CT urography: Review of technique and spectrum of diseases









Dr. Alderson is a Fellow in the Department of Radiology; Dr. Hilton is a Clinical Professor of Radiology, Co-Chief, CT Section, Department of Radiology; and Dr. Papanicolaou is Professor of Radiology and Co-Chief, CT Section, at the Hospital of the University of Pennsylvania, Philadelphia, PA.
Imaging of the upper urinary tract has traditionally been the purview of intravenous (IV) urography, but over the last decade, computed tomography urography (CTU) has become the modality of choice in imaging the urinary tract. With few exceptions, most notably that of the unenhanced CT performed for acute flank pain and stone disease, many urological symptoms and conditions are now investigated with CTU. Continuing improvements in the spatial resolution and speed of newer CT scanners, combined with advanced multiplanar and volume-rendered image reconstruction, have made CTU a comprehensive examination whereby the kidneys and upper collecting system, ureters, and urinary bladder can be evaluated in one setting.
Indications for CTU continue to evolve. Conditions commonly referred for CTU include urinary calculus disease, hematuria, flank and abdominal pain, suspected renal or urothelial neoplasm, a variety of inflammatory conditions, and congenital anomalies of the kidneys and ureters. Experience with patients who have undergone cystectomy with urinary diversion, most often for treatment of bladder carcinoma,continues to increase, and CTU is commonly used for surveillance of the urothelium in at-risk patients. Currently, CT urographic evaluation of the urinary bladder generally is not considered accurate enough to exclude small superficial urothelial tumors, and cystoscopy is indicated for complete bladder evaluation. The ability to biopsy and resect lesions are added benefits of cystoscopy.
The American Urological Association Best Practices Policy guidelines recommend IV or CT urography as the initial imaging test for patients with asymptomatic microscopic hematuria.1 Likewise, the American College of Radiology rated CTU as the most appropriate imaging procedure in the evaluation of hematuria.1 Furthermore, extraurinary findings, some of them clinically important, can be found in a percentage of patients undergoing CTU.1 Contraindications to CTU are generally limited to those patients who cannot receive iodinated contrast because of renal insufficiency, prior severe reaction, or pregnancy.
Technique
Most CTU protocols are triphasic examinations that include noncontrast, enhanced, and delayed images.1-3 Noncontrast images extending from the top of the kidneys through the bladder are obtained to evaluate for calculi, fat-containing lesions and parenchymal calcifications, and to provide baseline attenuation for assessment of lesion enhancement. Intravenous contrast is administered and, following a 90- to 100-sec delay, scanning of the abdomen and pelvis is performed during the nephrographic phase. Homogeneous enhancement of the kidneys during the nephrographic phase optimizes small renal mass detection. The final acquisition is during the excretory phase after a 12- to 15-min delay, when there is opacification and distention of the collecting systems, ureters, and bladder. Excretory images allow for evaluation of the urothelium. While diagnostic unenhanced and nephrographic acquisitions are relatively easy to obtain, optimal opacification and distention of the ureters during the excretory phase can be more problematic. Suboptimal distention of the ureters and intermittent peristaltic waves may result in limited visualization of one or more segments. A variety of techniques have been proposed to improve visualization, including oral and IV hydration, diuretic administration, use of a compression belt, prone positioning, and a log-rolling prior to the excretory acquisition.1,3-7
As an alternative to the triphasic single bolus CTU technique, the split contrast bolus technique has been designed whereby the contrast is given as 2 boluses before a single enhanced-scan is acquired.1 The aimed cumulative effect is that the earlier smaller bolus provides excretory information, while the second, larger bolus provides information on vascular anatomy and the renal parenchyma. The benefit of this protocol is the elimination of an additional acquisition, resulting in a decreased radiation dose; the disadvantage is less reliable opacification of the ureters. While the ideal protocol is not agreed upon, many centers hydrate patients prior to contrast administration (oral and/or IV route) and inject furosemide IV at contrast injection.
The large number of very thin section images allows for isotropic presentation of the axially acquired data set in any chosen plane. Often on a workstation, CT urograms are frequently reviewed utilizing multiplanar reformations, maximum intensity projections (MIP), and 3-dimensional volume-rendered images. Curved planar reformations along the length of the ureters are also often helpful. Not only do these postprocessing techniques allow the radiologist to interact with the data to more fully appreciate pathologic changes, but they also aid in communicating findings to referring physicians. If desired, IVU-like projectional images can be created for those more familiar with these images.
