Contrast-enhanced Ultrasound of Renal Masses
Images
SA-CME credit available at appliedradiology.org/SAM2
More than one-half of patients over age 50 have at least one renal mass. Up to 70% of these renal masses are discovered incidentally while undergoing an imaging examination for another indication. Most renal masses are benign simple cysts that can be classified using cross-sectional imaging alone. Conversely, most solid, contrast-enhanced renal masses, excluding inflammatory masses, vascular abnormalities, and pseudotumors, are regarded as malignant.1 However, there are a large variety of complicated cystic lesions, ranging from clearly benign to clearly malignant. These lesions are classified using the Bosniak system, which comprises four main categories: Bosniak 1, 2, 3, and 4.2,3
Contrast-enhanced ultrasound (CEUS) can be used to characterize simple and complicated cystic lesions and can provide information on kidney vascularity and perfusion. Kidney perfusion can be determined by using a microbubble contrast agent and contrast-specific imaging software. Microbubble contrast agents consist of encapsulated microscopic bubbles of gas, which oscillate when exposed to low-intensity ultrasound. They have a non-linear response to low MI (MI<0.4) ultrasound, which allows for a contrast-only image with excellent background tissue subtraction. When exposed to high-intensity ultrasound, these bubbles burst, giving a large acoustical signal that allows for exceptional representation of the vascularity and perfusion of the kidney lesion.
CEUS has several advantages over other modalities such as computed tomography (CT) and magnetic resonance imaging (MRI). The diameter of the microbubble is between 3 and 5 micrometers, which is much larger than contrast agents used for MRI and CT. The increased size of the contrast agent prevents it from escaping through the endothelium, keeping it