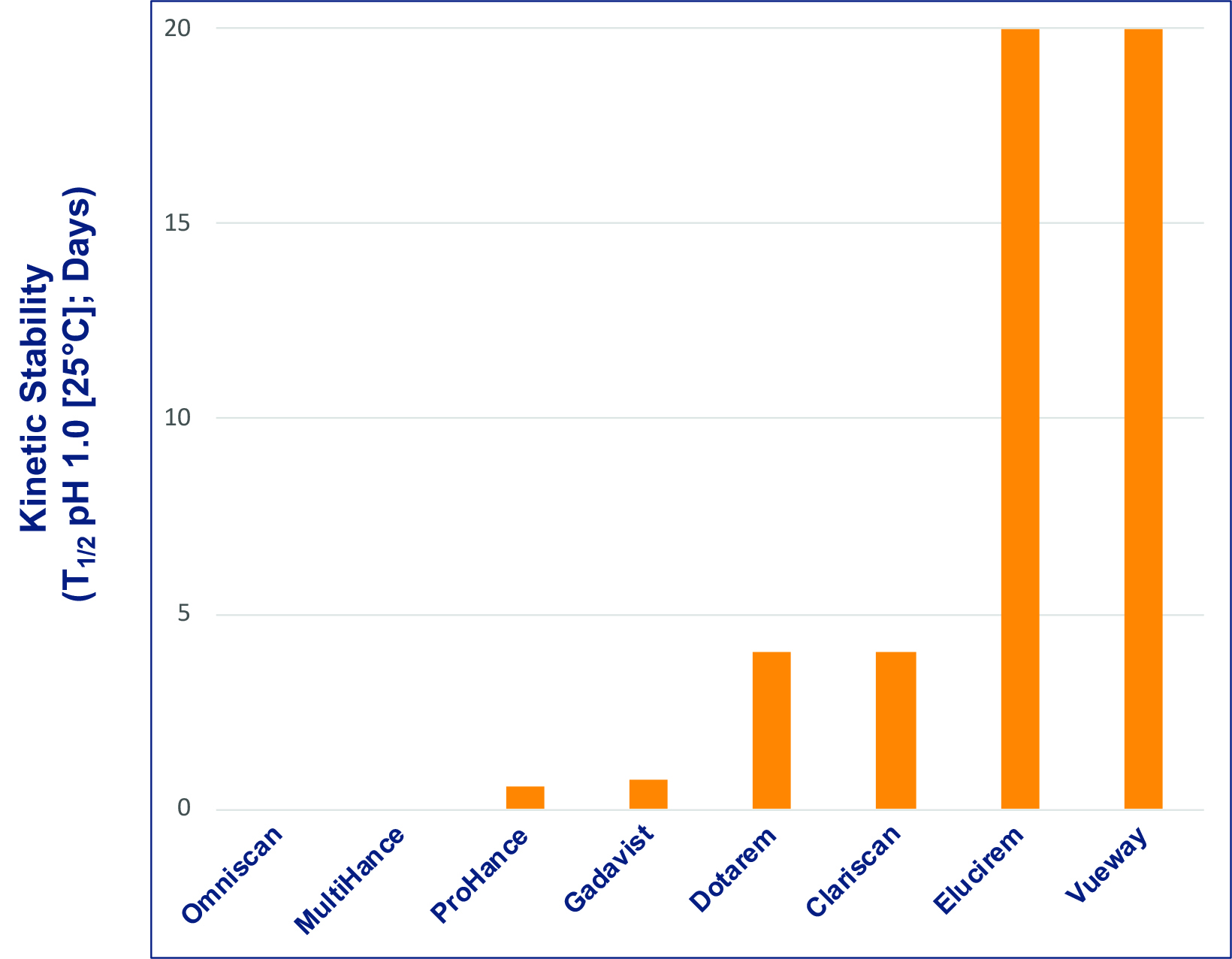
The precise stability of GBCAs is measurable in vitro and is typically represented by the conditional thermodynamic and kinetic stability constants. The conditional thermodynamic stability constant can be thought of as depicting how much Gd is released, and the kinetic stability constant as how fast Gd is released.13,14 The kinetic stability constant is perhaps the more important of the two; if dissociation is slow enough, GBCA elimination is likely to occur before dissociation. Figure 1 shows the kinetic stability constant for each of the available GBCAs: the constants are higher for the macrocyclic GBCAs.4 Notably, the constant of the new macrocyclic GBCA, gadopiclenol, is much higher than that of the other macrocyclic agents, indicating that the new agent has a higher dissociation half-life compared to the other GBCAs.4
GBCA Relaxivity
Relaxivity is the property that most relates to the efficacy of a GBCA: the higher the relaxivity, the greater signal-to-noise that will be produced on MR images. All GBCAs had comparable relaxivity until 2004, when MultiHance, a higher-relaxivity GBCA received FDA approval. MultiHance possesses high relaxivity owing to its ability to bind to protein, specifically albumin, which increases the effective molecular weight and slows the molecular tumbling, resulting in higher relaxivity.25 As a result, MultiHance possesses at least twice the relaxivity of conventional GBCAs.4 (Table 3) Many randomized, intrapatient, crossover trials comparing MultiHance with conventional GBCAs for a wide array of applications showed that, at equivalent Gd doses, MultiHance enhanced significantly more brightly.26-40 In addition, many similarly designed studies showed that at half the Gd dose, MultiHance provided enhancement equivalent to a full dose of a conventional GBCA.41-47 The ability to obtain brighter enhancement or, alternatively, to use lower doses, provided a breakthrough in flexibility for contrast-enhanced MRI (CE-MRI).
Because MultiHance is linear, many radiologists who had moved toward macrocyclic agents for safety reasons have been reserving it for certain niche applications where the higher relaxivity may be of particular benefit. There has clearly been a need for a highly stable, macrocyclic agent with high relaxivity that could be used to increase contrast or reduce Gd dose.
