Clinical Utility of Barium Sulfate Products: Formulation Determines Appropriate Use
Images

To receive CE/CRA credits click here.
Barium (Ba) is an alkaline earth metal found in the second column of the periodic table.1 In pure form, it is a soft, silvery-white metal with a slight golden shade.1 Based on its atomic number (56), barium is excellent for blocking X-rays of the energies used in medical imaging. However, barium is too reactive to exist as a pure metal in nature1 and, therefore, cannot be administered in pure form for the purposes of medical imaging.
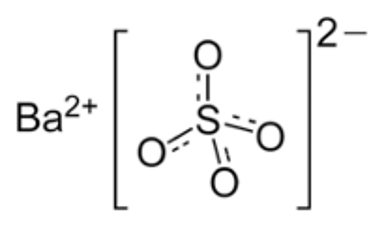
Barium sulfate (BaSO4) is a common, stable, inorganic, insoluble mineral salt, used industrially as a filler for rubber, plastics, and resins2 (Figure 1). Although difficult to dissolve in water, BaSO4 can be suspended in water with the help of additives, providing radiodensity (ie, radio-opacity) and physical density (g/mL) to the suspension. Concentrated suspensions ranging from 40% up to 240% weight-to-volume (w/v) can be used in fluoroscopy and radiography for a variety of clinical applications. To use BaSO4 for computed tomography (CT), a technology that has a very exquisite discrimination of radiodensities, the suspension has to be greatly diluted, to approximately 2% w/v. Preparations in that low concentration range are made and sold for several specialized abdominal CT imaging procedures.
In addition to BaSO4, barium-containing contrast media have a number of additional ingredients (almost all common food additives) that provide the important physicochemical properties of each product. Here we describe the purpose of each ingredient/additive found in BaSO4 contrast media products currently available for fluoroscopic and radiographic examinations. In addition, we review the role of these barium products in both the anatomic radiographic evaluation of the gastrointestinal (GI) tract and in evaluation of the physiology of swallowing. Finally, we review barium preparations used in CT applications.