inside the blood vessels. In addition, microbubble contrast agents do not cause nephrotoxicity, and they have a half-life of approximately 5-10 minutes, allowing for multiple injections in a single session.2 Lastly, CEUS does not require ionizing radiation. Because of these advantages, CEUS is becoming increasingly popular clinically.
The Bosniak classification for CT and MRI has been recently revised.3 A Bosniak classification for CEUS has not been developed.4 The Bosniak system is intended for cystic renal masses after infectious, inflammatory, and vascular etiologies, as well as necrotic solid masses, are excluded. The classification of cystic renal masses using the Bosniak classification should not be made on a noncontrast study. Therefore, in patients with contraindications for CT or MRI contrast, CEUS may be the examination of choice for renal mass classification.
This review will cover the different types of renal masses, including simple and complicated cystic lesions, and provide a description and image examples of each.
Benign Renal Masses Pseudotumors
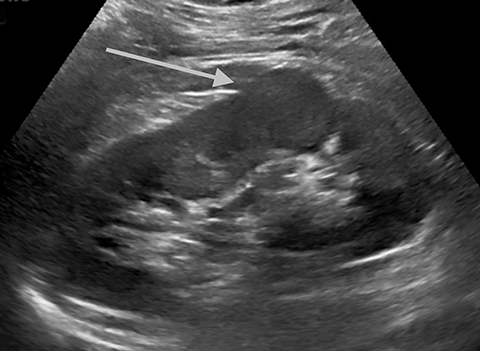
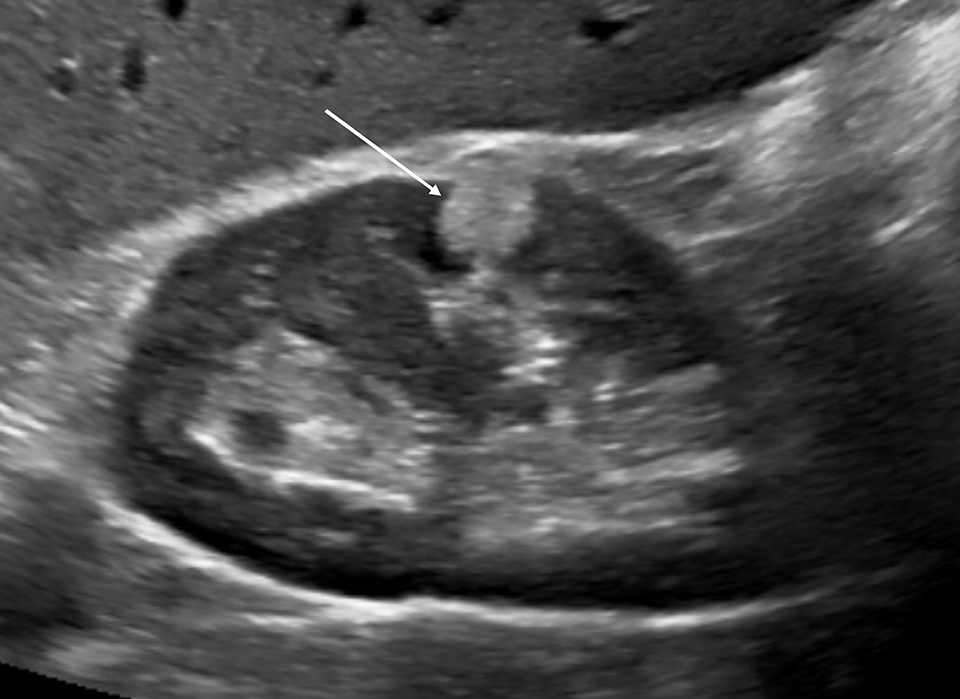
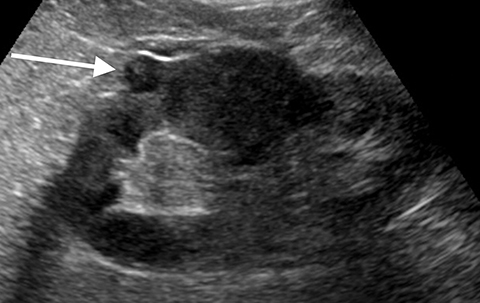
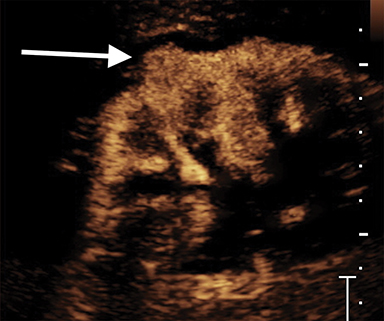
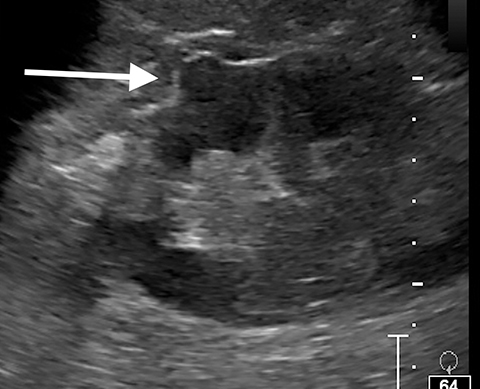
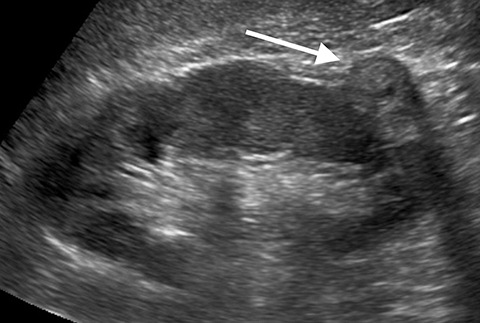
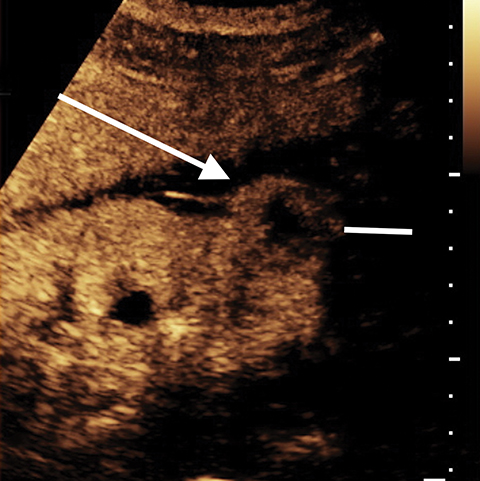
Pseudotumors (Hampton hump, fusion abnormalities) have equal enhancement in all phases of enhancement to normal renal cortex and have the presence of a medullary pyramid which has less enhancement than normal renal cortex. Pseudotumors are typically diagnosed on renal ultrasound, however further imaging may be performed in order to ensure that no concerning pathology is present. CT and MRI are both useful for ensuring the mass is benign; however, CEUS may represent a cheaper and safer alternative in confirming the diagnosis (Figure 1).