In 2022, the FDA approved Elucirem, the first high-relaxivity, macrocyclic GBCA with 2 water molecule exchange sites. In contrast to MultiHance, Elucirem achieves higher relaxivity via a different mechanism: a greater hydration number. Elucirem is capable of binding to two water nuclei (q=2), while all other GBCAs bind only one (q=1).4 Interaction with this greater number of water molecules results in higher relaxivity. In fact, as shown in Table 3, the relaxivity of Elucirem is approximately twice that of MultiHance and four times that of conventional GBCAs.4
Tolerability of GBCAs
Millions of doses of GBCAs have been administered worldwide, providing ample evidence of their excellent safety profile and very low rate of adverse reactions (0.07% to 2.4%).1,48 Most side effects are mild and allergic or physiologic, with hives, nausea, and vomiting the most commonly reported.1
Severe adverse events are exceedingly rare (0.001% to 0.01%).49 Moreover, small differences in adverse event rates among GBCAs have been demonstrated in large-scale analyses, with the linear, nonionic GBCAs demonstrating a slightly lower incidence of adverse events.49,50
GBCA Indications and Dosing
Most GBCAs are formulated at 0.5 M, indicated at the standard 0.1 mmol/kg dose in adults and children over the age of 2 years, and approved for a variety of neuro and body indications. An exception is Gadavist, which is formulated at 1 M.8 Given the concerns over Gd exposure, the clinical development program of Elucirem focused not on using this