Clinical Applications of Gadopiclenol in Neuro MRI
Images
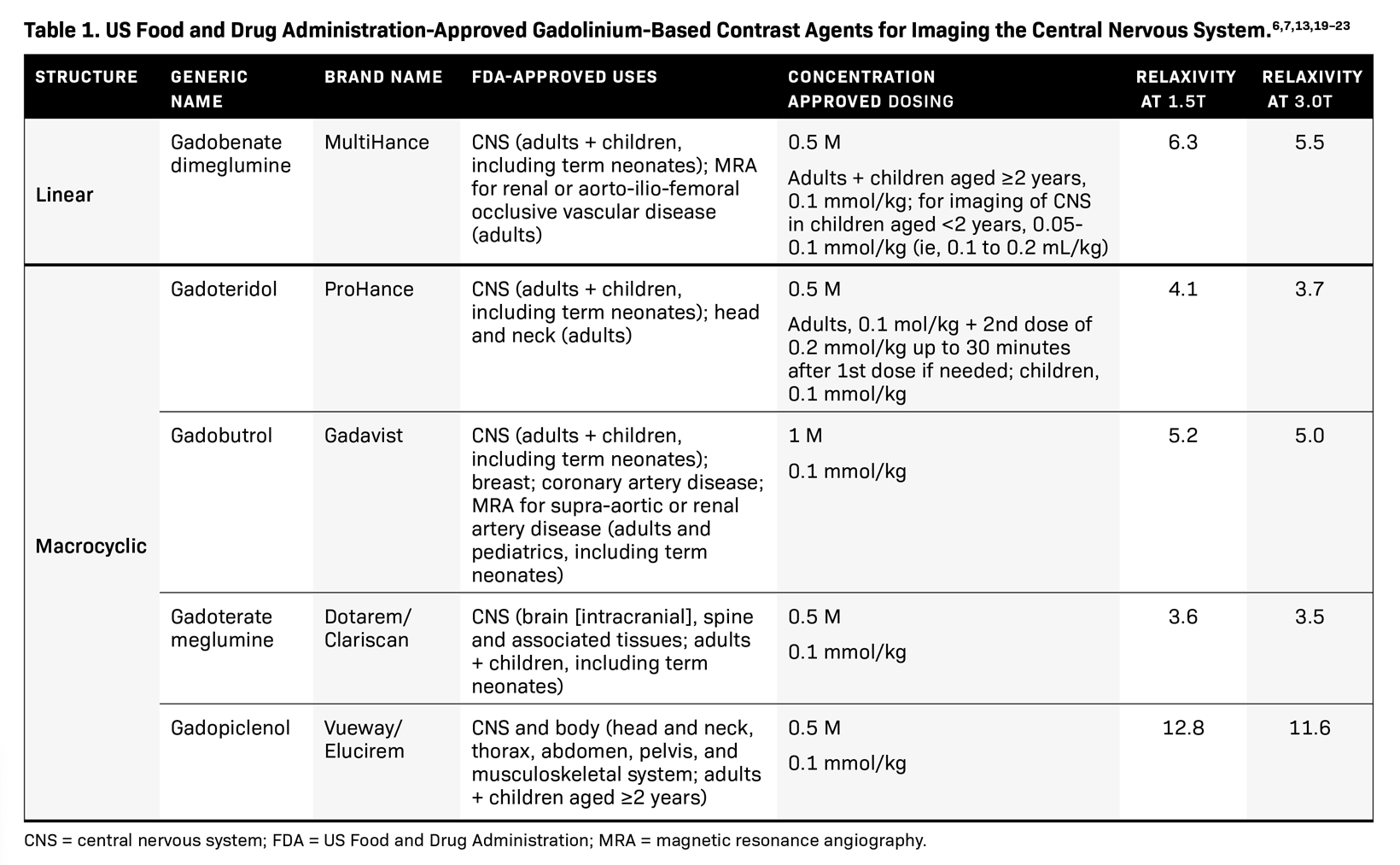
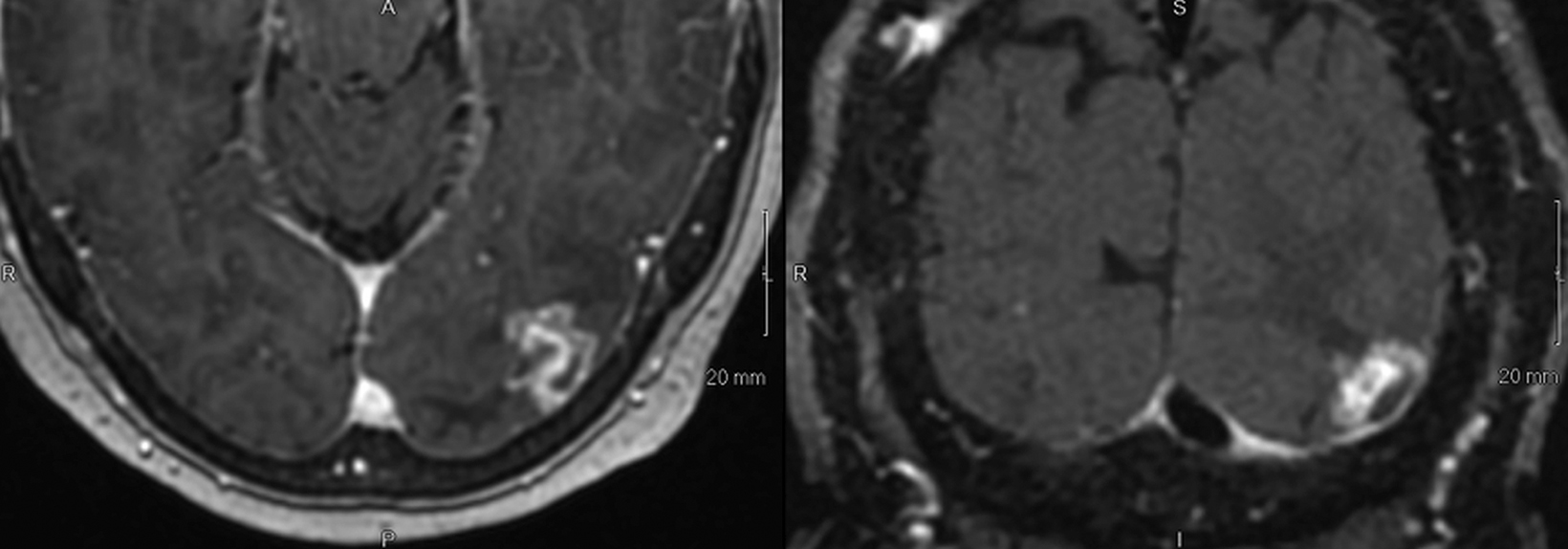
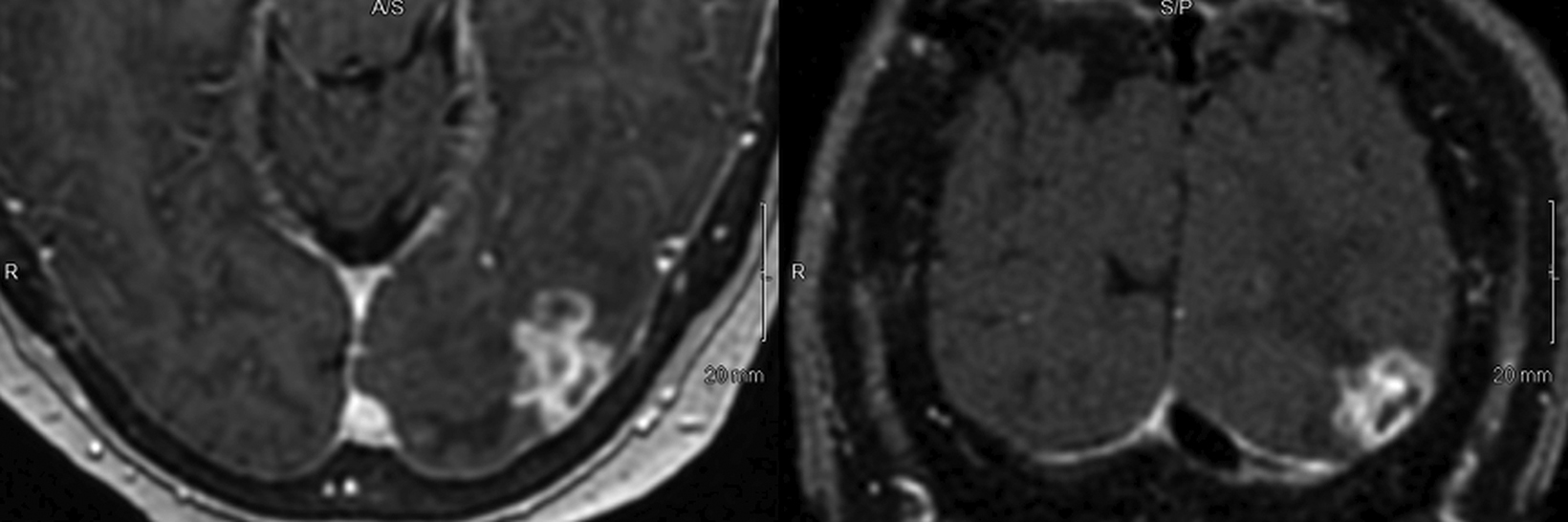
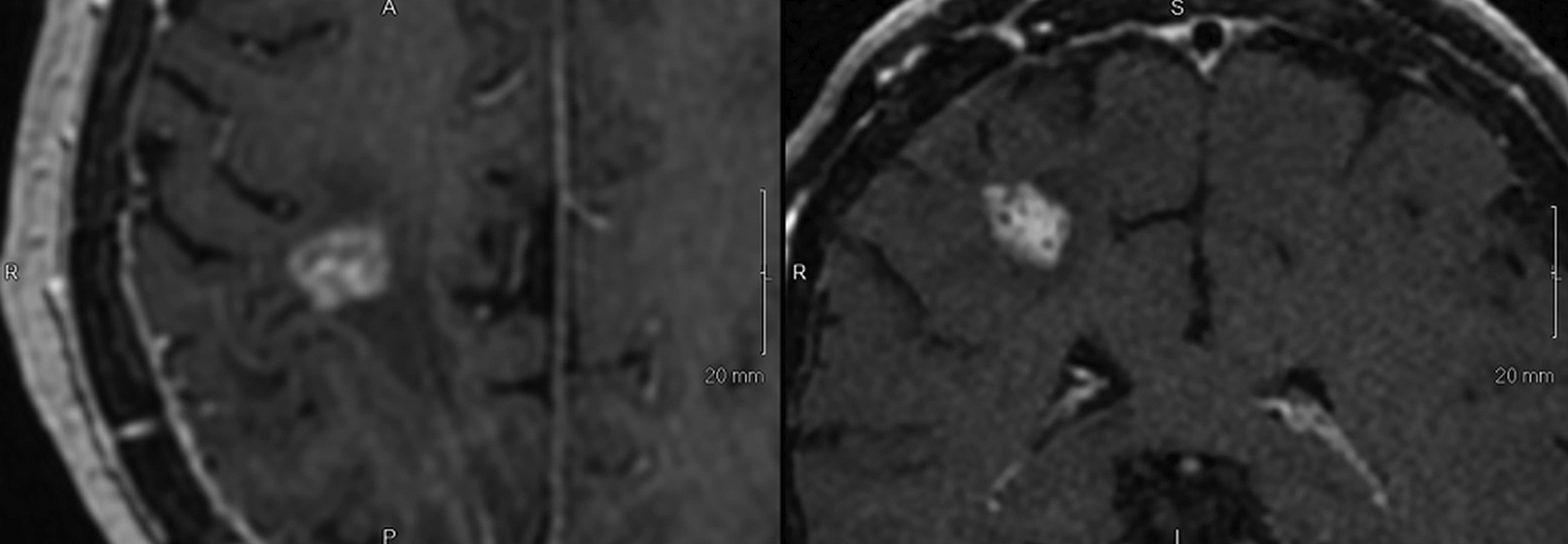
SPONSORED BY BRACCO
Introduction
Since their introduction in the late 1980s, gadolinium-based contrast agents (GBCAs) have revolutionized MR imaging, with more than 700 million doses administered worldwide.1,2 GBCAs contain gadolinium (Gd3+), a rare, heavy earth metal with paramagnetic properties that shortens the relaxation times of nearby water protons, thereby increasing signal intensity on T1-weighted MR images. In neuroimaging, this effect enables the detection of disruptions in the blood-brain barrier caused by pathological tissues and lesions, as well as vascular abnormalities.3
Despite widespread use and well-established safety profiles, GBCAs have been linked to conditions that have raised concern among the radiology community, including nephrogenic systemic fibrosis (NSF) in patients with renal impairment and, more recently, gadolinium retention in the brain and other tissues.4 While no clinical consequences related to gadolinium retention in the brain have been uncovered,4 there has been a push for the development of newer contrast agents with improved profiles and imaging capabilities. The most recently approved GBCA, gadopiclenol, is a promising alternative to older products due to its higher relaxivity and stability, lower gadolinium dose, and effective contrast enhancement.5–7 In this article, we discuss considerations for gadopiclenol use in neuroimaging.