Radiation dose
As 3 CT scans of the abdomen and pelvis are performed in the examination, radiation dose to the patient is of concern. One study reported a mean effective dose estimate of 14.8 mSv +/- 90 for CTU, and 9.7 mSv +/- 3 for IVU.2 Another study reported that the total effective dose for a triphasic CTU ranges between 20.1 mSv and 66.3 mSv.8 Thus, the total dose can be significantly higher than with IVU, and many report radiation doses for CTU to be 1.5 to 2 times higher.1,2 To limit the dose, the upper abdomen superior to the kidneys is excluded on the unenhanced and excretory acquisitions. Additionally, radiation reduction techniques, such as automated tube current modulation, can be employed, whereby tube potential and tube current-time product are automatically customized to each patient’s body habitus to limit overexposure while maintaining image quality. A recent study evaluating low-dose noncontrast CT for the evaluation of stone disease suggests that significant dose reductions can be realized in certain patient populations without sacrificing accuracy.9 Nonetheless, at this time, most authors report radiation doses associated with CTU to be greater than those with IVU, and continued refinement of patient selection criteria and imaging protocols should be performed to avoid unnecessary exposure.
Renal and urinary tract pathology
Renal parenchymal masses
A variety of benign and malignant renal masses are well depicted by CTU, particularly during the nephrographic phase. Noncontrast images are essential in identifying calcification, macroscopic fat within a lesion, and high attenuation of hemorrhagic or proteinaceous cystic lesions; they provide the baseline attenuation necessary for assessment of enhancement. In addition to simple and complicated/complex cysts, other common masses include renal cell carcinoma and angiomyolipoma. Less commonly encountered masses include renal lymphoma (primary or secondary), fat-poor angiomyolipoma, multilocular cystic nephroma, oncocytoma, and metastatic disease. A detailed description of the radiographic features of each lesion is beyond the scope of this review.
Renal papillary/medullary abnormalities
Papillary necrosis is associated with analgesic overuse, sickle cell anemia, diabetes, pyelonephritis, renal obstruction, and renal vein thrombosis.10(p89,p151),11(pt3,sec3,p46) Identified on the excretory phase, papillary necrosis may have several findings, including contrast-filled clefts in the medulla, contrast collections within one or more papillae, or caliceal-filling defects from sloughed papillae (Figure 1).10(p89,p151),11(pt3,sec3,p46)
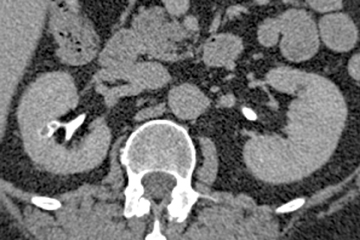
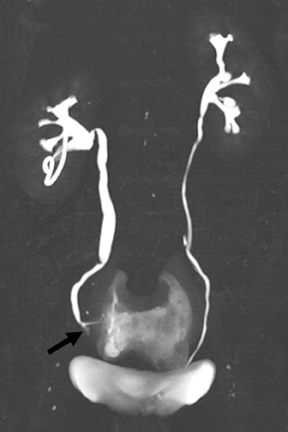
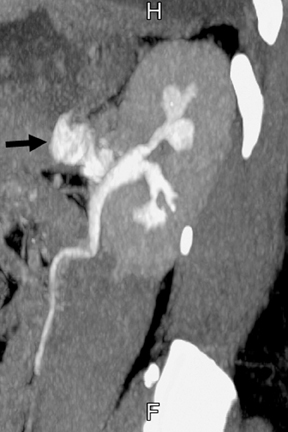
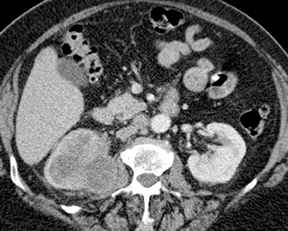
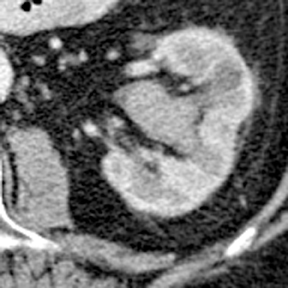
Renal tubular ectasia is characterized by noncalcified cystic dilatation of the collecting tubules.10(p40) Patients are frequently asymptomatic, but may have microhematuria. Classically described as having a “paint brush” appearance, contrast opacifies the dilated tubules, resulting in dense striations extending from the papillary tips into the medulla.3,10(p40) Patients with medullary sponge kidney disease, part of the spectrum of medullary cystic diseases, have associated medullary nephrocalcinosis with or without urolithiasis (Figure 2).