high-relaxivity GBCA to further increase contrast enhancement, but rather on using it at half dose to reduce Gd exposure. Therefore, Elucirem is approved at 0.05 mmol/kg for adult and pediatric patients over age 2 years to detect and visualize lesions with abnormal vascularity in the central nervous system (brain, spine, and associated tissues) and the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).7
Elucirem in Adult MR Neuroimaging
For the vast majority of adult neuroimaging applications, contrast is deemed necessary, and a conventional GBCA is considered sufficient. The potential to utilize Elucirem at the approved 0.05 mmol/kg dose and thereby reduce patient exposure to Gd is in line with health agency recommendations. Specifically, the FDA recommends “limiting GBCA dosing and allowing for adequate GBCA clearance between doses.”3 The agency also added a recommendation to the black box warning for all GBCAs to “avoid use of GBCAs in these (at-risk) patients unless the diagnostic information is essential and not available with noncontrasted MRI or other modalities.”5-12 Such at-risk patients include those with impaired elimination of drugs attributable to severe chronic kidney disease or acute kidney injury.
The ACR, meanwhile, recommends that “the lowest dose of GBCA required to obtain the needed clinical information … be used in at-risk patients, and it should generally not exceed the recommended single dose.”1 Reduced Gd exposure is particularly important in children, patients with renal impairment, and those requiring routine surveillance with CE-MRI; eg, patients with multiple sclerosis, where cumulative doses can become quite high over the patient’s lifetime.