Why Relaxivity Matters in Neuroimaging
Relaxivity is an inherent property that enables a GBCA to differentiate between abnormal lesions and background tissues. When deemed necessary for a specific exam, a higher relaxivity contrast agent can provide greater signal enhancement. This capability can be particularly beneficial in neuroimaging protocols where fine anatomical details and subtle pathological changes must be accurately identified, such as in tumors, infectious processes, inflammation, and stroke.3 In fact, randomized, multicenter, intraindividual, crossover studies have shown that when administered at equivalent doses, a GBCA with high relaxivity provides significantly better lesion enhancement and more diagnostic information than an agent with standard relaxivity for imaging the central nervous system (CNS).8–12
Gadopiclenol has gained attention for its exceptional relaxivity, which is more than twice that of its macrocyclic counterparts, gadoterate meglumine, gadobutrol, and gadoteridol (Table 1).13 This higher relaxivity enables the use of a gadolinium dose that is half that of other GBCAs approved by the US Food and Drug Administration (FDA) for imaging the CNS without compromising image quality.5–7 In a randomized, double-blind, intraindividual, crossover phase 3 study, PICTURE, a 0.05 mmol/kg dose of gadopiclenol demonstrated noninferiority to 0.1 mmol/kg of gadobutrol for all evaluated parameters and by all 3 blinded readers for imaging CNS lesions.5 The percentage of enhancement and lesion-to-background ratio were significantly higher with gadopiclenol for all readers, and the contrast-to-noise ratio was significantly higher for 2 readers. All 3 readers preferred gadopiclenol-enhanced images for almost half of the evaluations despite being administered at one-half the dose of gadobutrol.5
Gadopiclenol also demonstrates the highest stability among agents, imparted by the cage-like configuration characteristic of macrocyclic agents that binds the Gd3+ ion more tightly than linear GBCAs.13,14 Owing to the higher stability, it can be postulated that macrocyclic agents have a lower potential for dissociation of the Gd3+ ion from the chelate structure, reducing the risk for NSF development or gadolinium retention in the body.15 Although all GBCAs are associated with some degree of gadolinium retention, evidence suggests variability based on structure, with macrocyclic agents demonstrating less retention.15 Indeed, animal studies indicate that gadopiclenol behaves like other macrocyclic GBCAs, with less gadolinium retention in tissues compared with linear GBCAs.16,17 Moreover, when administered at the approved clinical dose, 0.05 mmol/kg of gadopiclenol is associated with less gadolinium retention than 0.1 mmol/kg of gadobutrol, suggesting that the extent of gadolinium retention is proportional to the administered dose.18 Although no evidence to date links gadolinium retention with clinical consequences, the flexibility to expose patients to less gadolinium without compromising diagnostic performance makes gadopiclenol an appealing GBCA.
