Cosmetic Injection-induced Hypercalcemia
Images
Case Summary
An elderly patient presented with ∼5-year history of chronic hypercalcemia. Etiology remained unknown despite extensive radiology and laboratory work-up, including negative nuclear medicine bone and parathyroid scans, negative CT and MRI scans, and numerous laboratory tests. Relevant medical history includes a history of gluteal cosmetic injections more than five years prior.
Laboratory investigations were significant for an elevated creatinine level of 1.13 mg/dL (reference range: 0.8-1.2 mg/dL); elevated calcium level of 14 mg/dL (reference range: 8.4-10.2 mg/dL); 1,25 dihydroxy vitamin D level of 88 pg/mL (reference range: 19.9-79.3 pg/mL); 24hr urine collection 404 mg calcium (100-300 mg/24hr); low parathormone (PTH) level of 11pg/mL (reference range: 12-88 pg/mL); and high parathormone-related protein (PTHrp) at 11pmol/L (reference range: 0.0-2.3 pmol/L).
Imaging Findings
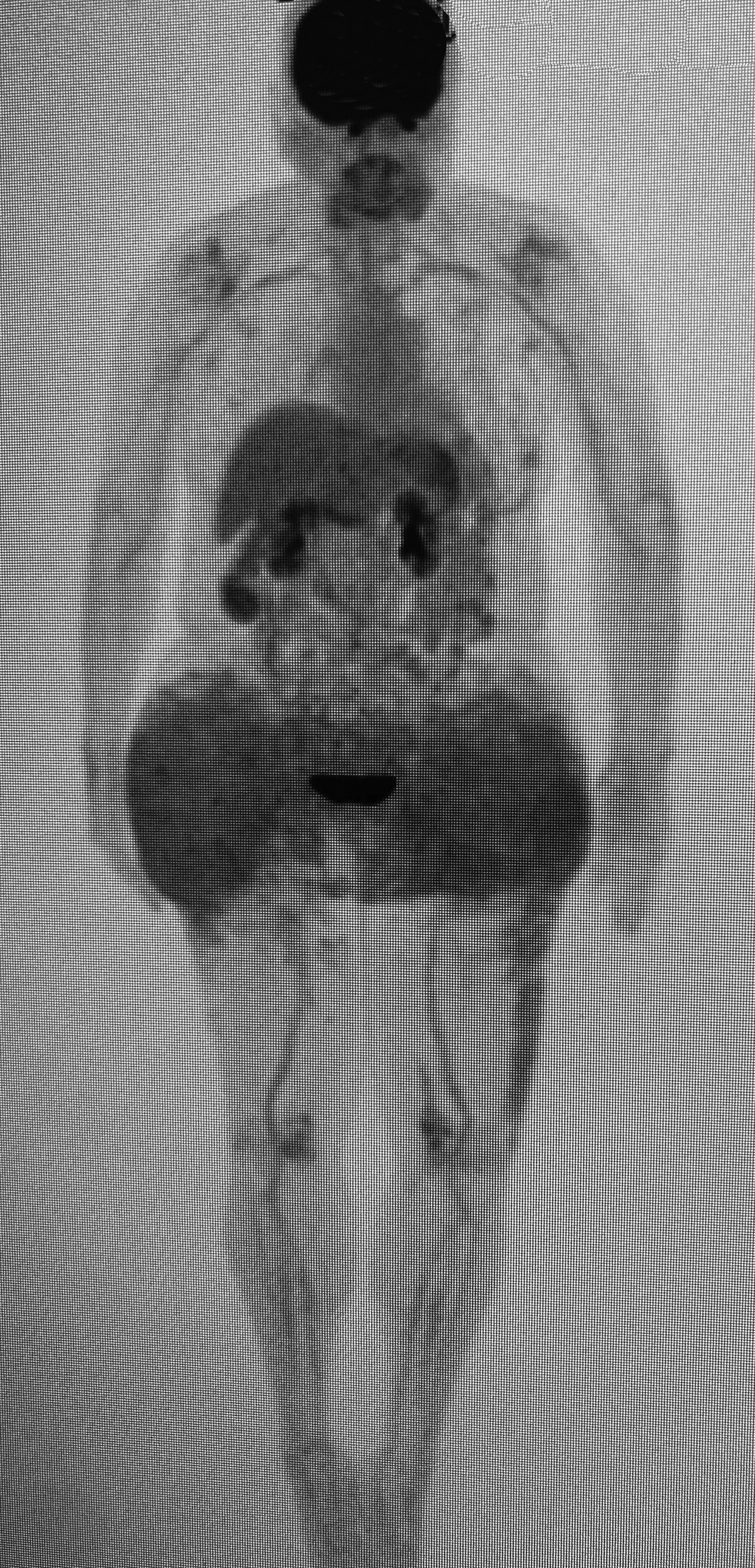
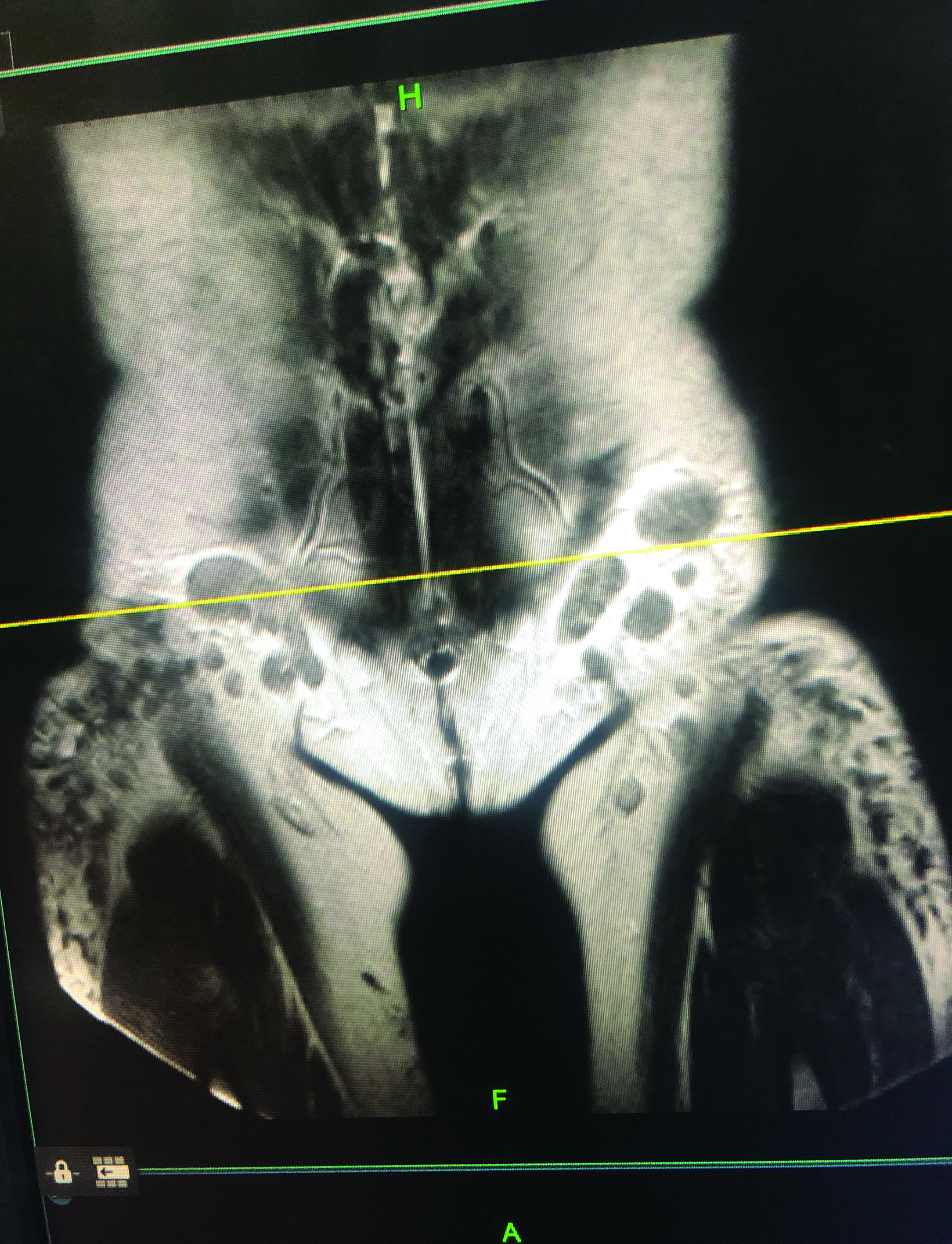
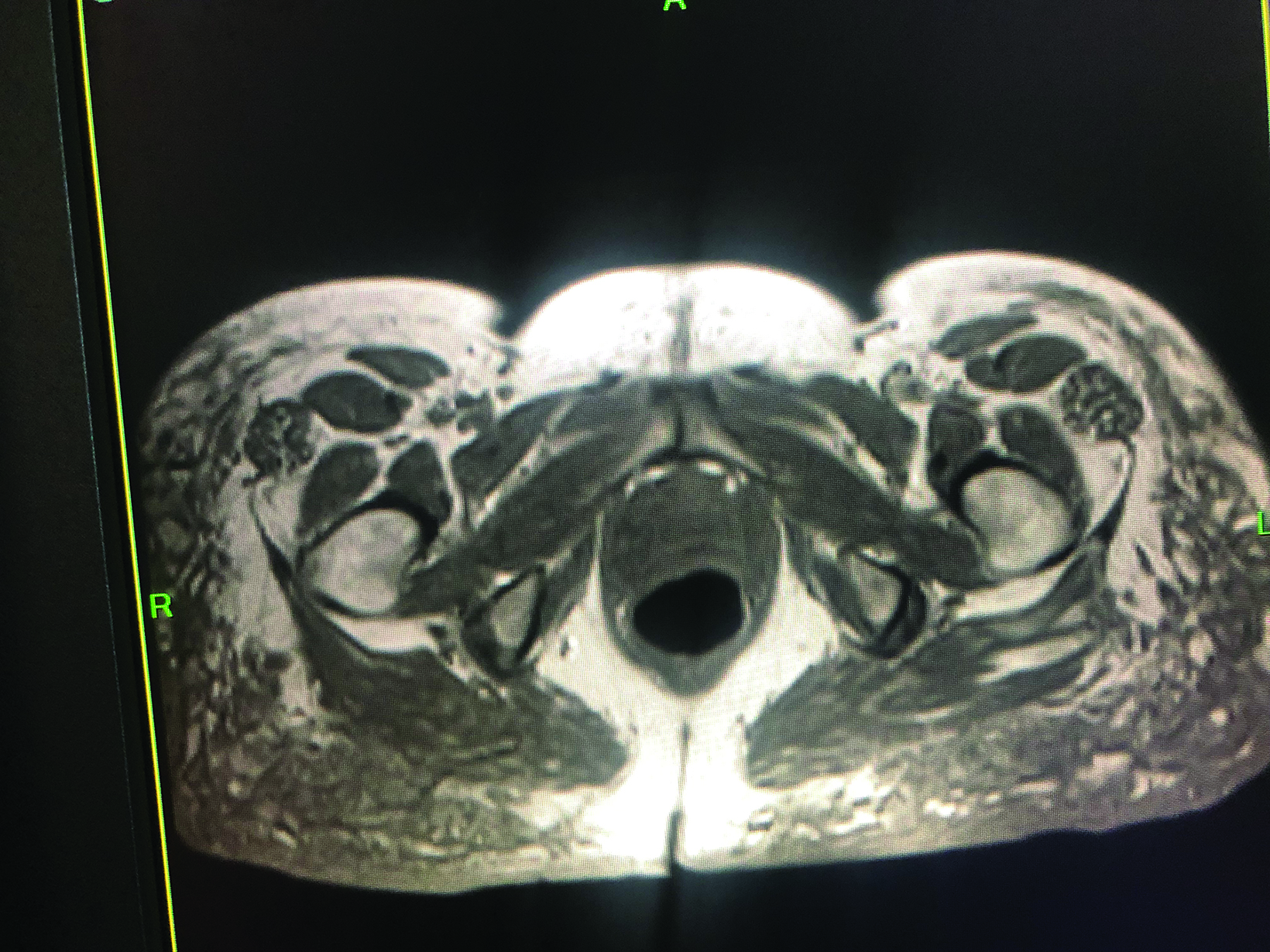