Elucirem Clinical Development Program for Neuroimaging
A Phase 2b international, multicenter, double-blind, randomized, controlled, parallel-dose groups, crossover study was performed in 272 adult subjects with known or highly suspected lesions with a disrupted blood-brain barrier, including at least one previously detected enhancing lesion.51 Patients were randomized to 1 of 4 doses of gadopiclenol (0.025, 0.05, 0.1, 0.2 mmol/kg) and to 1 series of 2 MRI scans: gadopiclenol then MultiHance (gadobenate dimeglumine) 0.1 mmol/kg, or vice versa. The qualitative and quantitative efficacy evaluations were performed by 3 independent off-site blinded readers. Adverse events were monitored up to 1 day after the 2nd MRI.
The results showed that the relationship between CNR and the gadopiclenol dose was linear for all readers. Compared with MultiHance at 0.1 mmol/kg, the gadopiclenol doses of 0.05 and 0.1 mmol/kg gave similar or significantly greater contrast enhancement, respectively. Thus, the authors concluded that both doses could be considered for future phase 3 studies. However, as mentioned, the Elucirem clinical development program focused not on using this high-relaxivity GBCA to further increase contrast enhancement, but rather on using it at half the conventional dose to reduce Gd exposure.
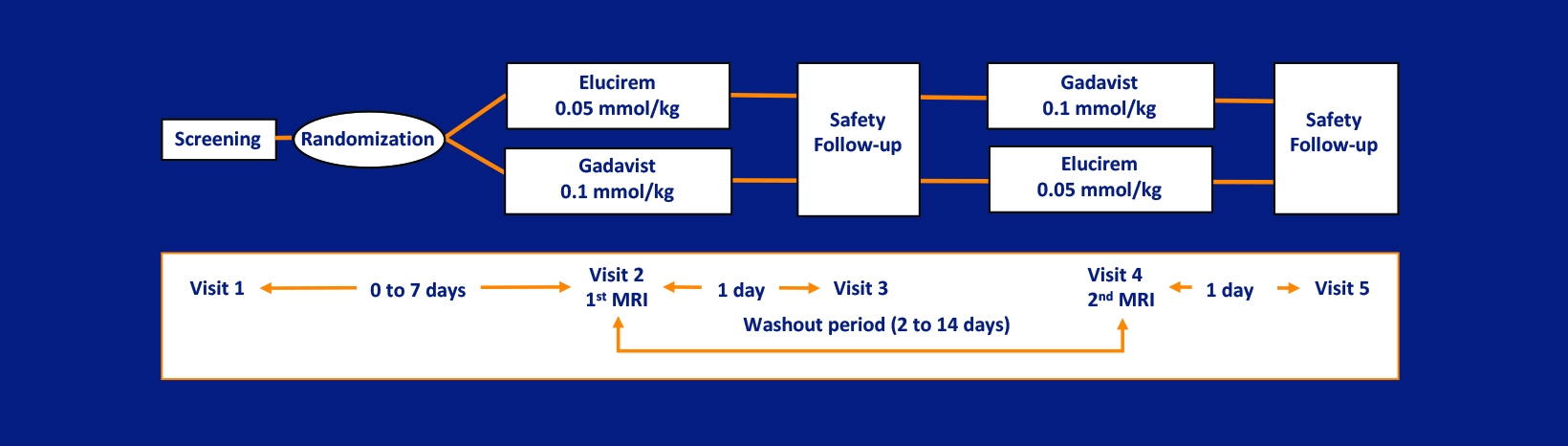
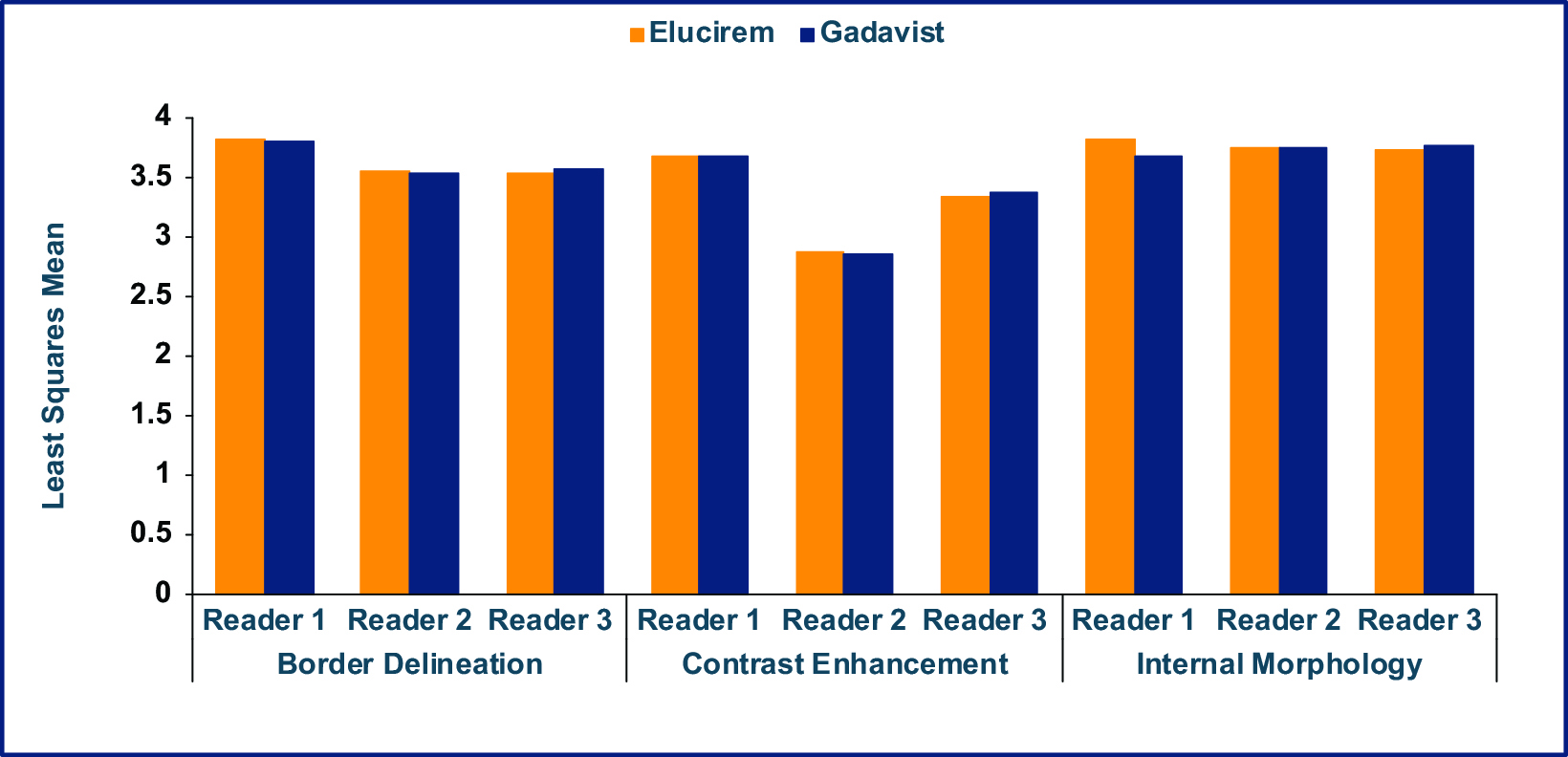
