Congenital Diaphragmatic Hernia
Images
Case Summary
A neonate presented with diminished breath sounds in the left chest. The infant’s mother did not receive prenatal care.
Imaging Findings
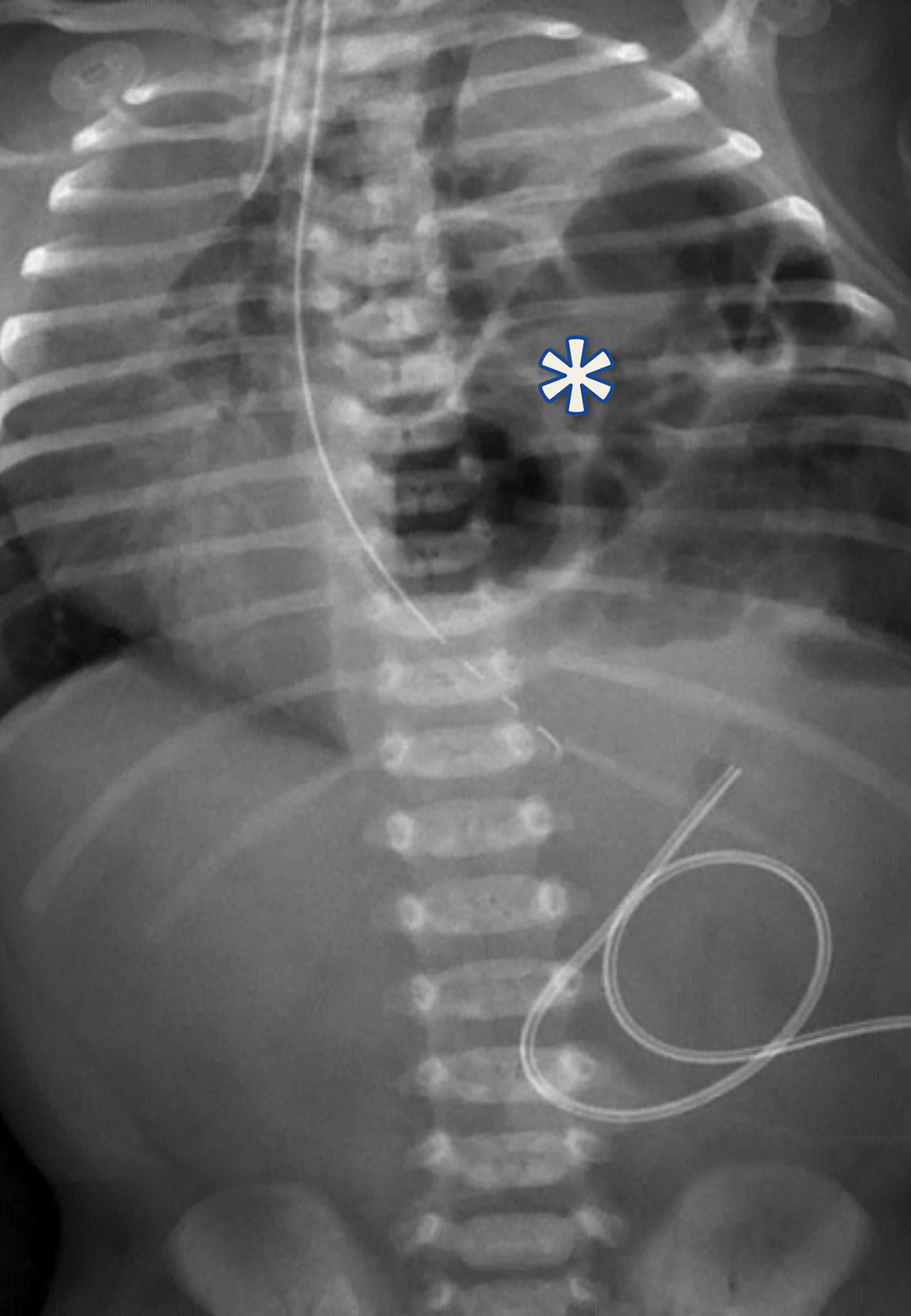
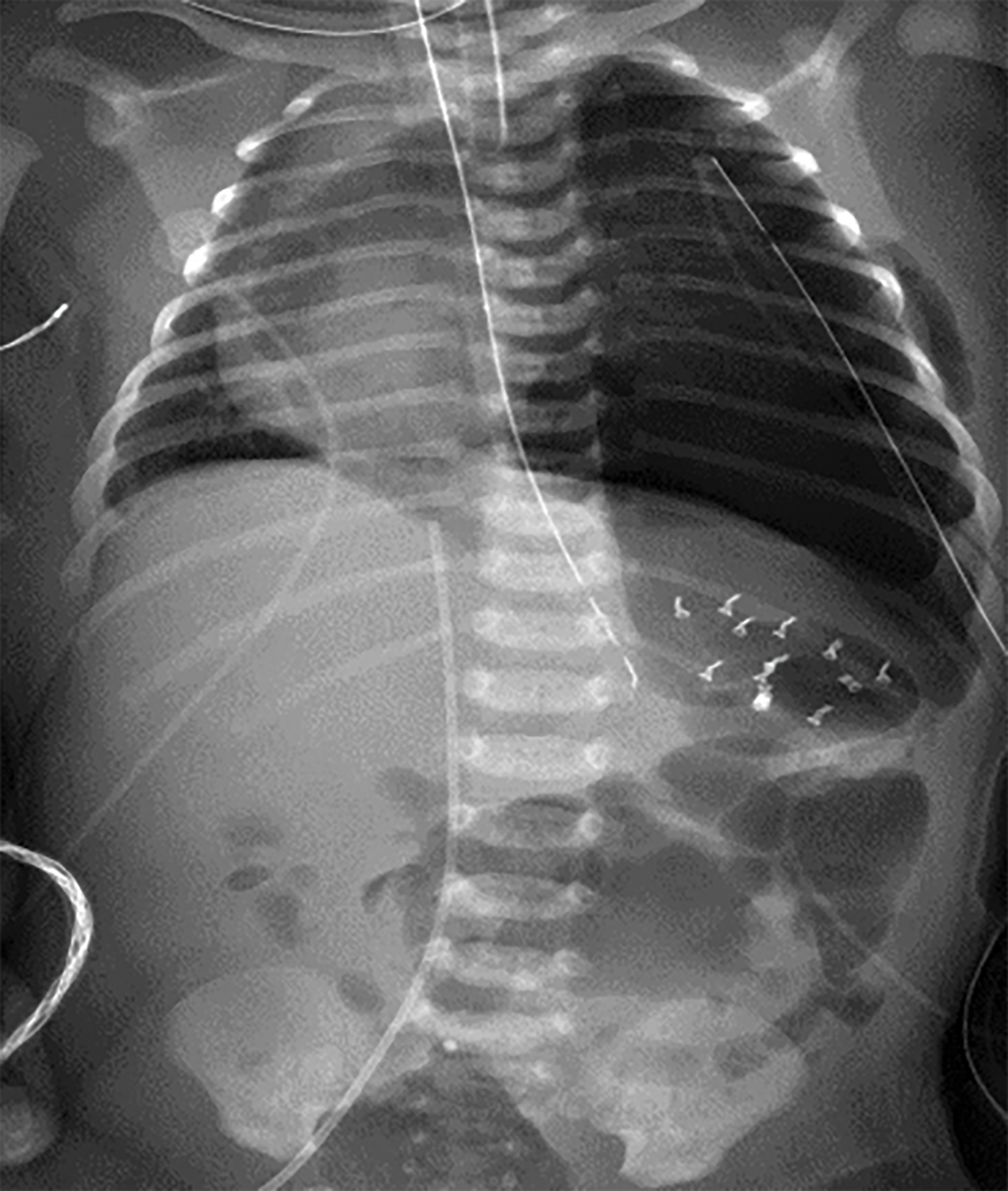
The initial chest radiograph (Figure 1) showed herniated loops of bowel in the left hemithorax. The herniated contents caused mass effect, shifting the heart and mediastinum from left to right. The left hemidiaphragm was not visible. They underwent repair with a good postoperative result (Figure 2).
Diagnosis
Congenital diaphragmatic hernia (CDH).