Barium Sulfate Formulations for Radiographic Procedures
BaSO4 itself is inert and not absorbed from the GI tract. It is present in contrast media to provide radio-opacity, which is determined by the BaSO4 concentration, and physical density. It has no pharmacologic effects, nor can it cause an allergic reaction. However, if BaSO4 leaks from the GI lumen, it can cause mediastinitis, peritonitis, or retroperitonitis.3 Importantly, these adverse clinical events result from a severe granulomatous reaction,4 and therefore are distinct from the concepts of both “hypersensitivity” and “toxicity.”
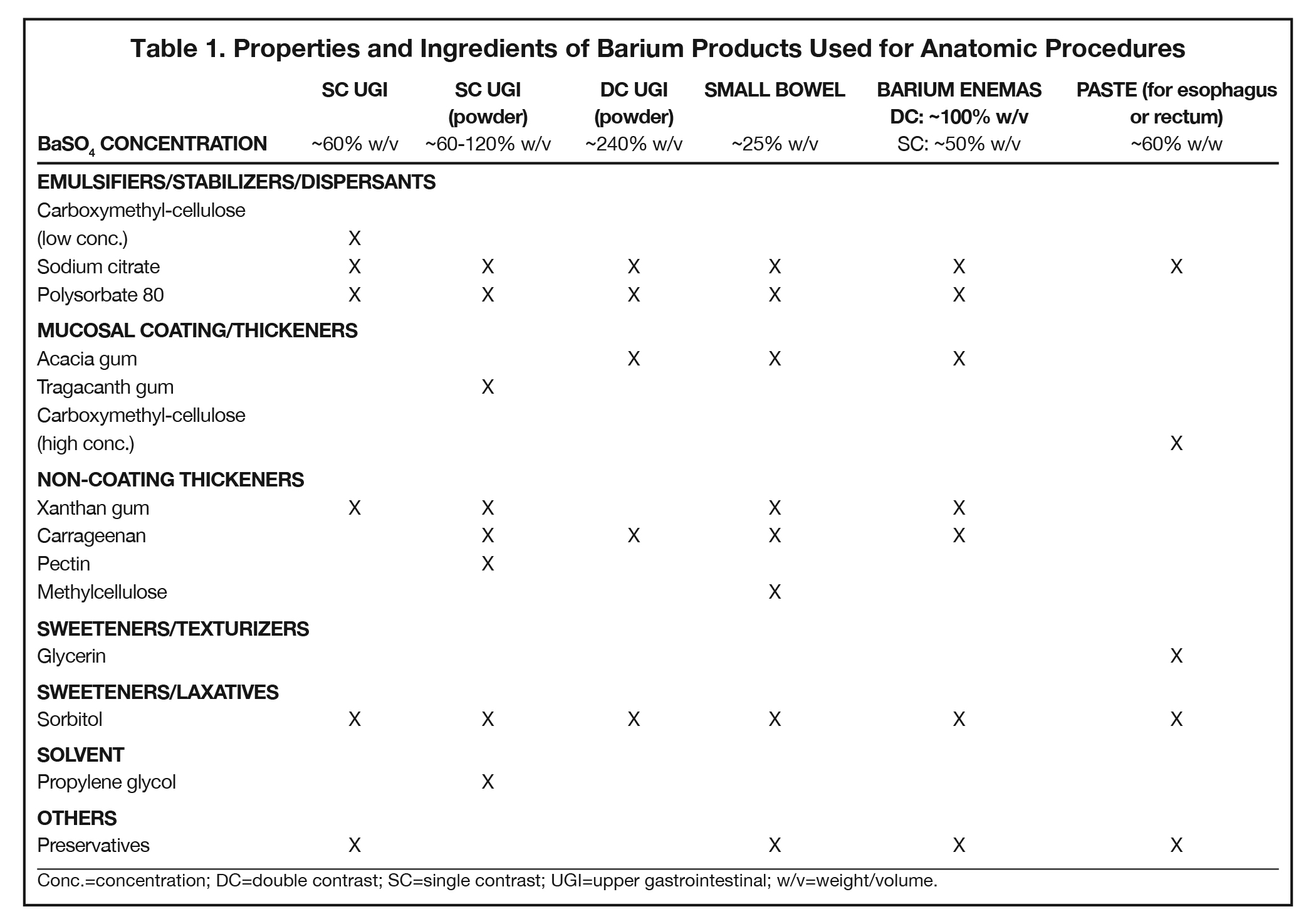
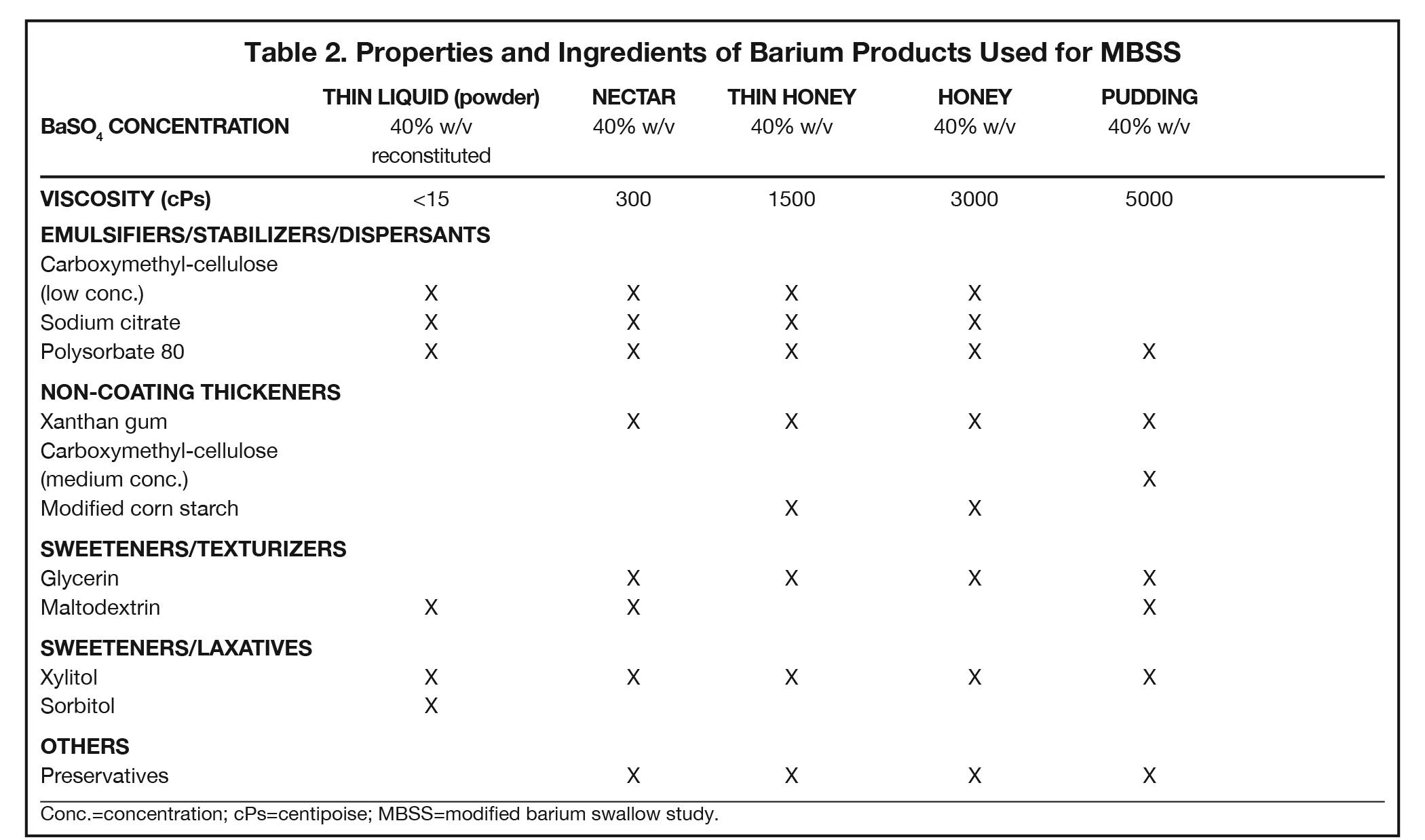
Additives present in BaSO4 contrast media formulations include emulsifiers, stabilizers, and dispersants to keep the BaSO4 particles smoothly suspended in water, without which the BaSO4 particles would simply sink to the bottom and clump. Mucosal-coating thickeners provide stickiness, while non-coating thickeners increase viscosity. Inclusion/exclusion of such thickeners is essential, as mucosal coating is vital for some applications (eg, double-contrast GI imaging), while detrimental to others (eg, modified barium swallow study [MBSS] to evaluate swallowing physiology, in which precise viscosities are critical). Finally, additives are included to ensure that BaSO4 products are palatable, readily eliminated, non-foaming, and long-lasting. Here we review the additives that provide each of these properties, to provide a better understanding of how they contribute to clinical utility of the various barium-containing contrast media (Tables 1 and 2).
Emulsifiers/Stabilizers/Dispersants