As part of the phase 3 trial, gadopiclenol-enhanced images were compared to unenhanced images and to Gadavist (gadobutrol)-enhanced images for MRI of the brain in 256 subjects (the PICTURE Trial; NCT03996447).7,52 The primary objectives were to demonstrate that Elucirem at 0.05 mmol/kg was superior to unenhanced MRI and noninferior to Gadavist at 0.1 mmol/kg. Lesion visualization parameters (border delineation, internal morphology, and degree of contrast enhancement) were assessed by 3 independent blinded readers. Up to 3 of the most representative brain lesions were scored for each parameter (from 1 to 4: 1 = none/poor, 2 = moderate, 3 = good, 4 = excellent), and the mean score was calculated. The main inclusion criteria were female or male adult with known CNS lesion(s) detected on a previous imaging study. The study design was a prospective, double-blinded, randomized, comparative, multicenter, controlled, cross-over study.7,52 (Figure 2) The trial disease diagnoses included meningiomas (32.1%), brain metastases (22.9%), and glial tumors (21.7%).53
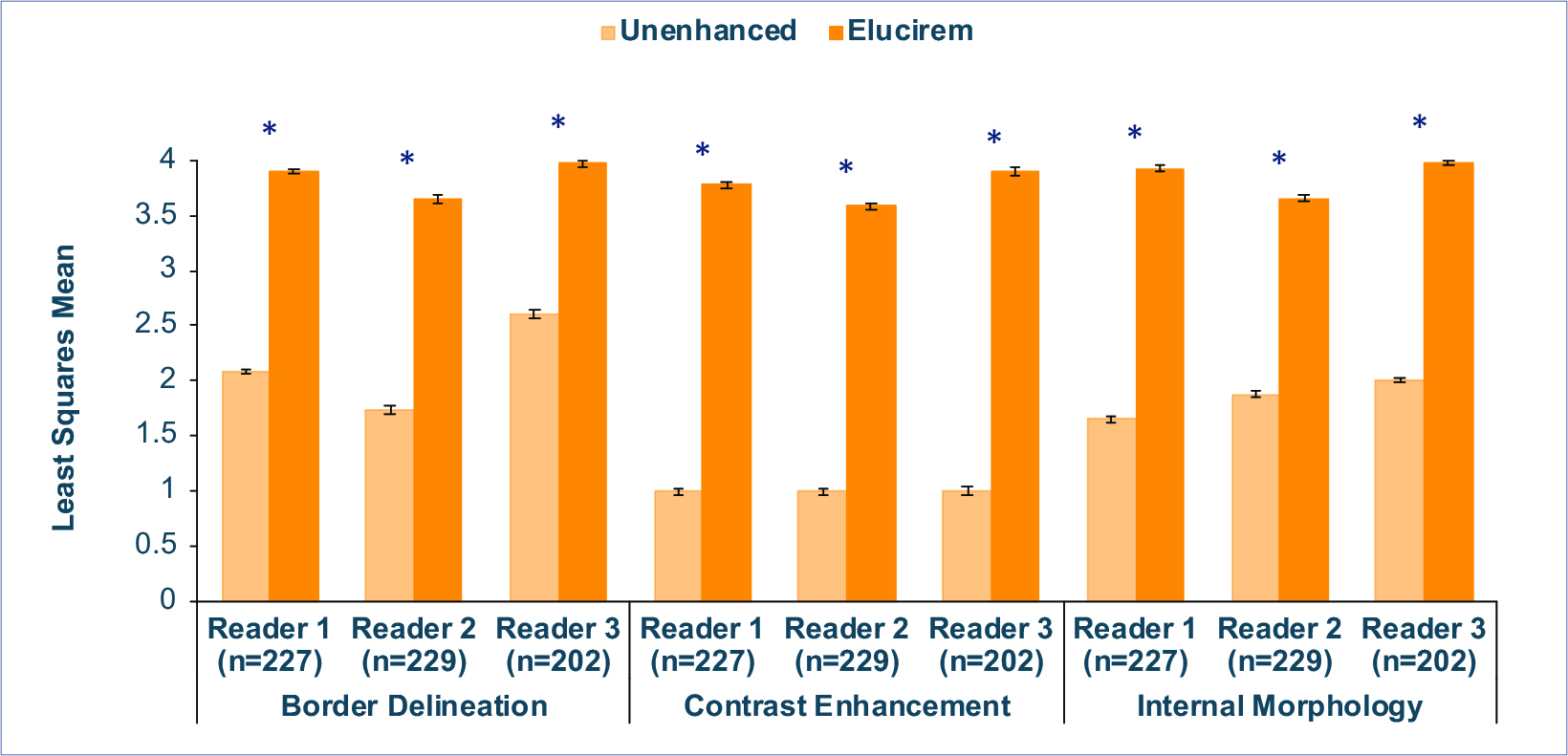
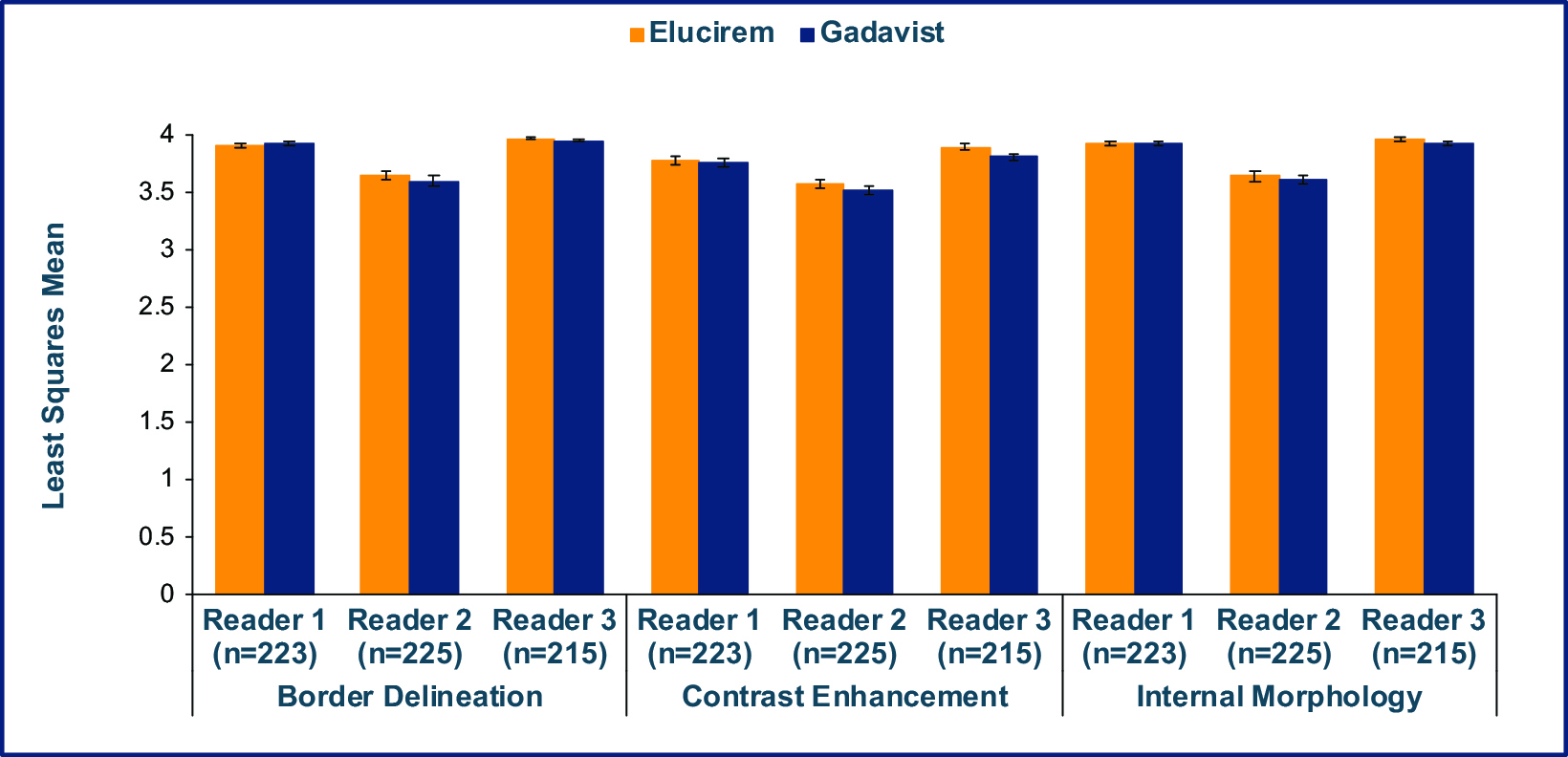
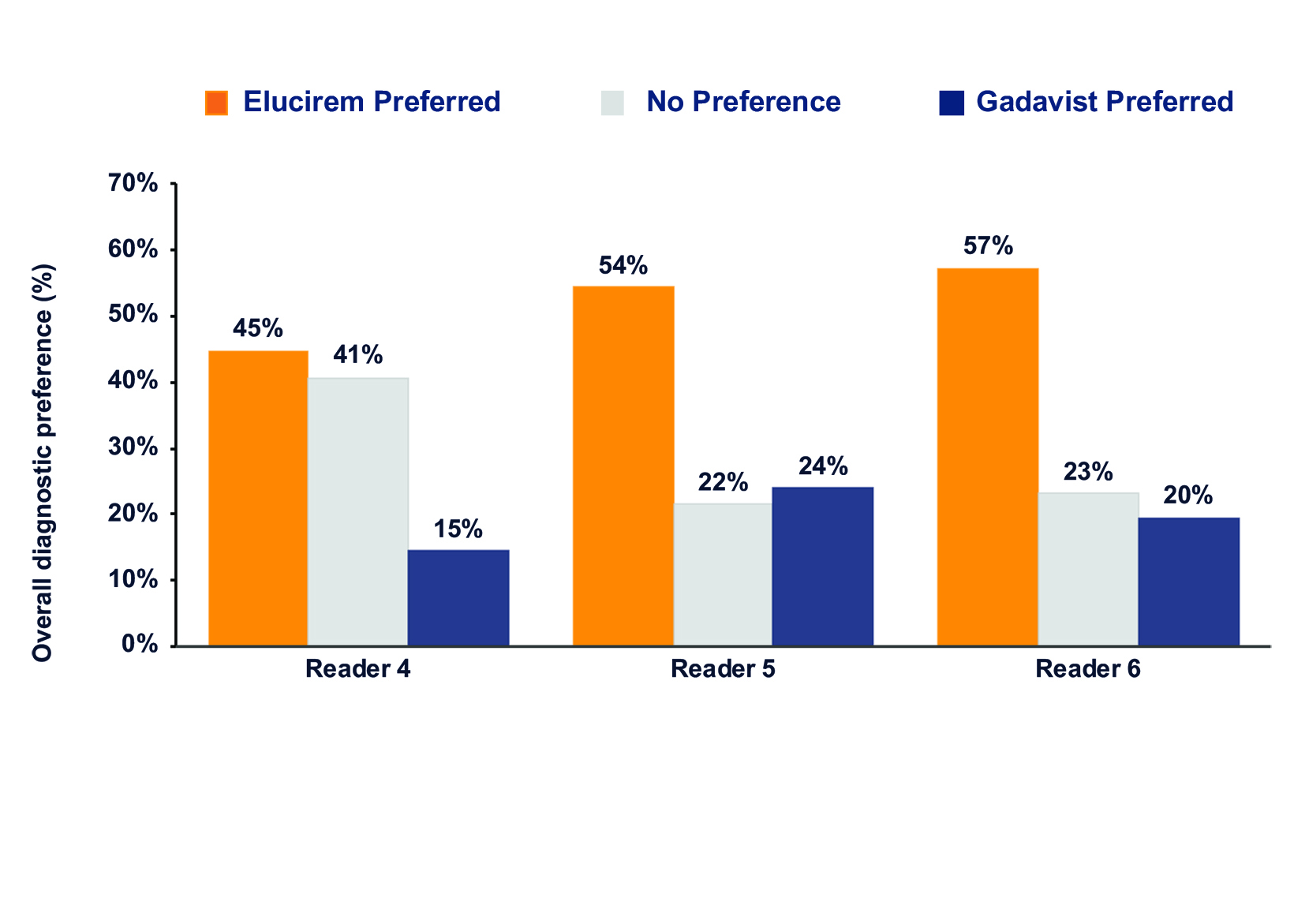
Qualitative and quantitative results are shown in Figures 3-6.7,52 In terms of lesion visualization, Elucirem-enhanced MRI was shown to be significantly superior to unenhanced MRI for all 3 parameters for all 3 readers. (Figure 3) Gadopiclenol was also shown to be noninferior to Gadavist for all 3 visualization parameters for all 3 readers. (Figure 