Pyelonephritis
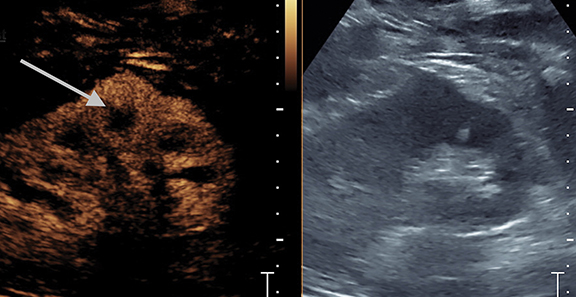
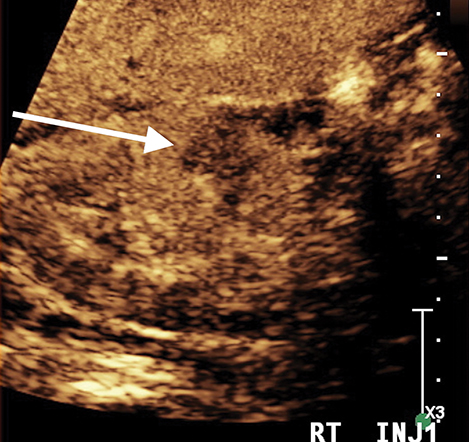
Complications of pyelonephritis may include acute papillary necrosis, impaired renal function, renal abscess, sepsis, and premature labor in pregnant women.5 Urinalysis, as well as CT, ultrasound, or magnetic resonance imaging can be used to help diagnose pyelonephritis.5 Imaging is usually not required for acute uncomplicated pyelonephritis. CEUS has a role to play as part of the initial US assessment, in patients with renal obstruction or impairment in whom CT and MRI contrast agents could have potential deleterious effects on renal function. Focal pyelonephritis may appear as a focal hypoechoic or echogenic area and may produce a mass-like lesion mimicking a tumor. The area may be hypovascular on CEUS compared to adjacent normal kidney. CEUS shows an abscess as a central defect with rim enhancement (Figure 2).
Angiomyolipoma
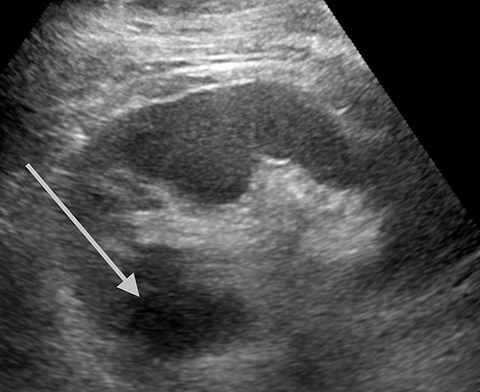
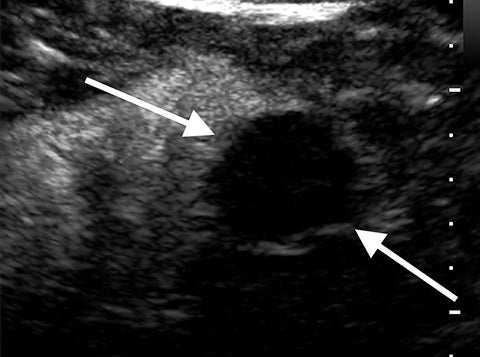
Angiomyolipoma (AML) appears as a uniformly echogenic mass on B-mode ultrasound. AMLs display less enhancement than the typical renal cortex; usually in a more peripheral distribution.2 Echogenic renal cell carcinomas tend to have marked enhancement more than renal cortex in the arterial phase. In one study all AMLs had peripheral mild enhancement.2 Obtaining an unenhanced CT or MRI to confirm macroscopic fat is recommended to confirm the diagnosis of AML. There are limited reports on fat-poor AMLs which may have more overlap with echogenic renal cell carcinomas (Figure 3).
