Bilateral Germ Cell Neoplasia In Situ with Left Testicular Seminom
Images
Case Summary
An adult presented to the urology clinic with persistent scrotal pain approximately four months after emergency evaluation for a traumatic scrotal injury. The clinical exam revealed a nontender right testis with a hydrocele and a normal-size left testis with a barely palpable mass. The patient ’s alpha-fetoprotein (AFP) level was 2.3 ng/mL and beta-human chorionic gonadotropin (b-HCG) level was <0.6 IU/L, both within normal range.
Imaging Findings
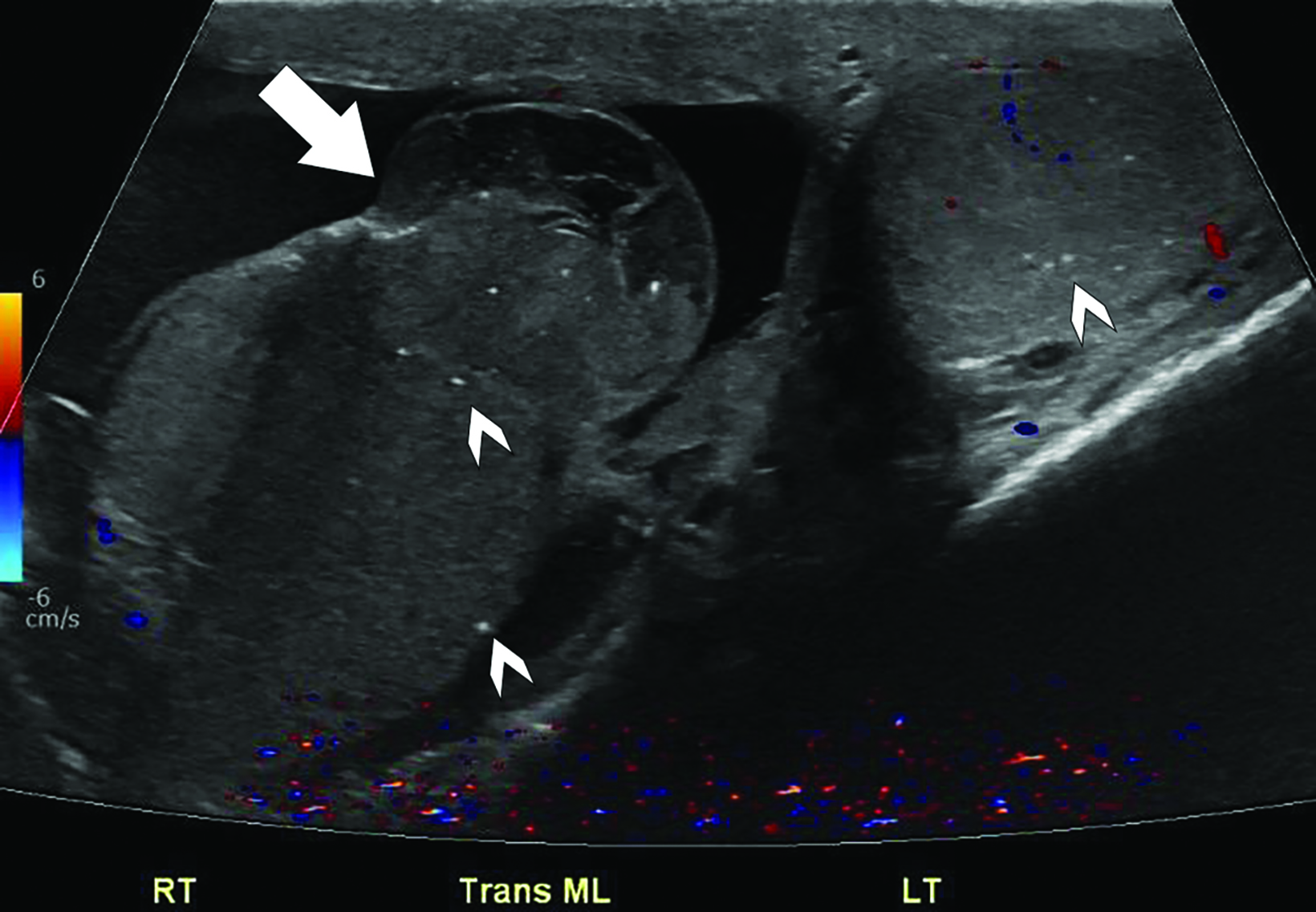
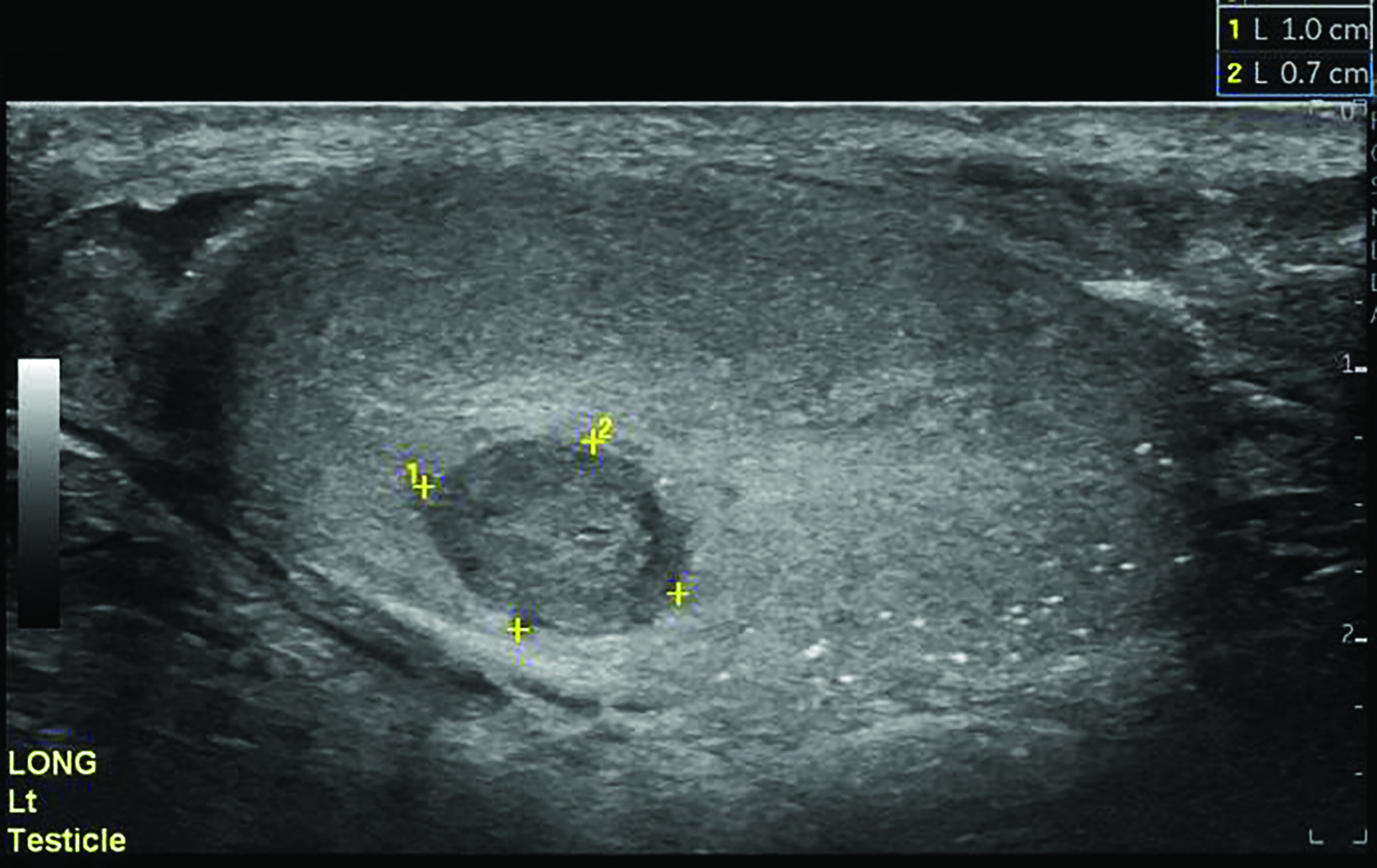
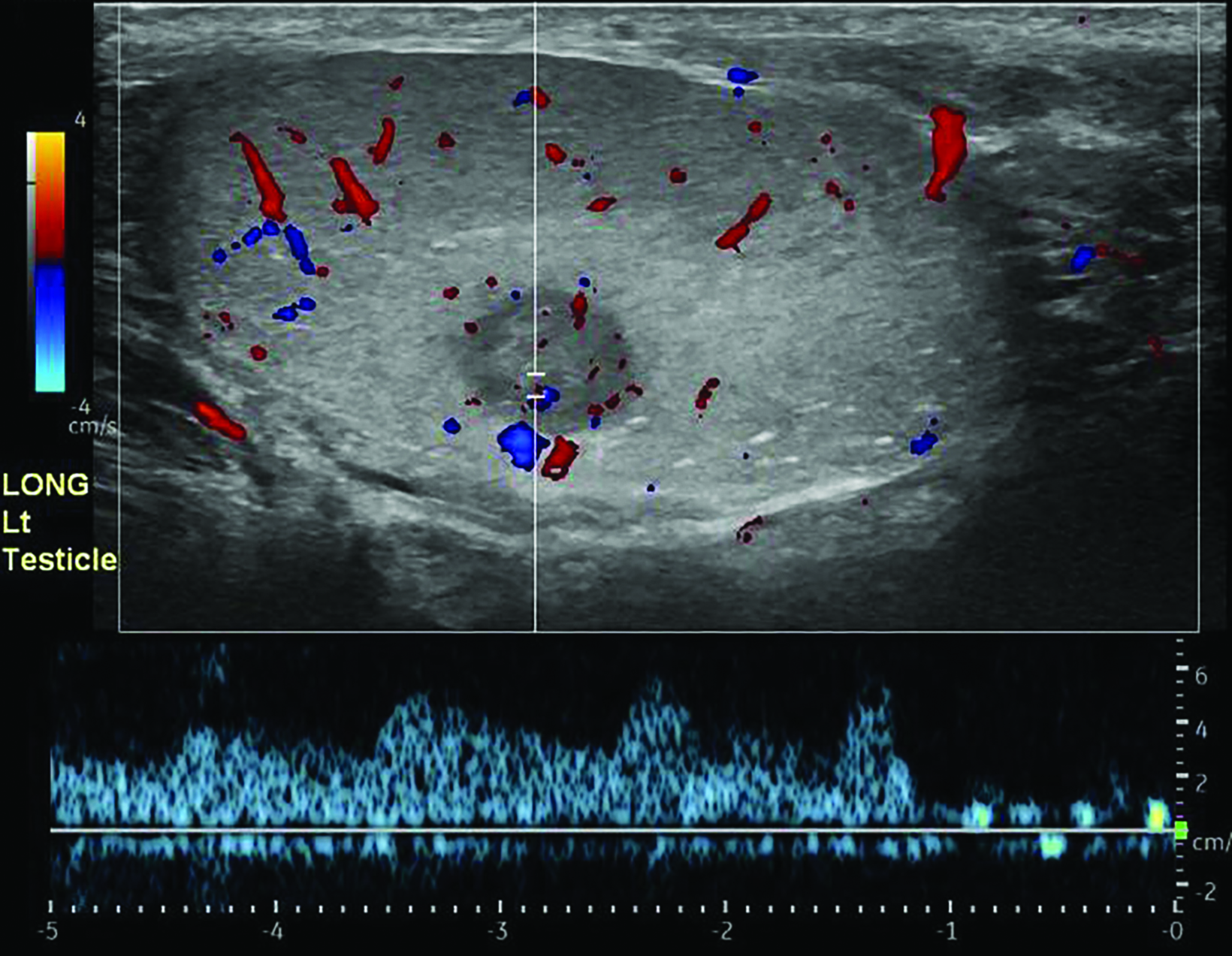
Scrotal ultrasound (US) demonstrated a heterogeneous, mass-like protrusion from the right testis consistent with the history of testicular trauma, and bilateral punctate echogenic foci consistent with microlithiasis (Figure 1). Ultrasound of the left testis showed microlithiasis and a hypoechoic mass measuring 1.0 x 0.7 cm with color Doppler flow (Figure 2).