Collecting system (calyces, renal pelves, ureters)
The calyces, renal pelves, and ureters may be affected by a variety of inflammatory, traumatic, congenital, and neoplastic processes.
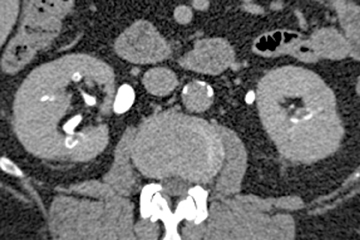
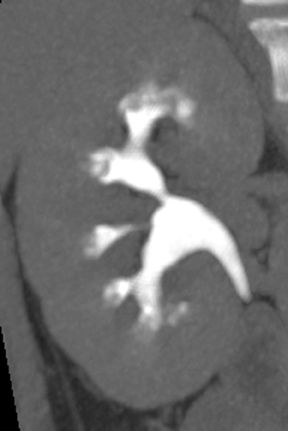
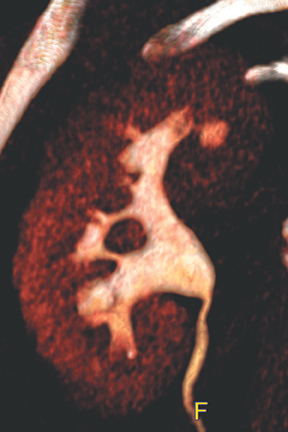
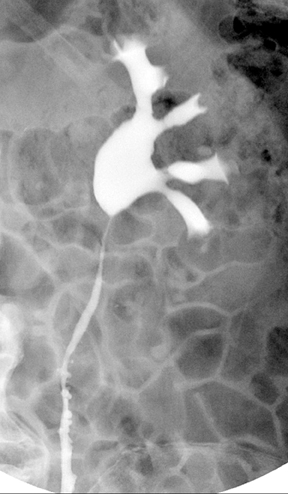
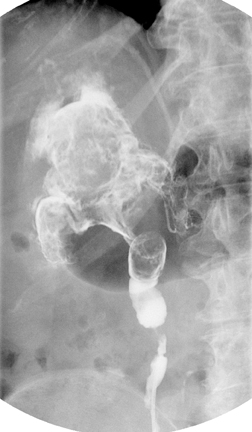
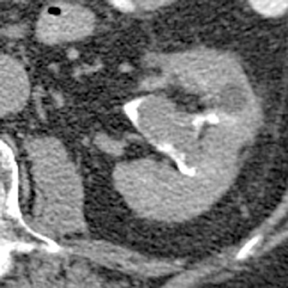
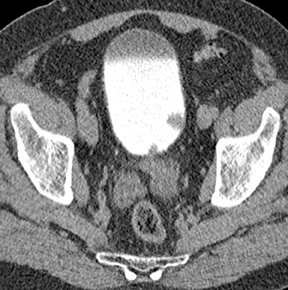
Caliceal diverticula are incidental findings of little or no significance. However, infrequently, they may contain stones and/or become obstructed, causing flank pain, hematuria, or urosepsis. Small outpouchings of the calyx are identified as contrast collections contiguous with a calyx (Figure 3).10(p43,pp105–106) Stones within diverticula are best identified on noncontrast images.