The differential diagnosis for bubbly lucencies in the neonatal chest includes congenital pulmonary airway malformation, loculated pneumothorax or pneumomediastinum, and pleuropulmonaryblastoma.1,2
Discussion
Congenital diaphragmatic hernia is an anomaly that occurs in 2.3-2.8 per 10,000 births.3 The condition is associated with a variable diaphragmatic defect ranging from diaphragmatic thinning to complete absence of the hemidiaphragm.3 Lung development is often affected, and patients with CDH may have pulmonary hypoplasia and associated pulmonary hypertension.3
Classically, a CDH is a Bochdalek-type hernia, most commonly occurring in the posterolateral diaphragm (70%) and on the left side (85%).1 It occurs because of inadequate closure of the posterior pleuroperitoneal membrane.3
CDH is usually diagnosed prenatally on a second- or third-trimester ultrasound. The diagnosis relies on observing abdominal contents within the chest and seeing the fluid-filled stomach posterior to the heart in the four-chamber view.1 While most cases are diagnosed via prenatal ultrasound, up to 11% are diagnosed postnatally.1
Fetal magnetic resonance imaging (MRI) allows radiologists to calculate morphometric features and provide useful prognostic information in the setting of CDH. Fetal lung volumes are the most important predictor of long-term survival.1 The prenatal observed/expected lung-to-head ratio is used for prognostic information and to select patients for fetal therapy.8 An observed/expected ratio < 25% indicates severe lung hypoplasia, while ratios of 25-35% indicate moderate hypoplasia.8 Fetuses with an observed/expected lung-to-head ratio between 25% and 35% have a 50-60% chance of survival.8
Postnatally, CDH patients may present with a barrel-shaped chest, absence of breath sounds on the affected side, a scaphoid abdomen, shifted cardiac sounds, and bowel sounds in the chest.4
Chest and abdominal radiographs often show an opacified hemithorax in the immediate postnatal period before distal bowel gas is present. As bowel gas progresses distally, bubbly lucencies fill loops of bowel. The herniated abdominal contents cause contralateral shift of the heart and mediastinum.5 Cross-sectional imaging may be used to more clearly visualize the associated anatomical defects in cases of suspected CDH and help to confirm the diagnosis.5
Whether the pulmonary hypoplasia associated with CDH stems from the diaphragmatic defect or an underlying issue with pulmonary development is unknown, but a “dual-hit” hypothesis based on animal models has been proposed.6 Although the underlying cause of pulmonary hypoplasia is poorly understood, upward-herniating abdominal contents in CDH are known to compress the ipsilateral lung, affecting terminal bronchiole, alveoli, and pulmonary vascular development.4 Additionally, a diaphragmatic defect causes a loss of negative pressure in the intrathoracic cavity, affecting lung development.5
Recent studies have sought to stratify CDH patients based on mortality risk. Features such as a very low birth weight, low 5-minute Apgar score, cardiac or chromosomal anomalies, and supra-systemic pulmonary hypertension help to separate patients into low (10%), intermediate (20%), or high (50%) risk of mortality before discharge from the neonatal intensive care unit.9
Several different in utero approaches have been used to treat CDH in an attempt to promote lung growth. One of the most promising is fetal endoscopic tracheal occlusion (FETO).7 By occluding the trachea with a balloon from about 26 weeks to 34 weeks of gestation, FETO causes a local increase in volume of fetal lung fluid which, in turn, causes lung growth.7
Infants with CDH are intubated and managed medically in preparation for corrective survery.6 Typically, surgery is delayed until the neonate is physiologically stable, usually within a week of delivery.4 Management includes treatment of accompanying medical issues such as pulmonary hypertension, a major cause of mortality in these patients. In those with severe CDH, additional therapies such as ECMO may be utilized to help provide oxygen to the end-organs.4 Once the patient is stable, the CDH may be surgically closed through primary or patch repair using an open or minimally invasive technique.4 Current data suggests that open surgical repair may lead to fewer postoperative complications.4
Patients treated for a CDH are at increased risk for long-term development of pulmonary disease related to pulmonary hypertension and pulmonary hypoplasia. Other long-terms effects include gastrointestinal reflux, poor growth, neurodevelopmental delay and behavioral disorders, sensorineural hearing loss, and chest wall deformities.4
Conclusion
Congenital diaphragmatic hernia is a developmental defect often detected prenatally, with clinical symptoms presenting soon after birth. Imaging plays a key role in the diagnosis of CDH. In this case, CDH was diagnosed postnatally upon presenting with diminished left-chest breath sounds, and successfully repaired with surgery.
References
- Marlow J, Thomas J. A review of congenital diaphragmatic hernia. Australas J Ultrasound Med. 2013;16(1):16-21.
- Zobel M, Gologorsky R, Lee H, Vu L. Congenital lung lesions. Semin Pediatr Surg. 2019;28(4):150821.
- Gaxiola A, Varon J, Valladolid G. Congenital diaphragmatic hernia: an overview of the etiology and current management. Acta Paediatr. 2009;98(4):621-627.
- Leeuwen L, Fitzgerald DA. Congenital diaphragmatic hernia. J Paediatr Child Health. 2014;50(9):667-673.
- Taylor GA, Atalabi OM, Estroff JA. Imaging of congenital diaphragmatic hernias. Pediatr Radiol. 2009;39(1):1-16.
- Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156(4):1299-1306.
- Kardon G, Ackerman KG, McCulley DJ, et al. Congenital diaphragmatic hernias: from genes to mechanisms to therapies. Dis Model Mech. 2017;10(8):955–970. doi:10.1242/dmm.028365
- Cordier AG, Russo FM, Deprest J, Benachi A. Prenatal diagnosis, imaging, and prognosis in Congenital Diaphragmatic Hernia. Semin Perinatol. 2020;44(1):51163.
References
Citation
JW L, RB T, DJ A, CM S, AJ T. Congenital Diaphragmatic Hernia. Appl Radiol. 2021;(6):8-10.
November 5, 2021