4) In the PICTURE Trial, overall diagnostic preference was assessed by 3 other independent blinded readers in a global matched-pairs fashion, and images with gadopiclenol at 0.05 mmol/kg were preferred to images with Gadavist at 0.1 mmol/kg by all 3 readers. (Figure 5)
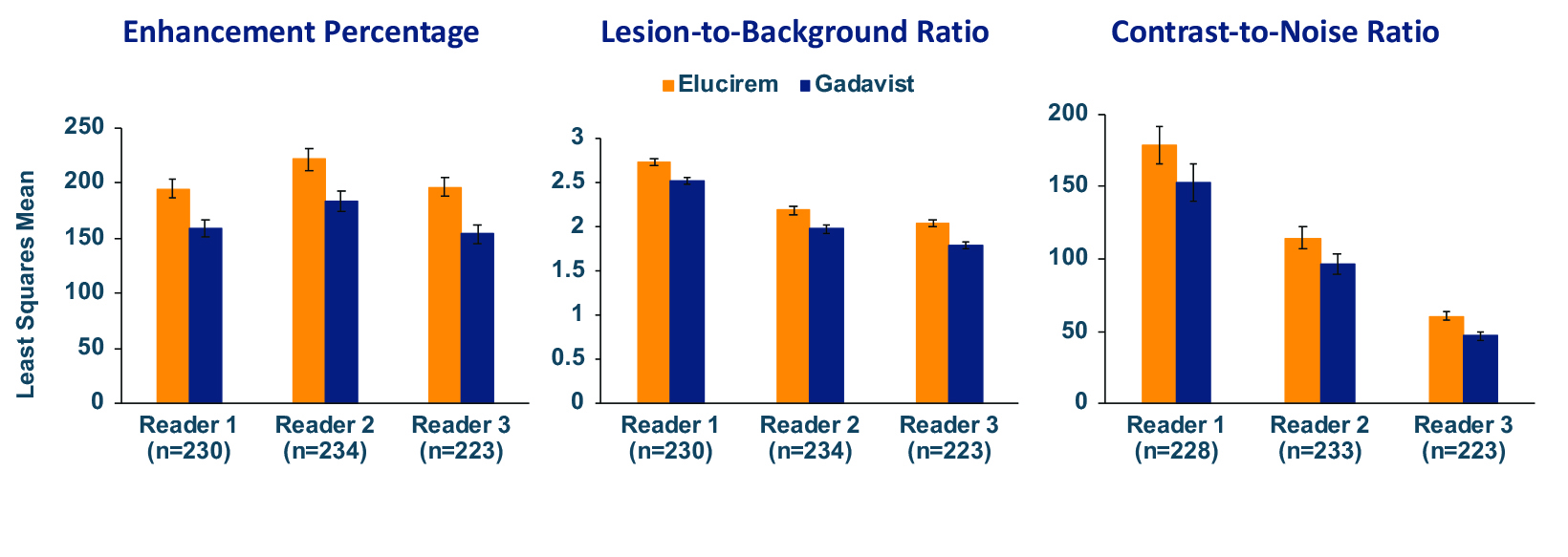
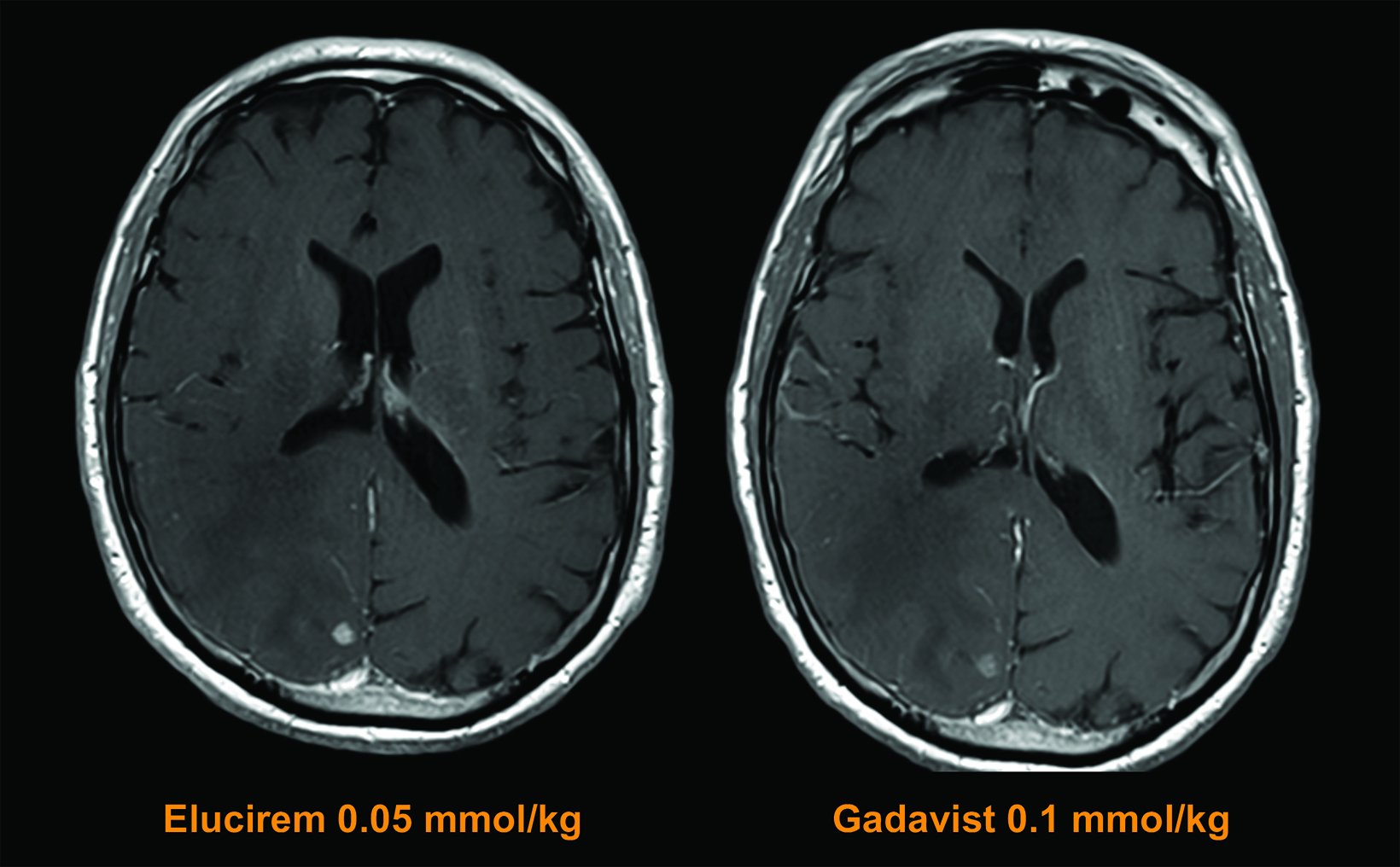
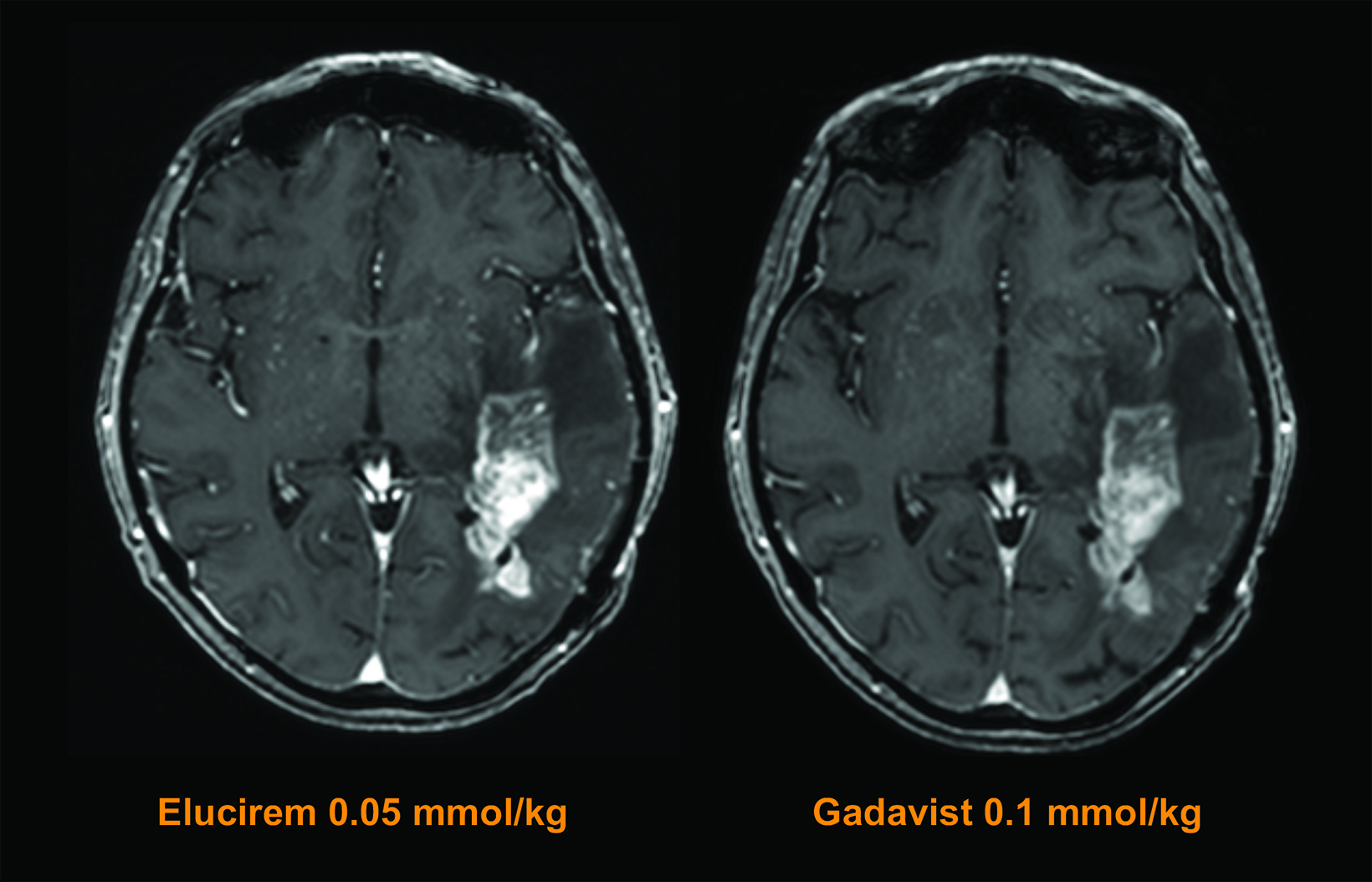
The assessed quantitative parameters included enhancement percentage (E%), lesion-to-background ratio (LBR), and contrast-to-noise ratio (CNR). For gadopiclenol at 0.05 mmol/kg, E% and LBR were significantly higher for all 3 readers (P<0.0001), and CNR was significantly higher with gadopiclenol for 2 of 3 readers (P<0.0001 and P=0.0182). (Figure 6) Examples of results from two patients enrolled in the PICTURE Trial are shown in Figures 7 and 8.52
Safety results from the PICTURE Trial demonstrated that gadopiclenol has a good safety profile, comparable to Gadavist. Post-injection treatment emergent adverse events (TEAEs) were reported in 14.6% of subjects after gadopiclenol administration and 17.6% of patients after Gadavist administration.7,52 Of those considered contrast related, 4.9% of subjects reported an adverse event after gadopiclenol and 6.9% after Gadavist. Most TEAEs were mild (94.2%).7,52 The most common adverse events with both GBCAs were injection site reactions.7,52 No safety concerns were detected regarding laboratory results and vital signs, and no cases of NSF were reported.7,52