Implementation of a New Contrast Agent
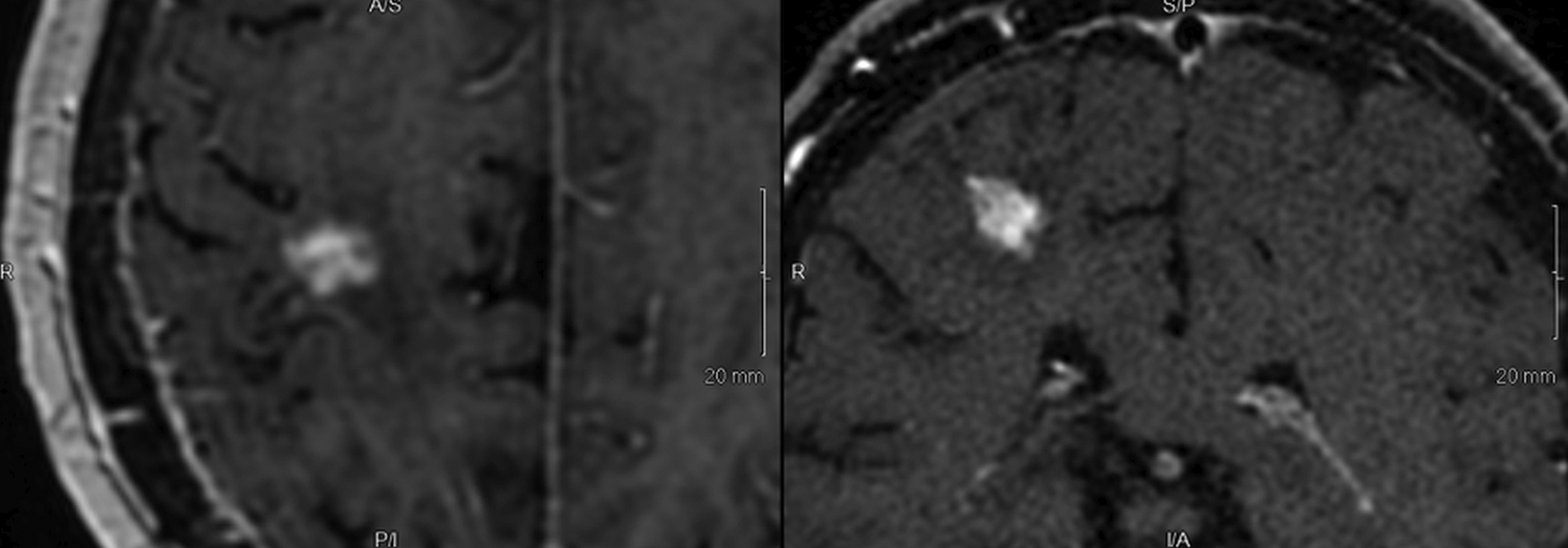
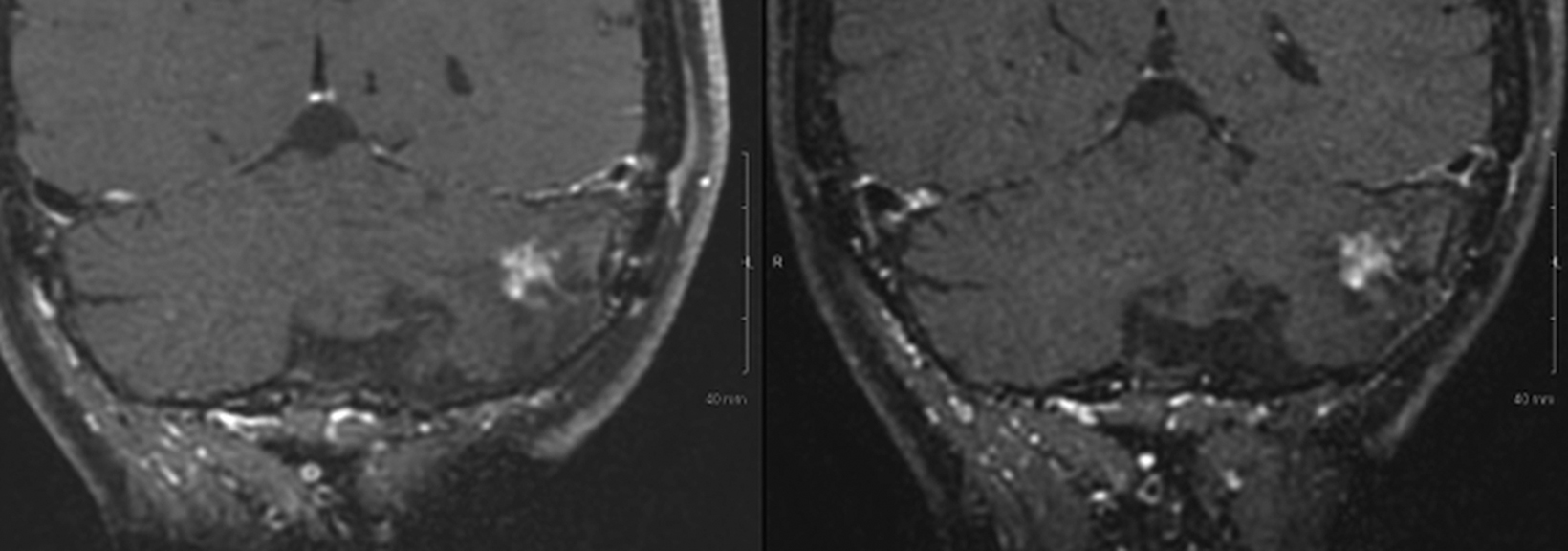
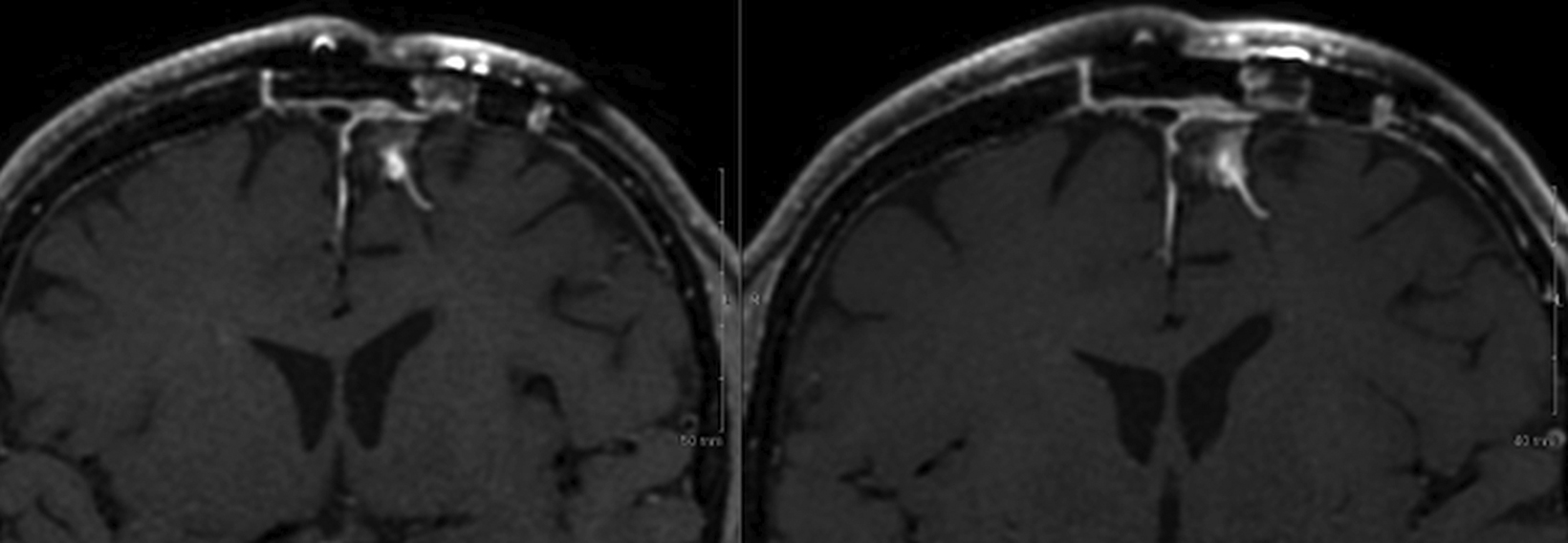
Introducing a new contrast agent within an institution requires a systematic approach that assesses its clinical utility and safety profile. As part of our neuroradiology section’s product evaluation at the University of Wisconsin School of Medicine and Public Health, we implemented a 2-week trial during which all enhanced neuroimaging protocols were acquired with gadopiclenol. For patients with previous exams enhanced with another agent, we compared studies to identify clinical changes and better understand how gadopiclenol behaved and was tolerated. The product evaluation also allowed us to educate residents and fellows on how to account for technical factors that can impact image quality, particularly when comparing images with prior enhanced studies. At the end of our trial period, we transitioned to gadopiclenol as our primary contrast agent after concluding that for T1-weighted imaging, a 0.05 mmol/kg dose performs as well as a full gadolinium dose (ie, 0.1 mmol/kg) of the standard GBCA we previously used (Figures 1–4).