Since BaSO4 is not soluble in water, in order for it to be used as a contrast medium, emulsifiers, stabilizers, and dispersants must be added to maintain smooth BaSO4 particle suspension, along with pH regulators to maintain suspension efficacy. One of these is carboxymethyl-cellulose sodium, a negatively charged polymer that coats BaSO4 particles. At low concentrations, carboxymethyl-cellulose sodium helps to suspend the particles in water via electrostatic repulsion. Other emulsifiers/stabilizers/dispersants include sodium citrate, a dispersant that also helps regulate pH, and polysorbate 80, a surfactant that also is anti-foaming. These 3 multi-purpose additives are present in many BaSO4 preparations.
Mucosal-coating and Non-coating Thickeners
Mucosal coating is achieved by increasing the stickiness of the barium product. Additives that increase stickiness include the plant saps acacia gum (aka, gum arabic, the sap of the acacia tree) and, to a lesser degree, tragacanth gum (the sap of the astragalus gummifer shrub). Electrostatic forces can also provide mucosal coating, and this can be achieved by adding high concentrations of carboxymethyl-cellulose sodium. These additives also perform more than one role: all 3 serve as thickeners, increasing viscosity; acacia gum and carboxymethyl-cellulose help suspend the BaSO4 particles.
Non-coating thickeners increase viscosity, but not stickiness, of the barium product; therefore, they do not result in mucosal coating. Products containing non-coating thickeners are suitable for the MBSS and single-contrast anatomic imaging, for which mucosal coating is undesirable. Non-coating thickeners include xanthan gum, a bacterial polysaccharide; carrageenan, a sulfated polysaccharide from red seaweed; pectin, a heteropolysaccharide from plant cell walls; modified corn starch (which is also anti-sticking/anti-caking), and methylcellulose, which are plant-fiber molecules bound to -CH3 moieties. Unlike the broader ranges of viscosity acceptable in anatomic imaging of the GI tract, the viscosities of the barium products used in the MBSS are tightly controlled and highly standardized, with increasing amounts of non-sticky thickeners present in correspondingly more viscous preparations.