Benign Renal Cysts
Simple Renal Cyst
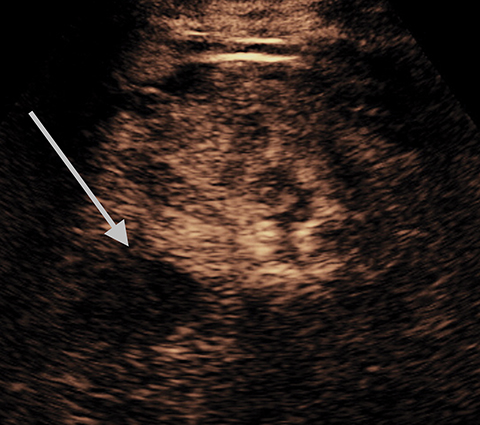
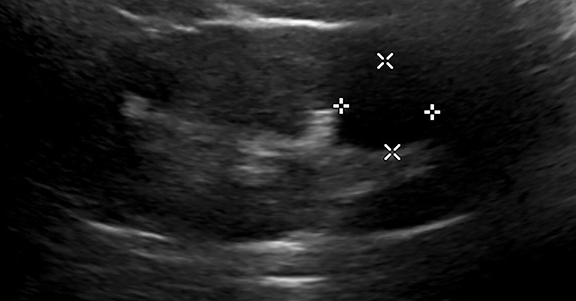
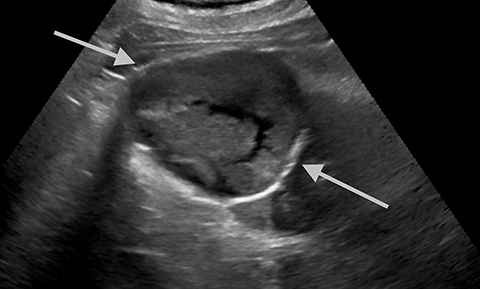
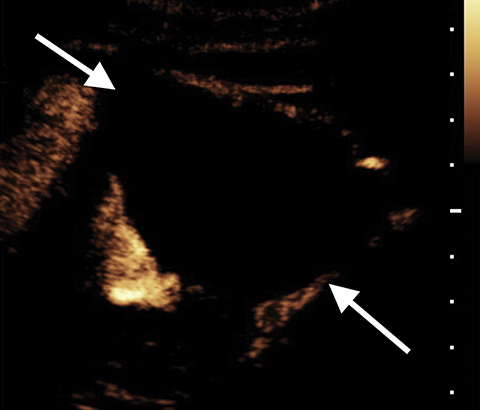
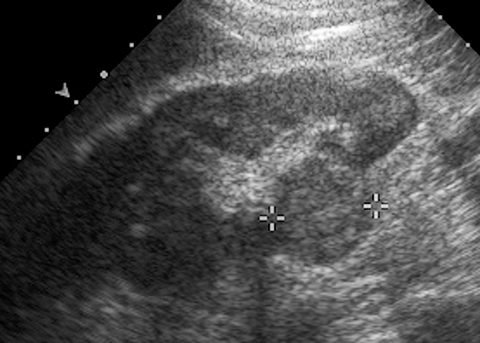
A Bosniak 1 lesion, a simple renal cyst (SRC), is characterized by a thin or imperceptible wall containing attenuated fluid.6 These cysts are typically asymptomatic, and no follow up is necessary in patients with normal renal function.6 SRCs are known to be non-enhancing when observed using CT imaging. By contrast, CEUS can be used to determine vascularity as well as to show nodular protuberances.6 These features can be used to better differentiate between benign and malignant renal cysts (Figure 4).
Bosniak Category 2 and 3 – Benign
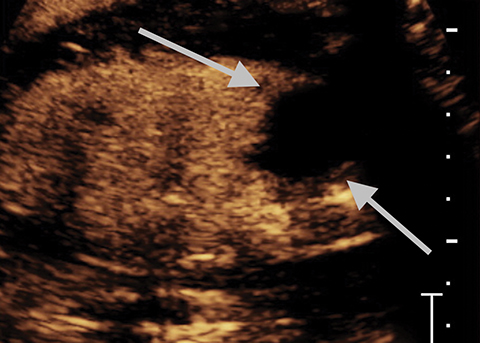
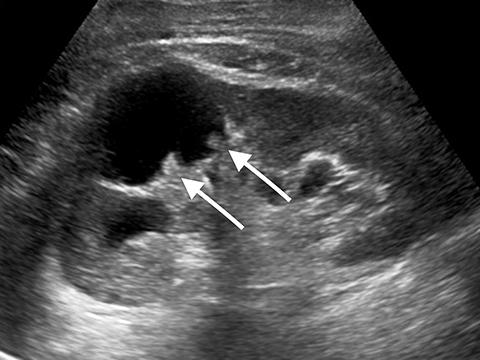
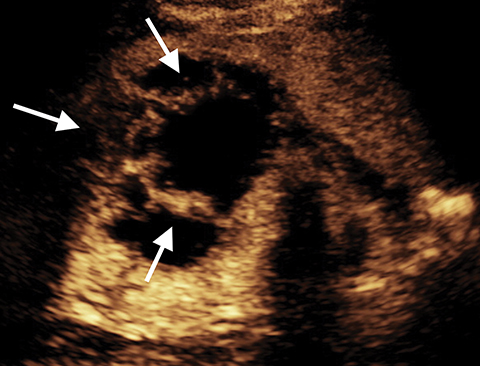
Lesions classified as Bosniak 2 are characterized by either thin, calcified walls, or by single thin septations (<1-mm).2 Only in rare cases are Bosniak 2 lesions found to be potentially malignant.2 As such, these lesions can typically be considered benign. In the case of Bosniak 2F renal lesions, a subset of Bosniak 2 lesions characterized by thick calcification of the walls or by thickened or enhanced septa, an increased malignancy rate of approximately 5% necessitates additional follow-up after imaging.2 Bosniak category 3 lesions are characterized by multiple thick septations as well as by mural modules. Bosniak 3 lesions can be hyperdense when imaged using computed tomography (CT).2 Approximately half of all observed Bosniak 3 lesions in a series were found to be benign, and half were found to be malignant.7,8 In benign Bosniak 2 or 3 lesions there is no enhancement within the cyst or the cyst wall. In a large study the lack of enhancement of the septations or solid components in a cyst had an NPV of 100% (Figure 5).2
Malignant Renal Masses
Bosniak Category 2 and 3 – Malignant
In malignant Bosniak 2 and 3 lesions, the septations or internal components show enhancement with CEUS (This finding has a PPV of 95%.2]). Diagnosis of Bosniak category 3 lesions as malignant or benign may require surgical resection or percutaneous biopsy on CT or MRI characterization.2 In the case of surgical resection, calculated malignancy rates have ranged between 31% and 100%.1,2 With the high NPV of CEUS biopsy or surgery are probably not required. Further studies are needed to determine if a CEUS non-enhancing indeterminate renal mass will not require additional follow-up. Some infected cysts demonstrate enhancement of a thickened cyst wall and will be a false positive on CEUS (Figure 6).
Bosniak Category 4