In summary, phase 2 and phase 3 studies demonstrated that for neuroimaging, the higher relaxivity of Elucirem could be used safely to reduce Gd exposure in patients without reducing the qualitative or quantitative efficacy one would expect from a contrast-enhancing GBCA.
Elucirem in Body MR Imaging
In addition to neuroimaging, many body imaging applications require contrast, and the vast majority of radiology practices use a conventional GBCA. However, higher relaxivity may be beneficial in certain specific applications. For example, contrast can be helpful for dynamic imaging of patients at increased risk for hepatocellular carcinoma (HCC) in the late arterial phase.54 In addition, for breast imaging, it has been demonstrated that more lesions can be seen with a higher-relaxivity GBCA.37 Notably, both patients at increased risk for HCC and for breast cancer typically require frequent CE-MRI; therefore, the ability to use a macrocyclic, high-relaxivity agent such as Elucirem to obtain the enhancement needed at half the conventional dose of gadolinium will reduce a patient’s exposure to Gd.
Another potential use for high-relaxivity, macrocyclic GBCAs is in low magnetic field strength scanners. Such scanners are becoming more common and typically provide images with lower signal-to-noise ratios. Therefore, a high-relaxivity GBCA may, in part, compensate for the low signal noise for detection and visualization of lesions with abnormal vascularity. The sensitivity and specificity of Elucirem for detection of specific breast pathology are not known.