Sweeteners/Texturizers/Laxatives
BaSO4 contrast agents should be palatable; thus, they require sweeteners, such as glycerin (aka, glycerol, a polyol), maltodextrin (a variable short-chain polysaccharide of glucose), and xylitol and sorbitol (poorly digestible sugar alcohols). Of note, such sweeteners play other important roles. Xylitol and sorbitol are also osmotic laxatives that hold water in the bowel, maintaining suspension and speeding transit time through the GI tract; at least one of them will be found in all BaSO4 preparations. Sweeteners can also act as texturizers and/or thickeners, creating a food-like texture (“mouthfeel”) and increasing viscosity. Glycerol also functions as a surfactant.
Others
Other important additives include: anti-foaming agents, such as simethicone and polysorbate 80, to ensure that the product doesn’t get bubbly when shaken; pH regulators, including citric acid, sodium citrate, HCl, and KCl, to maintain proper acid/base balance for successful suspension; and preservatives such as sodium benzoate, potassium sorbate, methylparaben, and propylparaben to increase the shelf life of liquid BaSO4 preparations.
Preservatives are not added to powdered BaSO4 preparations, which should be used shortly after reconstitution, based on manufacturer’s recommendations. Further additives include flavorants such as ethyl maltol, ethyl vanillin, natural/artificial fruit flavors, and saccharin, and the nontoxic solvent propylene glycol to help mix a powdered preparation with water.
Imaging with BaSO4 Preparations
Anatomic Radiographic Evaluation of the GI Tract
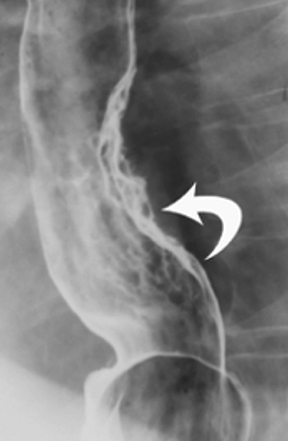
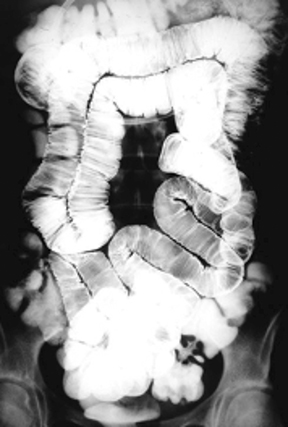
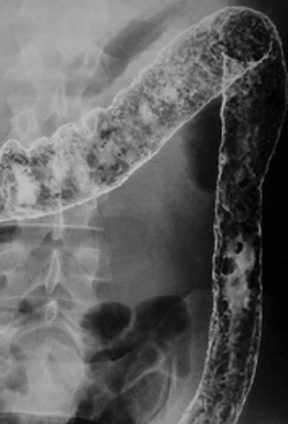
Anatomic evaluation of the GI tract with barium products includes single- and double-contrast upper GI (UGI) examinations (esophagus, stomach, and duodenum), as well as evaluation of the small bowel, colon, and rectum (Figure 2). The purpose of these exams is to identify and characterize anatomic lesions such as tumor, inflammation, obstruction, malformation, and others with an opacifying contrast agent that can either coat or fill the lumen. BaSO4 products used for all these applications must have high physical and radiographic density, ranging from 24% to 240% w/v.
Double-contrast UGI imaging is used to reveal detail of the GI tract’s mucosal inner lining to reveal ulcers, polyps, inflammatory patterns, etc. In order to see these structures, the mucosal lining must get a thin, even coating of a high-density, sticky BaSO4 preparation while the lumen is filled with air or gas. (Table 1) Therefore, the BaSO4 contrast agent must contain a sticky gum (eg, acacia gum) for mucosal coating, and be highly radiodense (100-240% w/v).
On the other hand, single-contrast upper GI imaging is used to see general anatomy and anatomic lesions. The GI structures must be filled with contrast, which is typically not problematic in AP or mildly oblique projections, owing to relatively little overlapping of upper GI structures in those projections. Therefore, for this exam, the lumen must be filled with a well-mixed, moderate-density (60-120% w/v) solution of a non-sticky (ie, no sticky gums) BaSO4 preparation.
