Cerebral Amyloid Angiopathy-related Inflammation
Images

Case Summary
An elderly patient presented to the emergency department with confusion, upper-extremity weakness, and slurred speech. Noncontrast head CT revealed a right temporoparietal lesion with surrounding vasogenic edema, which was confirmed by MRI. The patient was admitted and anticoagulation for their atrial fibrillation was discontinued.
The patient experienced improvement and was discharged after several days. Follow-up outpatient MRI four weeks later demonstrated persistent vasogenic edema and multiple new petechial lesions, necessitating a second hospitalization and steroid treatment.
Imaging Findings
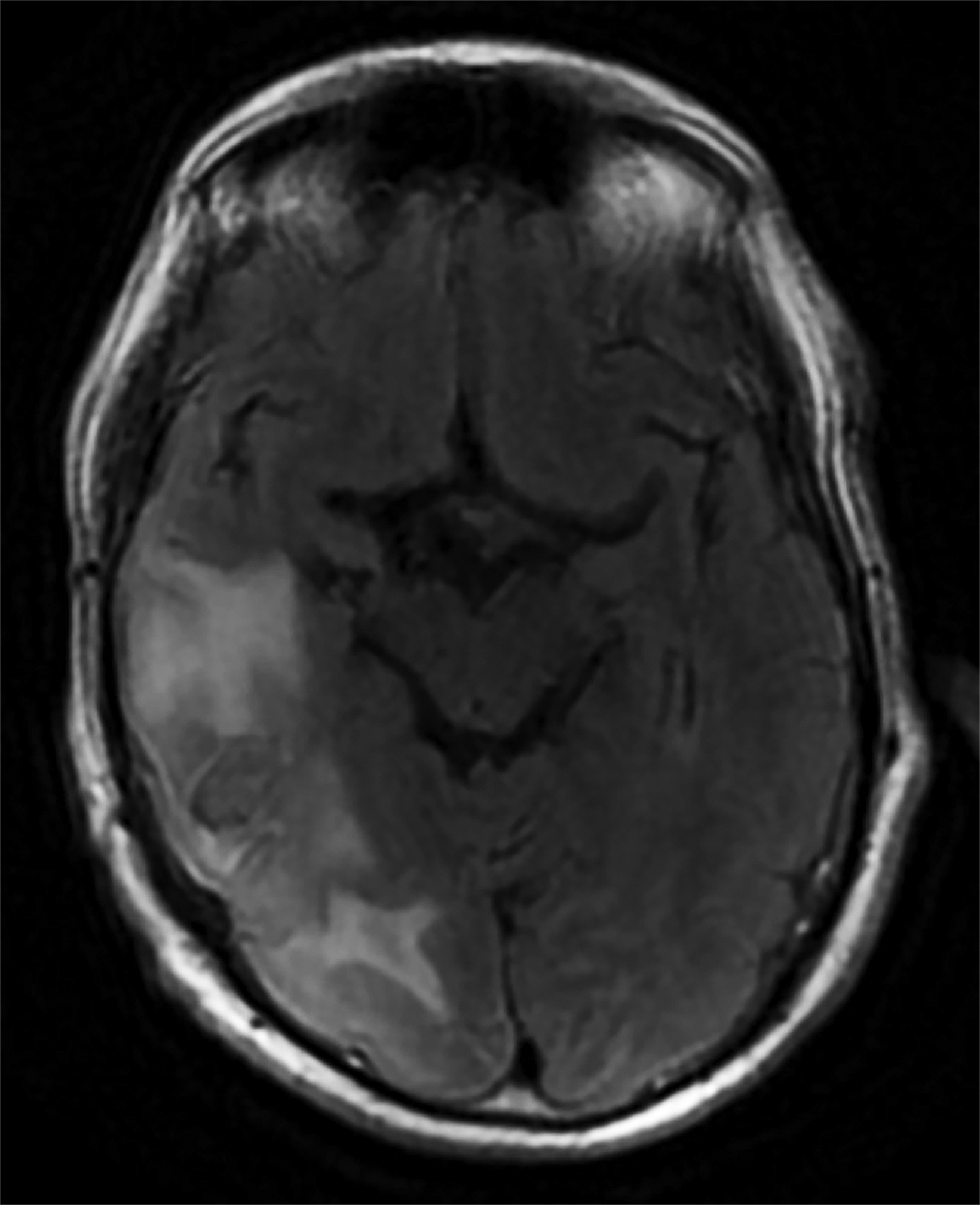
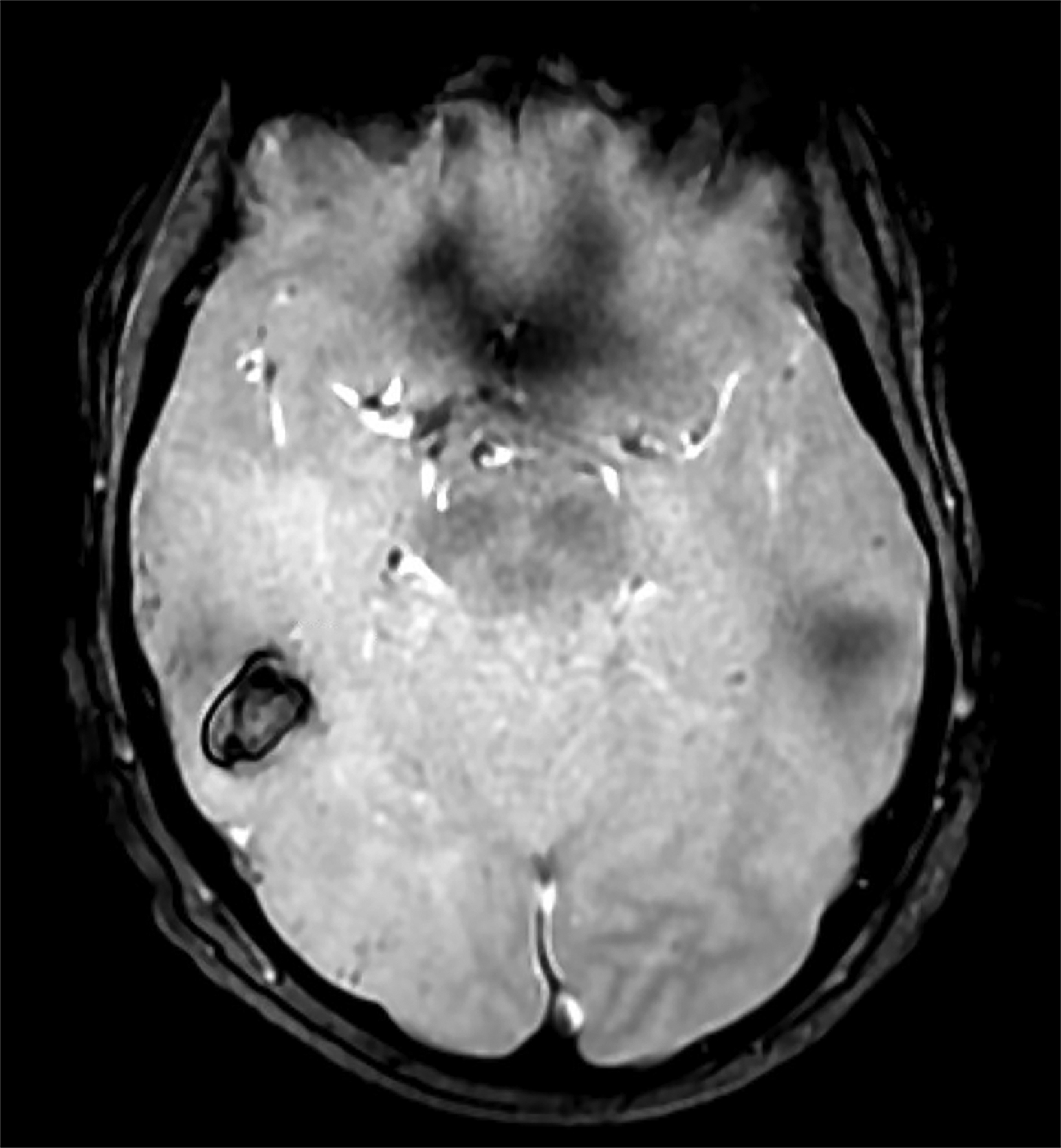
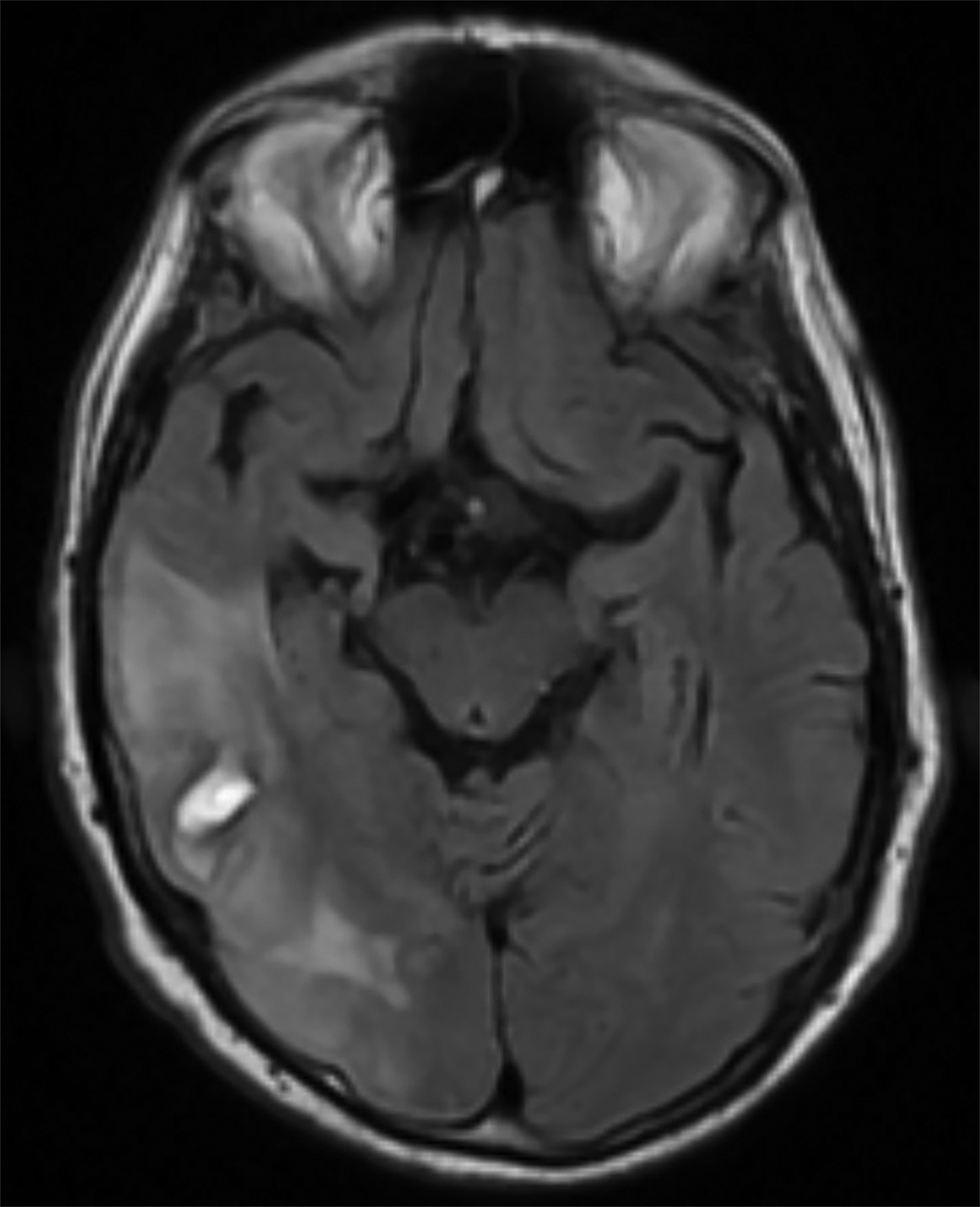
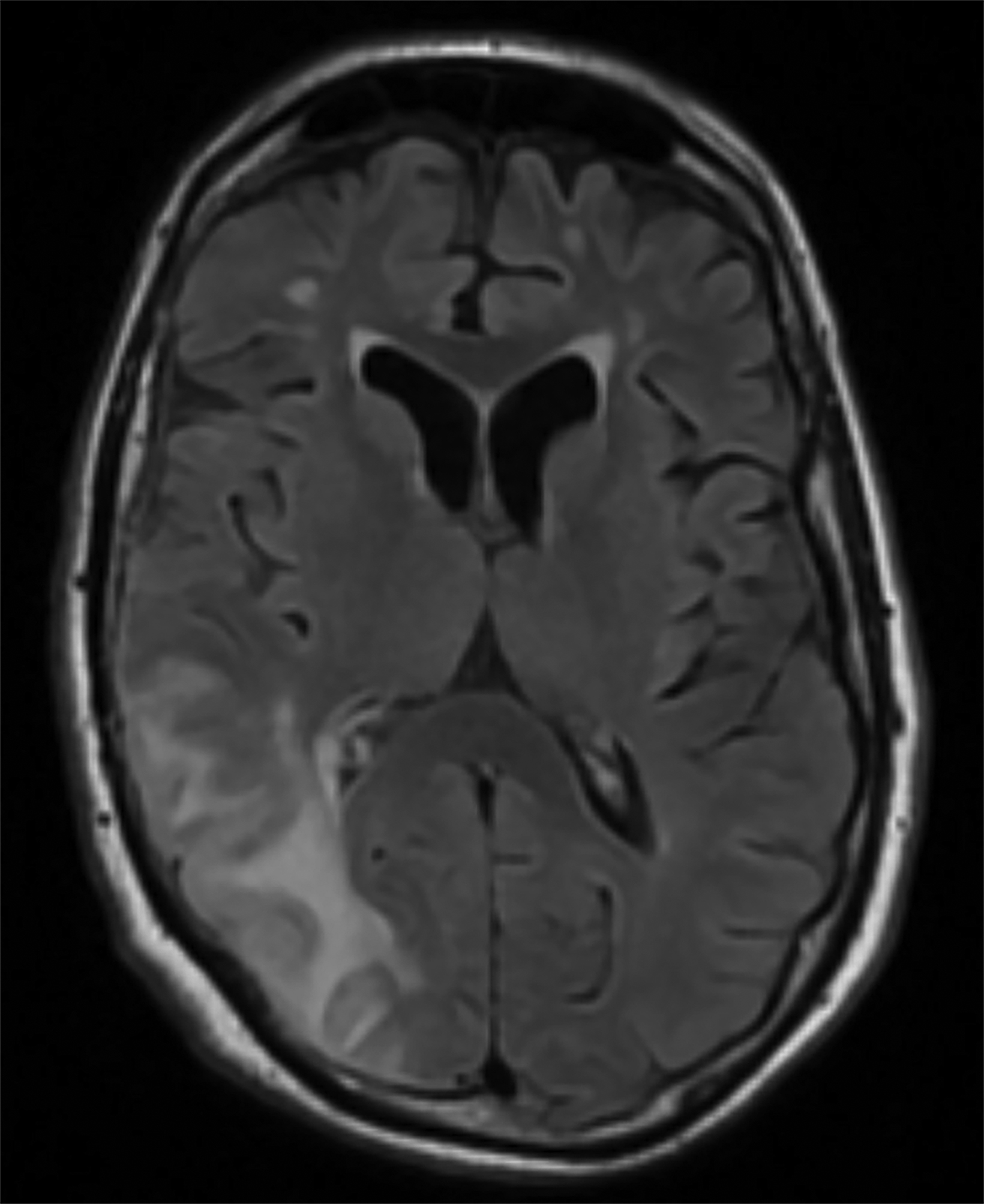
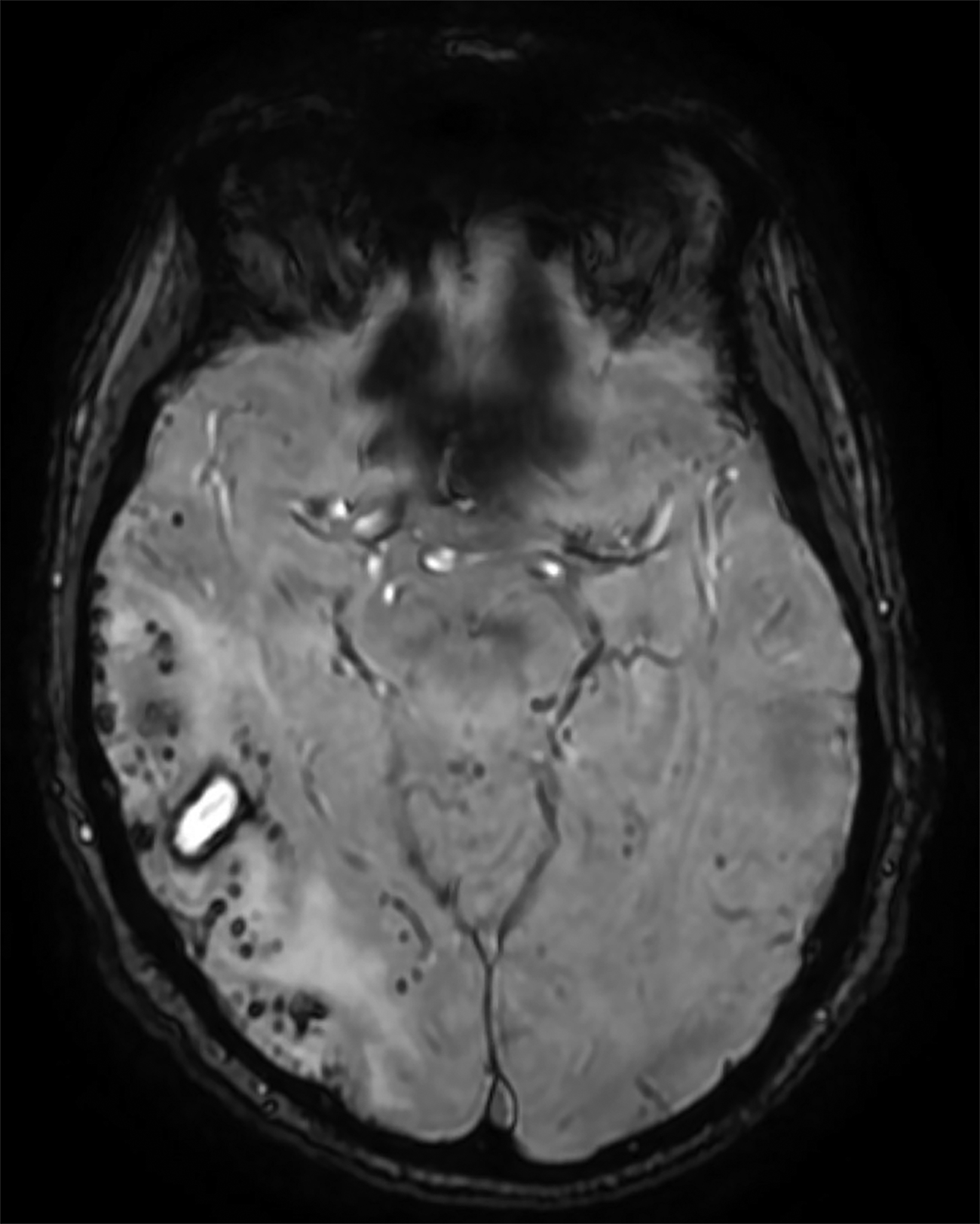
Initial noncontrast head CT revealed a 1.5 × 2.3 cm hyperdense ovoid lesion in the right temporoparietal region surrounded by wide-spread, frond-like areas of hypodensity. T2 FLAIR MRI further characterized this temporoparietal lesion as a subcortical hemorrhage with out-of-proportion hyperintense vasogenic edema. T2* GRE demonstrated concentric