Some complicated cystic masses may appear solid. If these are benign, they can easily be diagnosed with CEUS as there is no enhancement of the mass. Solid masses with enhancement have a very high probability of being malignant. Oncocytomas, which are benign, will demonstrate enhancement on CEUS and therefore be false positive cases. These are often surgically removed as some pathologists will not classify them as benign unless they examine the entire specimen.
Renal Cell Carcinoma
Approximately 3% of all adult malignancies and more than 90% of all renal neoplasms are a result of renal cell carcinoma (RCC).9 Over 12,000 deaths in the United States resulted from RCC in 2005.9 Despite its prevalence, most diagnoses remain incidental, discovered during imaging for other complications. CEUS has been used to diagnose RCC when neither MRI nor CT have indicated the presence of a renal mass.9 CEUS and CEUS-guided radiofrequency ablation (RFA) can be used to diagnose and treat, respectively, patients with reduced renal function as well as those whose circumstances require minimally invasive operation.9
Clear cell RCC (ccRCC) cases comprise between 75% and 80% of all RCCs.10 It is believed to develop from the proximal tubule, presenting as a highly vascularized tumor several centimeters in size.10,11
Papillary RCC may be missed on CT and MRI, owing to mild enhancement using these modalities. However, it can be identified using CEUS enhancement in the arterial phase.
Echogenic RCC usually demonstrates marked rapid enhancement more than the renal cortex in the arterial phase. They can be distinguished from AML as AML tend not to have marked enhancement and usually patchy enhancement as the areas of fat do not enhance significantly.
All renal cell carcinomas demonstrate enhancement on CEUS. Papillary RCCs tend to have milder enhancement and washout and often appear as complicated cysts on enhanced CT and MRI due to contrast timing issues. As a real-time imaging technique, CEUS will reveal enhancement in the arterial phase, confirming the lesion is not a benign lesion. Clear cell RCCs and echogenic RCCs tend to have marked arterial enhancement and may have washout. Necrotic areas within the RCC will not demonstrate enhancement (Figure 7).
Transitional Cell Carcinoma
Transitional Cell Carcinoma (TCC) is most commonly found in the bladder, though in rare cases TCC can be found in the renal pelvis or ureter.12 TCC accounts for approximately 95% of all malignant tumors in the epithelial lining of the urinary tract.13 TCC is known for its high rate of recurrence, as well as its multifocality.13 For these reasons, it is important to closely monitor the urinary system following detection of TCC.