Positron emission tomography (PET)/CT maximum intensity projection image (Figure 1) demonstrated marked fluorodeoxyglucose (FDG)-avid uptake in the gluteal regions and thighs with associated mild inguinal adenopathy. Magnetic resonance imaging demonstrated nodular, low T1 and T2 masses in the subcutaneous fat and inguinal lymphadenopathy (Figure 2).
Diagnosis
Cosmetic injection-induced granulomas causing calcitriol-mediated hypercalcemia.
Discussion
Many different varieties of cosmetic fillers are used today, several of which are not approved for use by the US Food and Drug Administration (FDA). While collagen, hyaluronic acid, and polymethacrylate (PMMA) are approved for injection,1 liquid silicone injections were banned by the FDA in 1991 because of associated complications. Nonetheless, illicit usage of these injections remains high.2,3 The exact numbers are unknown, but complications from silicone injections are significant enough that there are calls for enhanced state-level enforcement of penalties.4
The first foreign body granuloma caused by a silicone injection was described as a “siliconoma” by Winer et al in 1964.5 The researchers described three cases of granuloma formation resulting from silicone injections and advised against itheir use. Foreign body granulomas associated with cosmetic injections may occur in up to 1% of all cases. Even when appropriately used, cosmetic injections may, albeit rarely, trigger calcitriol-mediated hypercalcemia. Silicone, as well as other cosmetic injections including PMMA and paraffin oil are used in plastic surgical procedures and can all cause granulomas that may induce hypercalcemia.6
Granulomatous conditions like sarcoidosis can lead to calcitriol-induced hypercalcemia. In these conditions, macrophages release 1-alpha-hydroxylase, which facilitates the conversion of inactive vitamin D to its active form. Calcitriol has an immunomodulatory action resulting in decreased T cell activity, most likely due to inhibition of IL-2 and γ-interferon.6 Under normal circumstances, renal 1-alpha-hydroxylase is responsible for producing calcitriol, an active form of vitamin D. High circulating calcitriol elicits negative feedback on 1-alpha-hydroxylase production and thus inhibits further calcitriol production. This regulatory pathway is lacking in macrophages, leading to uncontrolled calcitriol production.7
The radiologist who identifies cosmetic induced granulomas may be the first of the patient’s physicians to recognize them as a potential source of “idiopathic” hypercalcemia. This will aid in early diagnosis and may decrease the need for unnecessary medical tests.8
Conclusion
Cosmetic injections can cause foreign body granulomas resulting in life-threatening hypercalcemia, that may present years after initial injection. Cosmetic-induced granulomas which should be included in the differential diagnosis of causes of hypercalcemia, may first be recognized by radiologists. Hypercalcemia remains a diagnosis of exclusion after evaluating for and excluding malignancy, autoimmune disease, paraneoplastic syndromes, and other granulomatous diseases.
References
Citation
R G, M B. Cosmetic Injection-induced Hypercalcemia. Appl Radiol. 2023;(4):34-35.
June 23, 2023