Small bowel follow-through (SBFT) examination or enteroclysis (direct contrast administration into the small bowel) can be done as a single- or double-contrast procedure. The main challenge is to fill and “see through” a long stretch of heavily overlapped bowel loops. So, the barium contrast must provide significant substance and should be of relatively low concentration. In addition, some mucosal coating may be helpful. Therefore, the formulation should consist of non-sticky thickeners/fillers (such as methylcellulose, xanthan, or carrageenan) and a small amount of sticky gum, and be of low radiodensity (<25% w/v).
For a double-contrast barium enema, the anatomic consideration is capacious bowel loops with some overlapping, especially on oblique views of the sigmoid and splenic flexures. The primary barium contrast requirement is mucosal coating (filling is achieved with insufflated air); however, too much stickiness may interfere with instilling the coating all the way to the cecum. Therefore, the barium preparation has a moderate concentration of sticky gum, and a moderate radiodensity (100% w/v).
For the single-contrast barium enema, filling is the priority, and overlapping loops can be a considerable problem. The barium preparation should have non-sticky thickeners and fillers such as xanthan or carrageenan, and low radiodensity (50% w/v).
When performing defecography, the primary anatomic consideration is that the rectum may be capacious, and therefore, the radiodensity should be relatively low. The contrast, moreover, must imitate the consistency of normal feces so it can be retained in the rectum. Consequently, the barium preparation should be a paste, which can be attained with a high concentration of carboxymethyl-cellulose, and be of relatively low radiodensity (60% w/v).
Evaluating Swallowing Physiology
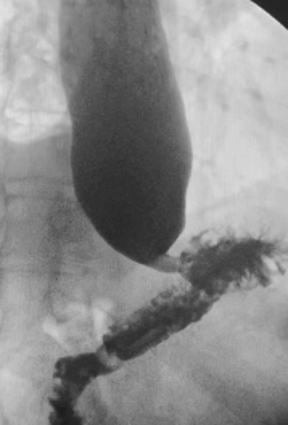
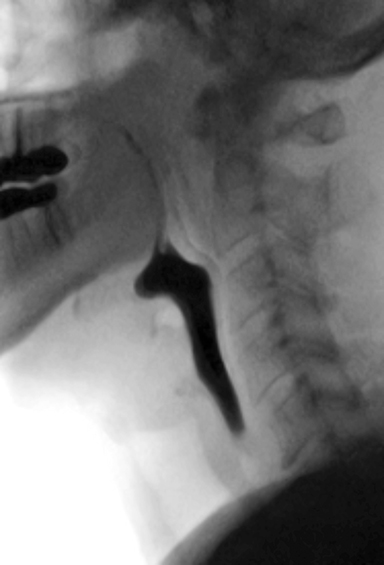
The MBSS, also known as the videofluoroscopic swallow study (VFSS), is a real-time fluoroscopic radiologic exam used to assess the physiology of swallowing (Figure 3). Its purpose is to obtain a systematic and reproducible assessment of the structure and function of the swallowing mechanism, and determine compensatory strategies for any abnormalities. This is a single-contrast examination, so the BaSO4 preparations must contain a low-to-moderate radiodensity (40% w/v). (Table 2) Perhaps more importantly for BaSO4 preparations for the MBSS/VFSS, a consistent selection of standardized viscosities must be available. Typical consistencies include thin liquid, nectar, thin honey, honey, puree/pudding, and solid. Moreover, these viscosities must be achieved with non-sticky starches and sugars---no acacia or tragacanth gum, to avoid mucosal coating. Finally, they must be agreeable and food-like, providing adequate “mouthfeel,” or texture, and include sweetness and flavoring for palatability.
Note that carboxymethyl-cellulose is present as a thickener in all liquid BaSO4 preparations used for the MBSS. However, the low concentrations found in these liquid preparations are not sufficient to provide BaSO4 particles with enough electrostatic attraction to adhere to the inner lining of the GI tract, so there is no coating effect. When carboxymethyl-cellulose is added in a concentration sufficient to create a pudding for MBSS, the electrostatic attraction to the GI inner lining is counteracted by other additives.
Why can’t “regular” GI barium products suitable for single-contrast imaging be used for the MBSS? Because these products lack the precise, standardized viscosities that the MBSS products possess. During development, the viscosity of each MBSS barium product, from thin liquid to pudding, was selected to best reveal physiologic deficiencies in the swallowing mechanism. The use of these products is critical to performing an accurate, reproducible MBSS;5 they also improve patient safety, increase efficiency, and reduce waste. Simply diluting standard BaSO4 preparations for the MBSS does not eliminate mucosal coating, creates different (and possibly excessively low) radiodensities, creates unknown viscosities, and dilutes the helpfulness of the additives in unpredictable, and likely unfavorable, ways.