deoxyhemoglobin around the hemorrhage (Figure 1). One month later, T2 FLAIR demonstrated persistence of prior temporoparietal hyperintensities. Susceptibility-weighted imaging revealed interval development of punctate foci of signal loss, co-localized to the hyperintensities seen on FLAIR (Figure 2).
Diagnosis
Cerebral amyloid angiopathy-related inflammation (CAARI). The differential diagnosis includes posterior reversible encephalopathy syndrome, leukoencephalopathy, brain tumor, primary angiitis of the central nervous system, acute disseminated encephalomyelitis, and neurosarcoidosis.
Discussion
CAARI is a rare variant of cerebral amyloid angiopathy (CAA). In CAA, amyloid β (Aβ) is deposited in the walls of cortical and leptomeningeal vessels, producing vasculopathy.1 Hereditary CAA is associated with Down syndrome and mutations in genes encoding amyloid precursor and presenilin proteins, whereas sporadic CAA is age-related.2
Both types can present with acute, recurrent intracerebral hemorrhage (ICH), progressive dementia, and transient focal neurological episodes.1,2 CAARI shares these manifestations, in addition to specific features like subacute encephalopathy, cognitive changes, stroke-like deficits, headaches, and seizures. The average age of patients affected by CAARI is 67, with no clear gender predominance.2,3
In CAARI, Aβ is believed to trigger an autoimmune response, resulting in either perivascular inflammation (inflammatory cerebral amyloid angiopathy [ICAA]) or intramural/transmural inflammation (Aβ-related angiitis [ABRA]). ICAA and ABRA are reconciled as distinct histologic forms of CAARI that have equivalent clinicoradiologic presentations. Regardless of the form, brain biopsy is needed to definitively diagnose CAARI.2 Increasingly, MRI is preferred for noninvasive probable diagnosis of CAARI.