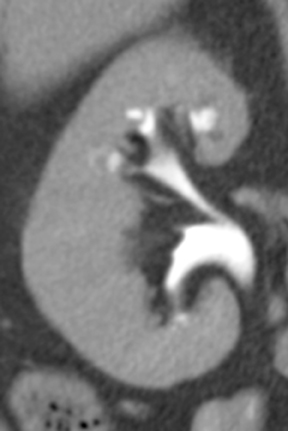
Small subepithelial inflammatory cysts in the renal pelvis and/or ureter characterize pyeloureteritis and ureteritis cystica, respectively.The cysts appear as multiple smooth filling defects most commonly in the proximal one-third of the ureter and are often seen in association with chronic urinary tract infections or stones.6,10(pp191-192),11(pt3,sec4,p10) Findings may be bilaterally symmetric or asymmetric or even unilateral.Involvement of the bladder is termed cystitis cystica. The radiolucent filling defects should be differentiated from multifocal uroepithelial carcinoma, in which they are usually not as numerous. Ureteral pseudodiverticulosis (Figure 4) is associated with chronic inflammation and urothelial neoplasms. Multiple small contrast-containing outpouchings are evident on excretory images. The significance of this finding is its reported association with an increased risk of urothelial carcinoma. 10(p89,p151)
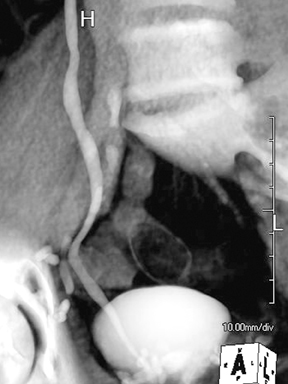
Ureteral strictures may be the result of prior surgery or instrumentation, stone passage, primary urothelial neoplasms, penetrating injuries, prior radiation therapy, ischemia, retroperitoneal fibrosis, endometriosis, metastatic tumor encasement, or an infectious disease (e.g., tuberculosis, schistosomiasis,etc.).10(pp92,96,183-184,201) CT urography may diagnose the focal wall thickening at the site of the stricture and demonstrate its cause. In contradistinction to malignant strictures, benign strictures should be smoothly tapered with little luminal irregularity. As a rule, proximal urinary tract dilation and obstruction coexist.
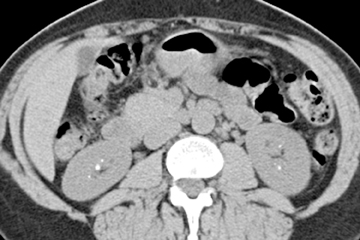
Traumatic injury to the ureter is most frequently iatrogenic, but it may result from blunt or penetrating traumatic injuries. Hematuria following traumatic injury may be an initial clinical clue. Intramural hematomas appear as wall thickening with stranding of the periureteral fat(Figure 5). There may be proximal dilatation if the ureteral lumen is narrowed. Rupture or tear of the ureter is identified on excretory imagesas excreted contrast accumulates outside of the collecting system and ureter (Figures 6 and 7). It should be noted, however, that the most reliable method to diagnose a suspected ureteral tear is a retrograde pyelogram.
Fibroepithelial polyps are benign mesodermal tumors of the ureter composed of a fibrovascular core lined by normal urothelium.6 Commonly solitary and in the proximal ureter, the tumor is usually identified as a smooth long filling defect that may be seen to move between the different acquisitions if it is attached by a long stalk.6 Nonetheless, the polyp may not be easily differentiated from urothelial malignancy and is therefore resected for pathological diagnosis.
Detection of urothelial carcinoma is arguably the primary role of CT urography, be it in patients with hematuria or those with a history of urothelial tumors of the bladder requiring surveillance of the upper tracts. Urothelial carcinoma, most commonly transitional cell carcinoma,most commonly affects males older than 60.11(pt3,sec3,p103) Risk factors include history of smoking, occupational exposure to certain dyes and plastics, prior cyclophosphamide and other medication use, and chronic infections, such as schistosomiasis.6,11(pt3,sec3,p104) Patients may report hematuria or flank pain, or present with obstructive uropathy. Various growth patterns have been described, including predominantly pedunculated or intraluminal growth and sessile with mural invasion.6,11(pt3,sec3,p103) Tumors may be localized, spread extensively along the urothelium, or present as a multifocal process throughout the urinary tract. If the tumor arises in the calyces or renal pelvis, differentiation from a central renal cell carcinoma may occasionally be difficult. Infiltrating urothelial carcinoma tends to invade the renal sinus fat and renal parenchyma as a soft tissue neoplasm of decreased attenuation compared to that of the normal kidney, usually without a significant change in the renal contour (Figure 8). If the tumor obstructs a calyx, the calyx may appear irregular or amputated. Lesions frequently demonstrate enhancement in the nephrographic phase and appear as filling defects (Figure 9) or as an irregular luminal contour in the excretory phase. Urothelial thickening often results from tumor spread.