Elucirem Clinical Development Program for Body Imaging
As part of the phase 3 trials, gadopiclenol-enhanced images were compared to unenhanced images and to Gadavist-enhanced images for body MRI in 304 adults with suspected enhancing abnormalities in at least one body region: the head and neck, thorax, abdomen, pelvis, and musculoskeletal system (the PROMISE Trial; NCT03986138). The study design was similar to that of the PICTURE Trial. Three readers assessed images of the head and neck, three readers assessed images of the musculoskeletal system, and three readers assessed other areas collectively referred to as the body (thorax, abdomen, and pelvis).7 Similar to those in the PICTURE Trial, images enhanced with 0.05 mmol/kg gadopiclenol were significantly superior to unenhanced images and noninferior to images enhanced with 0.1 mmol/ kg Gadavist.7,55 (Figure 9)
Elucirem in Pediatric MR Imaging
The considerations for contrast use in pediatric patients are, in general, similar to those in adults but of potentially greater clinical concern. These patients often require surveillance imaging that, given their age, can result in multiple administrations over their lifetime.56 To date, the only known adverse health effect related to gadolinium retention is the rare condition NSF that occurs in a small subgroup of patients with pre-existing kidney failure; the FDA continues to assess the health effects of Gd retention in the body.57
The overall trend in pediatric imaging has been to avoid linear contrast agents in favor of macrocyclic agents.58 The ability to use half of the conventional dose of an agent with similar contrast enhancement and safety profile is likely to be hailed as a welcome development by pediatric radiologists.
The safety and effectiveness of Elucirem have not been established in pediatric patients younger than 2 years.
Conclusions
Elucirem is the first macrocyclic, high-relaxivity GBCA approved by the FDA. It was developed to address the concerns raised by the regulatory agency and medical associations. The approved dose of Elucirem is 0.05 mmol/kg, providing half the Gd of the other GBCAs but with no loss of signal. At this lower dose, Elucirem has shown to be noninferior to 0.1 mmol/kg of another macrocyclic GBCA, Gadavist. In summary, Elucirem has a unique combination of properties (high relaxivity, high kinetic stability) that allows the agent to be used at half dose to reduce patient exposure to the toxic Gd ion.
ELUCIREMTM (gadopiclenol) injection Important Safety Information
Indications and Usage
ELUCIREMTM (gadopiclenol) injection is indicated in adult and pediatric patients aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in the central nervous system (brain, spine, and associated tissues), and the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).
Contraindications
History of hypersensitivity reactions to ELUCIREM.
Warnings and Precautions
- Nephrogenic Systemic Fibrosis: GBCAs increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease as well as patients with acute kidney injury.
- Hypersensitivity Reactions: With GBCAs, serious hypersensitivity reactions have occurred. In most cases, initial symptoms occurred within minutes of GBCA administration and resolved with prompt emergency treatment. Before ELUCIREM administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to ELUCIREM.
- Gadolinium Retention: Gadolinium is retained for months or years in several organs. Linear GBCAs cause more retention than macrocyclic GBCAs. Consequences of gadolinium retention in the brain have not been established. Pathologic and clinical consequences of GBCA administration and retention in skin and other organs have been established in patients with impaired renal function. While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients, and patients with inflammatory conditions. Consider the retention characteristics of the agent when choosing a GBCA for these patients. Minimize repetitive GBCA imaging studies, particularly closely spaced studies when possible
- Acute Kidney Injury: In patients with chronically reduced renal function, acute kidney injury requiring dialysis has occurred with the use of GBCAs. The risk of acute kidney injury may increase with increasing dose of the contrast agent. Do not exceed the recommended dose.
- Extravasation and Injection Site Reactions: Injection site reactions such as injection site pain have been reported in the clinical studies with ELUCIREM. Extravasation during ELUCIREM administration may result in tissue irritation. Ensure catheter and venous patency before the injection of ELUCIREM.
- Interference with Visualization of Lesions Visible with Non-Contrast MRI: As with any GBCA, ELUCIREM may impair the visualization of lesions seen on non-contrast MRI. Therefore, caution should be exercised when Gadopiclenol MRI scans are interpreted without a companion non-contrast MRI scan.
Adverse Reactions:
In clinical trials, the most frequent adverse reactions that occurred in > 0.2% of patients who received ELUCIREM included: injection site pain, headache, nausea, injection site warmth, injection site coldness, dizziness, and localized swelling
Adverse reactions that occurred with a frequency ≤ 0.2% in patients who received 0.05 mmol/kg BW ELUCIREM included: maculopapular rash, vomiting, worsened renal impairment, feeling hot, pyrexia, oral paresthesia, dysgeusia, diarrhea, pruritus, allergic dermatitis, erythema, injection site paresthesia, Cystatin C increase, and blood creatinine increase.
Use in Specific Populations
- Pregnancy: GBCAs cross the human placenta and result in fetal exposure and gadolinium retention. There are no available data on ELUCIREM use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
- Lactation: There are no data on the presence of ELUCIREM in human milk, the effects on the breastfed infant, or the effects on milk production. However, published lactation data on other GBCAs indicate that 0.01 to 0.04% of the maternal gadolinium dose is excreted in breast milk.
- Pediatric Use: The safety and effectiveness of ELUCIREM have not been established in pediatric patients younger than 2 years of age.
- Geriatric Use: This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function.
- Renal Impairment: In patients with renal impairment, the exposure of gadopiclenol is increased compared to patients with normal renal function. This may increase the risk of adverse reactions such as nephrogenic systemic fibrosis (NSF). Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities. No dose adjustment of ELUCIREM is recommended for patients with renal impairment. ELUCIREM can be removed from the body by hemodialysis
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see the full Prescribing Information, including the Medication Guide, for additional important safety information.
References
Citation
C S. Development and Use of Elucirem™ (gadopiclenol) Injection, a Macrocyclic, High-relaxivity GBCA for CE-MRI. Supplement to March-April 2023 Applied Radiology. 2023; (2):
March 7, 2023