Although infrequent, symptomatic, large ICH is a classic imaging finding of CAA. Other characteristic MRI findings include white matter hyperintensities (WMHs), cerebral microhemorrhages or microbleeds (CMBs), cortical superficial siderosis (CSS), sulcal subarachnoid hemorrhage, and cortical atrophy.1-3 With CAARI, these findings are preserved but may differ slightly from those in traditional CAA. For example, WMHs in CAARI are unifocal or multifocal, patchy, asymmetric, and confluent.2-4 These hyperintensi- ties have vasogenic edema with or without mass effect and contiguous gray matter extension.2,4
Hyperintensities in CAARI usually reverse with resolution of edema.4 Comparatively, hyperintensities in traditional CAA are spot-like when subcortical and have periventricular and posterior cerebral predominance without vasogenic edema.1,5 Similarly, hyper-intensities reflecting chronic small-vessel ischemia (caused by CAA or hypertensive arteriopathy) should be distinguished from the reversible, patchy hyperintensities characteristic of CAARI, as both types may be seen in some patients.
Compared to CAA, the incidence of CMBs is much higher in CAARI, and their distribution is not necessarily occipital dominant.3,6 Cerebral microhemorrhages are best seen using T2* GRE/SWI sequences.2,4 Hemosiderin from hemorrhage is paramagnetic and produces magnetic field inhomogeneity enhanced by T2* sequences, resulting in blooming artifact. Cerebral microhemorrhages or microbleeds are thus visualized as punctate areas of signal loss.
SWI is more sensitive to CMB depiction, owing to its high spatial resolution, three-dimensional capability, and lower section thickness compared to standard T2* GRE.7 In CAARI, CMBs appear in a lobar distribution and can colocalize with WMHs.3,4,6 T2* GRE/SWI also depict CSS, a chronic finding which appears as linear signal loss along pial surfaces of the brain.7 In the acute stage, in place of siderosis, sulcal subarachnoid hemorrhage may appear as T2 FLAIR hyperintensities.1
White matter hyperintensities of CAARI might resemble those of posterior reversible encephalopathy syndrome and some leuko-encephalopathies. T2* GRE/SWI is thus critical for differentiation, as it can depict CMBs specific for CAARI.2 Additionally, WMHs of CAARI have a tumefactive appearance that may mimic a brain tumor.2,6
In the absence of brain biopsy, a diagnosis of tumor may be missed in patients with coexisting CAARI. Accordingly, CAARI is diagnosis of exclusion, and biopsy should be performed based on the clinical circumstance.2 Moreover, diseases like primary angiitis of the central nervous system, acute disseminated encephalomyelitis, and neurosarcoidosis can imitate CAARI, but gradient-echo MRI can again help suggest a CAARI diagnosis.2,3
Patchy, confluent WMHs and concomitant hemorrhagic manifestations (CMBs, CSS, or prior ICH) compose the imaging criteria needed to diagnose CAARI with MRI. Besides imaging, patients must also be older than 40, have one or more typical symptoms of CAARI, and lack neoplastic or infectious causes for proper diagnosis.8 Empiric treatment with high-dose glucocorticoids is indicated in suspected CAARI. In select cases, immunosuppressants like cyclophosphamide and azathioprine may be considered as supple- mental therapy.2,4,6
In our case, the WMHs of CAARI were confounded by the effects of local vasogenic edema from the temporoparietal hemorrhage, making diagnosis more difficult. Additionally, initial T2* GRE did not reveal substantial evidence of CMBs, which further delayed diagnosis and treatment. Glucocorticoid treatment was begun when these white matter hyperintensities persisted on follow-up outpatient MRI and new CMBs were identified using susceptibility-weighted angiography.
This illustrates the importance of prompt follow-up imaging, especially with three-dimensional susceptibility-based sequences with high spatial resolution, in unclear cases like ours.
Conclusion
CAARI is a rare variant of CAA that causes subacute neurobehavioral symptoms, focal neurologic signs, headaches, and seizures. Patients can also have classic manifestations such as large, symptomatic ICH. Characteristic findings on MRI using T2 FLAIR and T2* gradient echo sequences permit probable diagnosis of CAARI. Brain biopsy is needed for definitive diagnosis, but empiric treatment should not be delayed when clinical and radiologic suspicion is high.
References
Citation
K M, S P. Cerebral Amyloid Angiopathy-related Inflammation. Appl Radiol. 2024; (3):44-46.
May 7, 2024