Brain Cholesterol Metabolism Visualized With New PET Radiotracer
Images
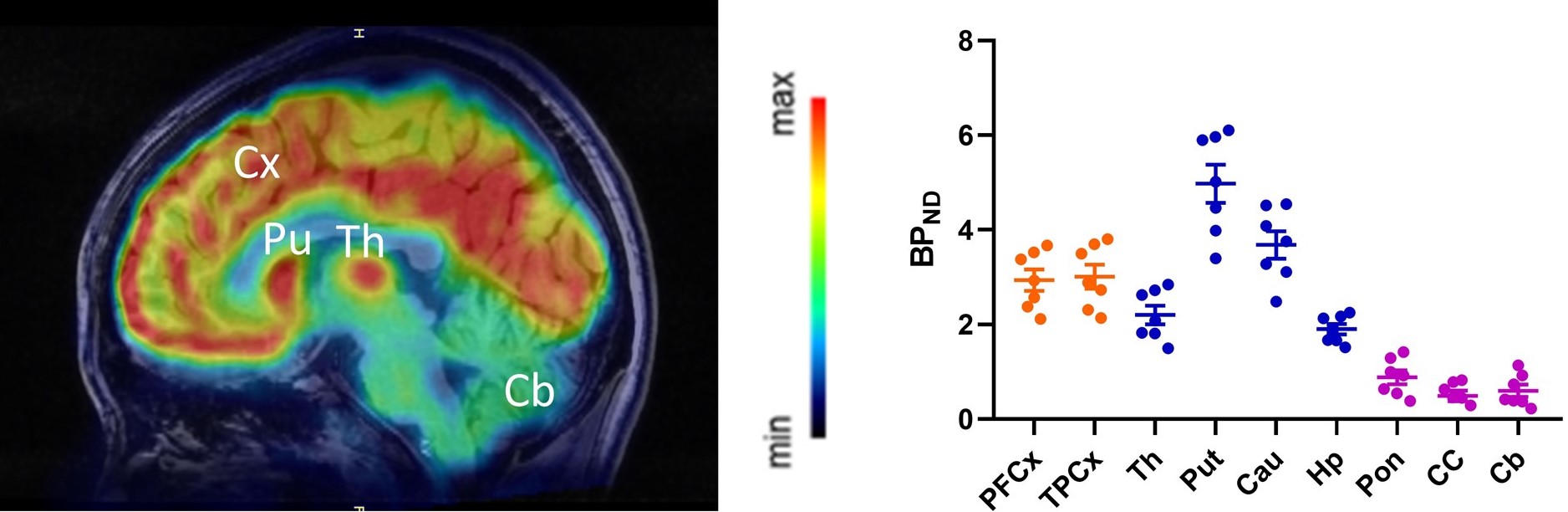
A new PET radiotracer,18F-Cholestify, holds the potential to substantially improve clinical neuroimaging by visualizing the enzyme primarily responsible for metabolic cholesterol degradation in the brain. Research validating8F-Cholestify was presented at the 2023 Society of Nuclear Medicine and Molecular Imaging Annual Meeting may lay the foundation for new image-guided trials to study neurodegenerative diseases and neurological disorders.
Brain cholesterol metabolism is impaired in many neurodegenerative diseases and neurological disorders, including Alzheimer’s disease. However, there are no validated methods currently available to visualize metabolic cholesterol clearance in the brain.
“Given the scarcity of tools to image brain cholesterol metabolism, a PET radioligand that is suitable for quantitative mapping of CYP46A—the enzyme that eliminates excessive brain cholesterol—could broadly affect disease diagnosis and treatment options,” stated Steven Liang, PhD, associate professor in the Department of Radiology and Imaging Sciences and head of the PET Imaging Center at Emory University in Atlanta, Georgia. “In response to this need, we developed a CYP46A1-targeted PET radioligand with high CYP4A1 binding affinity and tested in across different species to evaluate tracer performance characteristics.”
18F-Cholestify was created by radiolabeling the molecule CHL2205 with fluorine-18. To investigate its effectiveness, the affinities of CHL2205 in rats, non-human primates, and humans were determined by radioligand saturation/competitive binding assays. Autoradiographies were performed on rodent brain tissue, and PET imaging studies were conducted in rats, non-human primates and healthy volunteers.
Preclinical evaluation of 18F-Cholestify in rats showed remarkable selectivity for CYP46A-rich brain regions such as the striatum, cortex, thalamus, and hippocampus. Regional selectivity for CYP46A1-rich brain areas was confirmed in non-human primate PET studies and in a first-in-human study. Kinetic modeling revealed excellent correlation of the PET signal with non-displaceable binding potentials as well as with Western-blot results in post-mortem specimens in non-human primates.
“In this study we successfully performed cross-species validation of our target structure, CHL2205, using a variety of radioligand binding assays. Remarkably, the PET signal intensity observed in vivo in mice, non-human primates and humans exhibited a direct correlation with protein expression levels detected in postmortem brain specimens across all species,” stated Liang. “The compelling findings warrant the imminent integration of 18F-cholestify PET imaging into clinical studies for neurodegenerative diseases and neurological disorders.”
Looking to the future, Liang noted that there is accumulating evidence that brain cholesterol is implicated in early Alzheimer’s disease pathology, making it a target of interest. “While amyloid-targeted antibodies hold promise for modifying the disease process, their clinical efficacy and long-term effects are still being evaluated. As such, it is of paramount importance to foster research into exploring the potential of combination therapies, as well as investigating other approaches targeting different aspects of Alzheimer's disease pathology,” he said.