An oft-stated concern with the MBSS is aspiration of BaSO4 preparations. Per the ACR Manual on Contrast Media,3 aspirated barium contrast agent is usually mobilized proximally by the ciliary action of normal bronchial epithelium. However, damaged epithelium from bronchial disease delays this process. If not completely expectorated, retained BaSO4 particles can remain indefinitely and may cause inflammation. As is true for any liquid, high-volume aspiration of barium-containing contrast can lead to acute respiratory distress or pneumonia.6 To minimize risk, preparations approved for the MBSS have a relatively low BaSO4 concentration (40% w/v) and have no sticky gums, both of which assist in clearance. Iodinated contrast media are also not recommended for the MBSS. Ionic high-osmolar contrast media (HOCM), if aspirated, may cause life-threatening pulmonary edema.3 Nonionic low-osmolar CM (LOCM), while much safer to aspirate by themselves, are all “thin liquids;” therefore, for a full swallowing evaluation, they would have to be mixed with various foods/food additives, whose viscosities are not standardized, and would be nonsterile and possibly dangerous to aspirate.
Barium Preparations for CT
Three CT applications utilize barium contrast: bowel marking, for standard CT of the abdomen/pelvis; tagging residual solid stool, for CT colonography (CTC; residual liquid stool is tagged with iodinated CM); and bowel distention at near-water density, for CT enterography (CTE).
The function of barium preparations for standard CT of the abdomen/pelvis is to mark bowel loops in order to distinguish them from adjacent structures such as vessels, tumors, and nodes. BaSO4 preparations for standard CT should: be at 2% w/v concentration, which is sufficient to reveal enhancement; be highly palatable for patient compliance; and contain sorbitol to maintain filling and promote bowel transit.
Barium preparations for CTC are used to tag residual solid stool fragments to distinguish them from polyps. BaSO4 preparations for this application should: be at 40% w/v concentration; contain carboxymethyl-cellulose (for tagging); be highly palatable; and come packaged and labeled for home use.
Barium-containing contrast for CTE is used to distend bowel loops at near-water density, facilitating the assessment of IV contrast-enhanced bowel wall for involvement with inflammatory bowel disease. For this application, BaSO4 preparations should: be at no more than 0.1% w/v concentration; include natural gum to provide substance; and contain sorbitol to maintain filling and promote bowel transit.
Conclusions
Barium sulfate provides barium-containing contrast agents with two specific properties: radiodensity and physical density. Radiodensity, determined by the BaSO4 concentration, is adjusted for the imaging technology, technique, and structure(s) of interest. All other ingredients provide the other useful properties present in BaSO4 products: BaSO4 particle suspension; mucosal coating; viscosity and texture, which are both important for MBSS preparations; sweetness; filling and laxative effect; preservation; foaming prevention; and flavoring. Selecting the right BaSO4 product for the right exam requires an understanding of the body structures being imaged and the diseases being evaluated. Each imaging technology and clinical application carries not only its own optimal BaSO4 concentration or concentration range, but also an optimal combination of additives that permit evaluation of the anatomy and/or function of interest.
References
- National Center for Biotechnology Information. PubChem Periodic Table of Elements. Barium (Element). Available at: https://pubchem.ncbi.nlm.nih.gov/element/56. Accessed February 7, 2021.
- Miller-Keane Encyclopedia & Dictionary of Medicine, ;Nursing & Allied Health. Revised Reprint, 7th Edition, Elsevier, Amsterdam.
- American College of Radiology (ACR) Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, 2020. Available at: https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf. Accessed February 7, 2021.
- Goldner RD, Adams DO. The structure of mononuclear phagocytes differentiating in vivo. III. The effect of particulate foreign substances. Am J Pathol. 1977;89:335-350.
- Hazelwood RJ, Armeson KE, Hill EG, Bonilha HS, Martin-Harris B. Identification of Swallowing Tasks From a Modified Barium Swallow Study That Optimize the Detection of Physiological Impairment. J Speech Lang Hear Res. 2017;60:1855-1863.
- Gelfand DW. Complications of gastrointestinal radiologic procedures: I. Complications of routine fluoroscopic studies. Gastrointest Radiol. 1980;5:293-315.