Congenital anomalies of the kidneys and ureters
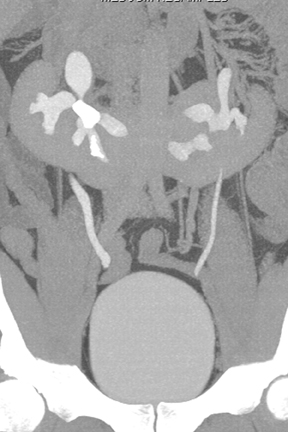
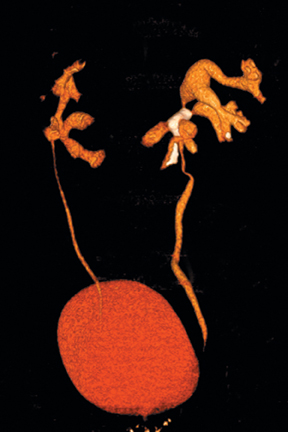
Congenital anomalies of the kidneys, including horseshoe kidney, renal ectopia with or without crossed-fusion,hypertrophied column of Bertin, and renal agenesis (sometimes with a genesis or cystic dilatation of the ipsilateral seminal vesicle), are all well depicted by CT urography.10(pp29-48),11(pt3, sec3,pp6-15) Anomalous kidneys may be complicated by duplicated ureters, stone disease, vesicoureteral reflux, traumatic injury, and ureteropelvic junction obstruction (Figure 10). CT urography is well suited to detect such complications.
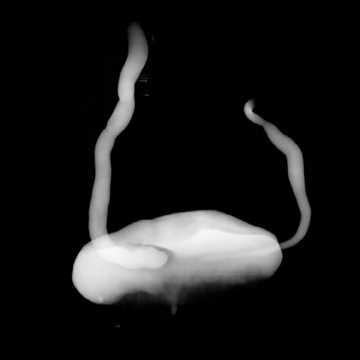
Congenital anomalies of the ureters include complete and partial duplication, ectopic ureteral insertion, and orthotopic or ectopic ureteroceles (Figure 11). Often, the ureter draining the upper pole moiety of a duplicated system drains into an ectopic ureterocele located inferomedial to the insertion of the lower pole ureter. Classically, the ureterocele of the upper pole ureter results in obstruction, while abnormal insertion of the lower pole ureter results in vesicoureteral reflux (Weigert-Meyer rule).
Diseases of the urinary bladder
CT urography is useful in detecting and characterizing multiple bladder abnormalities. Calculi within the bladder appear similar to those in the upper tract and are easily seen on noncontrast images. Often, stones are found at or near the ureterovesical junction, and it may be necessary to differentiate a stone lodged in the distal ureter from one that has passed into the bladder lumen and which is lying on the dependent wall. In these situations, the patient can be placed in the prone position and sections through the pelvis can be repeated. Stones lodged at the ureterovesical junction will not change position, while those free within the bladder will shift to the new dependent wall.
When hematuria is the indication for imaging, particularly in the setting of gross hematuria, intraluminal blood clots within the bladder usually can be differentiated from urothelial tumors. Prior to contrast administration, clots will be of soft tissue attenuation. After intravenous contrast injection, clots do not enhance, whereas urothelial tumors usually do.
Diverticula of the bladder are fairly common and appear as focal outpouchings of the bladder wall contiguous with the lumen. They maybe congenital, as in paraureteral (Hutch) and urachal diverticula, or acquired. Calculi may form within a diverticulum because of urinary stasis. Diverticula are also predisposed to infection and development of urothelial tumors.
Urinary bladder rupture may be seen in cases of pelvic trauma, although the recommended imaging method to detect a urinary bladder rupture is a cystogram, using direct instillation of contrast into the bladder and imaging with either conventional radiography or CT. It is important to correctly identify the type of bladder injury. Extraperitoneal rupture, the most common type, is treated conservatively with Foley or suprapubic catheter drainage. Intraperitoneal rupture requires immediate surgical repair. Mixed type injuries also need to be correctly identified. CTU and/or CT cystography can diagnose the type of bladder injury as well as posterior urethral ruptures. Furthermore, CTU is used to diagnose fistulae between the lower ureter or bladder and adjacent intestinal tract or vagina. Patients with bladder catheters should have their catheters clamped during the examination to achieve adequate bladder distention.
Urothelial tumors of the bladder, similar to those involving the collecting system or ureters, may have a variety of appearances. Superficial tumors may be undetectable, which is one of the reasons why cystoscopy remains the gold standard for evaluation of the bladder. Accumulating data, however, indicates that CTU is reliable in detecting bladder cancer.13,14 More advanced tumors may be polypoid, sessile, or present as focal or diffuse bladder wall thickening (Figure 12). Invasion into the perivesical fat may be revealed as infiltration or irregular fat-bladder wall interface.