As gadopiclenol use expands to other imaging areas in our institution, we remain poised to foster a collaborative approach between neuroradiology and other sections, as well as with technologists. Interdisciplinary meetings and case discussions can further facilitate the exchange of knowledge and promote best practices for gadopiclenol use in different clinical scenarios.
Lastly, we continue to educate patients about the benefits and risks of gadopiclenol to improve transparency and build trust. Patients are more knowledgeable about their health care options than ever before. By addressing questions and providing reassurance, we help alleviate patient anxiety and encourage participation in the decision-making process.
As of October 2024, 1 million doses of VUEWAY® (gadopiclenol) solution for injection — one of the trades names for gadopiclenol — have been administered, a testament to its clinical utility and a sign of growing acceptance by the radiology community.24
Summary
Gadopiclenol represents a significant advancement in the development of GBCAs, offering the highest relaxivity among the FDA-approved agents and excellent diagnostic performance with lower gadolinium exposure. Its clinical applications in tumor imaging, vascular pathology, and stroke evaluation highlight its potential to improve patient outcomes. By adopting best practices for product evaluation and implementation, institutions can work to seamlessly integrate gadopiclenol into their imaging protocols and ensure optimal workflow. With additional research and collaboration, the clinical benefits of gadopiclenol are expected to further expand into neuroradiology applications.
VUEWAY® (gadopiclenol) solution for injection
Indications
VUEWAY injection is indicated in adults and children aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in:
- the central nervous system (brain, spine and associated tissues),
- the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).
IMPORTANT SAFETY INFORMATION
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. VUEWAY is not approved for intrathecal use.
NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
- The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2), or
- Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended VUEWAY dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration.
Contraindications
VUEWAY injection is contraindicated in patients with history of hypersensitivity reactions to VUEWAY.
Warnings and Precautions
There are risks associated with intrathecal use of GBCAs that can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of VUEWAY have not been established with intrathecal use and VUEWAY is not approved for intrathecal use.
Risk of nephrogenic systemic fibrosis is increased in patients using GBCA agents that have impaired elimination of the drugs, with the highest risk in patients with chronic, severe kidney disease as well as patients with acute kidney injury. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities.
Hypersensitivity reactions including serious hypersensitivity reactions, could occur during use or shortly following VUEWAY administration. Assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders, administer VUEWAY only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, and observe patients for signs and symptoms of hypersensitivity reactions afteradministration.
Gadolinium retention can be for months or years in several organs after administration. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (brain, skin, kidney, liver and spleen). Minimize repetitive GBCA imaging studies, particularly closely spaced studies, when possible.
Acute kidney injury requiring dialysis has occurred with the use of GBCAs in patients with chronically reduced renal function. The risk of acute kidney injury may increase with increasing dose of the contrast agent.
Extravasation and injection site reactions can occur with administration of VUEWAY. Ensure catheter and venous patency before the injection of VUEWAY.
VUEWAY may impair the visualization of lesions seen on non-contrast MRI. Therefore, caution should be exercised when VUEWAY MRI scans are interpreted without a companion non-contrast MRI scan.
The most common adverse reactions (incidence ≥ 0.5%) are injection site pain (0.7%), and headache (0.7%).
POST-MARKETING EVENTS
Acute pancreatitis within 48 hours of GBCA administration has been reported.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for VUEWAY® (gadopiclenol) solution for injection including BOXED WARNING on Nephrogenic Systemic Fibrosis.
Manufactured for Bracco Diagnostics Inc. by Liebel-Flarsheim Company LLC, Raleigh, NC, USA 27616.
VUEWAY is a registered trademark of Bracco Imaging S.p.A.
Bracco Diagnostics Inc.
Prospect Road, Building H
Monroe Township, NJ 08831 USA
Phone: 609-514-2200
Toll-Free: 1-877-272-2269 (U.S. only)
Fax: 609-514-2446
©2024 Bracco Diagnostics Inc. All Rights Reserved.
1/25 US-VW-2400034