Lymphoma
Renal lymphoma typically occurs as part of multisystemic lymphoma or as a result of tumor recurrence.14 Consequently, imaging often reveals multiple hypodense masses.14 Almost half of all cases of renal lymphoma are discovered during autopsy, while approximately 5% are detected using CT imaging.14
Monitoring of Interventional Procedures
CEUS can be used to monitor interventional procedures such as radiofrequency ablation and cryoablation.15 CEUS has a short enhancement life-time of about 5 minutes and multiple injections can be used during the procedure. CEUS monitoring of radiofrequency and cryoablations can be performed during the procedure to access if adequate ablation (no enhancement of the tumor) has occurred (Figure 8). It can also be used to guide biopsies and avoid areas of necrosis.
Discussion
CEUS is a very sensitive and specific method for characterizing renal masses. In one series of over 1000 renal masses CEUS had a sensitivity of 100%, specificity of 96.6%, PPV of 91.5%, and NPV of 100%. It has improved visualization of some features compared with CECT and contrast enhanced MRI due to its thin slice thickness (no volume averaging), real-time assessment of vascularity (can identify areas of enhancement that may be missed due to timing on CT and MRI) and superb background tissue suppression (allowing visualization of amounts of contrast enhancement). The lack of ionizing radiation, ability to use in renally impaired patients, and patients with renal obstruction are also advantages. The ability to use multiple injections is also helpful in performing biopsies and ablation procedures. The addition of CEUS to an imaging department is another tool in the toolbox to improve patient care.
References
- Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008; 249(1): 16–31
- Barr RG, Peterson C, Hindi A. Evaluation of Indeterminate Renal Masses with Contrast-enhanced US: A Diagnostic Performance Study. Radiology. 2014; 271(1): 133-142.
- Silverman SG, Pedrosa I, Ellis JH, Hindman NM, Schieda N, Smith AD, et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology. 2019; 292:475-488.
- Barr RG. Is There a Need to Modify the Bosniak Renal Mass Classification With the Addition of Contrast-Enhanced Sonography? J Ultrasound Med. 2017; 36:865-868.
- Patient. Pyelonephritis. https://patient.info/pdf/2689.pdf. Accessed June 9, 2019.
- Bell DJ, Morgan MA, et al. Renal cyst. Radiopaedia. 2019. https://radiopaedia.org/articles/renal-cyst-1?lang=us. Accessed June 5, 2019.
- Siegel CL, McFarland EG, Brink JA, Fisher AJ, Humphrey P, Heiken JP. CT of cystic renal masses: analysis of diagnostic performance and interobserver variation. Am J Roentgenol. 1997;169(3): 813–818.
- Aronson S, Frazier HA, Baluch JD, Hartman DS, Christenson PJ. Cystic renal masses: usefulness of the Bosniak classification. Urol Radiol. 1998; 13(2): 83–90.
- Sanchez K, Barr RG. Contrast-Enhanced Ultrasound Detection and Treatment Guidance in a Renal Transplant Patient with Renal Cell Carcinoma. Ultrasound Quarterly. 2009; 25(4): 171-173. Doi: 10.1097/RUQ.0b013e3181b4f9cf
- Cairns P. Renal Cell Carcinoma. Cancer Biomark. 2011; 9(1-6): 461–473.
- Nabi S, Kessler ER, Bernard B, Flaig TW, et al. Renal cell carcinoma: a review of biology and pathophysiology. F1000Research. 2018; 7: 307. Doi: 10.12688/f1000research.13179.1
- Cancer Research UK. Transitional cell cancer of the kidney or ureter. https://www.cancerresearchuk.org/about-cancer/kidney-cancer/stages-types-grades/types-grades/transitional-cell. Accessed June 10, 2019.
- Vikram R, Sandler CM and Ng CS. Imaging and staging of transitional cell carcinoma: part 1, lower urinary tract. Am J Roentgenol. 2009; 192(6): 1481-1487. Doi: 10.2214/AJR.08.1318
- Urban BA, Fishman EK. Renal lymphoma: CT patterns with emphasis on helical CT. RadioGraphics. 2000; 20(1): 197-212.
- Lackey L, Peterson C, Barr RG. Contrast-Enhanced Ultrasound-Guided Radiofrequency Ablation of Renal Tumors. Ultrasound Quarterly. 2012; 28(4): 269-274. Doi: 10.1097/RUQ.0b013e318274de66
Citation
J B, C P, RG B,. Contrast-enhanced Ultrasound of Renal Masses. Appl Radiol. 2020;(6):10-16.
November 6, 2020