Evaluation of the postoperative patient
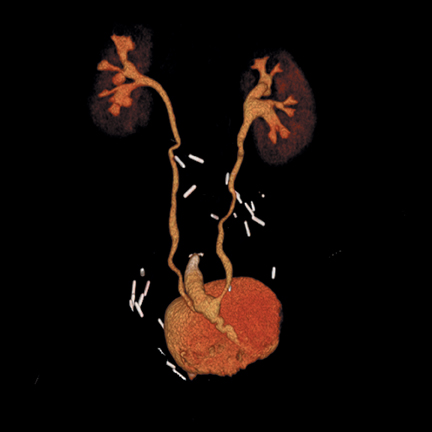
Superficial or noninvasive tumors are often treated locally, with cystectomy reserved for more advanced disease (muscle-invasive carcinomasor recurrent, extensive superficial disease). Many surgical techniques are available for urinary diversion following cystectomy and are created by fashioning a section of bowel into a conduit or reservoir to which the ureters can be anastomosed. Diversions may be formed utilizing an isolated loop of ileum to form a conduit (Bricker, incontinent diversion); a segment of colon to form a catheterizable reservoir (continent diversion); or an orthotopic neobladder (Studer pouch, continent) to which the urethra is anastomosed (Figure 13). These diversions may be imaged on CTU.15 Complications related to the surgery generally occur within the first 30 days and include hematoma, abscess, conduit/reservoir anastomotic leak,hydronephrosis, ischemia, and infection. The bowel anastomosis may leak, or a postoperative ileus or bowel obstruction may occur. Late complications include ureteral reflux with hydronephrosis, ureteral stricture, stone formation, pouch necrosis, and tumor recurrence.
RefeRences
- Silverman SG, Leyendecker JR, Amis ES. What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology. 2009;250:309-323.
- Nawfel RD, Judy PF, Schleipman AR, et al. Patient radiation dose at CT urography and conventional urography. Radiology. 2004;232: 126-132.
- Joffe SA, Servaes S, Okon S, et al. Multi-detector row CT urography in the evaluation of hematuria. Radiographics. 2003;23:1441-1456.
- Noroozian M, Cohan RH, Caoili EM, et al. Multislice CT urography: State of the art. Br J Radiol. 2004;77, Spec No 1:S74-S86.
- Nolte-Ernsting CC, Wildberger JE, Borchers H, et al. Multi-slice CT urography after diuretic injection: Initial results. Rofo. 2001;173:176-180.
- Kawashima A, Vrtiska TJ, LeRoy AJ, et al. CT urography. Radiographics. 2004;24 Suppl 1:S35-S54; discussion S55-S58.
- Kim S, Wang LL, Heiken JP, et al. Opacification of urinary bladder and ureter at CT urography: Effect of a log-rolling procedure and postvoiding residual bladder urine volume. Radiology. 2008; 247:747-753.
- Vrtiska TJ, Hartman RP, Kofler JM, et al. Spatial resolution and radiation dose of a 64-MDCT scanner compared with published CT urography protocols. AJR Am J Roentgenol. 2009;192:941-948.
- Ciaschini MW, Remer EM, Baker ME, et al. Urinary calculi: Radiation dose reduction of 50% and 75% at CT--effect on sensitivity. Radiology. 2009;251:105-111.
- Silverman SG, Cohan RH, eds. In: CT Urography: An Atlas. 1st ed. Philadelphia, Pa: Lippincott Williams and Wilkins; 2007.
- Federle MP, Jeffrey RB, Desser TS, et al. In: Diagnostic Imaging: Abdomen. Salt Lake City: Amirsys; 2004.
- Wasserman NF, La Pointe S, Posalaky IP. Ureteral pseudodiverticulosis. Radiology. 1985;155:561-566.
- Sadow CA, Silverman SG, O’Leary MP, et al. Bladder cancer detection with CT urography in an academic medical center. Radiology. 2008;249:195-202.
- Park SB, Kim JK, Lee HJ, et al. Hematuria: Portal venous phase multi detector row CT of the bladder--a prospective study. Radiology. 2007;245:798-805.
- Sudakoff GS, Guralnick M, Langenstroer P, et al. CT urography of urinary diversions with enhanced CT digital radiography: Preliminary experience. AJR Am J Roentgenol. 2005;184:131-138.