Diagnosis
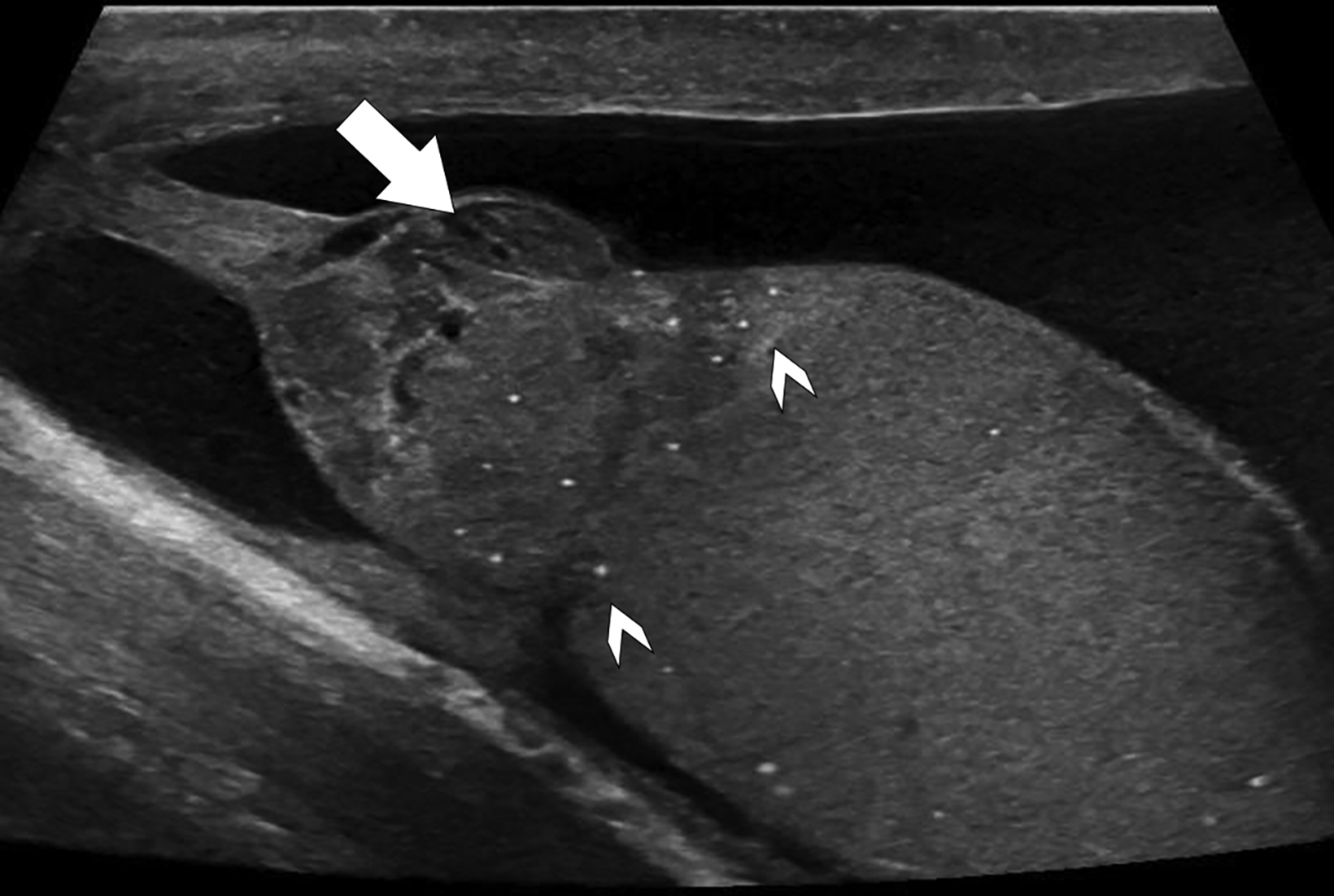
The patient was treated with trans-scrotal repair of the chronic posttraumatic right testicular rupture and with left partial orchiectomy via an inguinal approach. Pathology of the left testicular mass showed classic seminoma and tubular germ cell neoplasia in situ (GCNIS). Neoplastic cells were positive for CD117 and D2-40 and negative for keratin, CD30, and CD45, consistent with seminoma. Pathology of the right extruded testis showed infarct-like necrosis with granulation tissue. Tubular GCNIS was also present, with neoplastic cells positive for CD117. Six-month surveillance US showed expected changes after right repair and left partial orchiectomy (Figure 3); however, 19 months postoperatively, surveillance ultrasound identified two new left testicular lesions, indicating recurrent seminoma, confirmed after left inguinal orchiectomy.
Discussion
Testicular cancer most commonly occurs between the ages of 15 and 40 and accounts for 1% of all cancers in people with testes. Seminoma is the most common testicular tumor and typically presents as a painless mass between 30 and 40 years of age. Non-seminomatous tumors are often a mix of multiple germ cell subtypes and occur about a decade younger. Tumor markers should be obtained prior to treatment and include AFP, b-HCG, and lactate dehydrogenase (LDH). Seminoma can have elevated or normal b-HCG and LDH levels, while an elevated AFP level indicates nonseminomatous components.1,2
Germ cell neoplasia in situ, formerly intratubular germ cell neoplasia, increases the risk of germ cell tumor (GCT) formation. Other risk factors include history of contralateral testicular cancer, infertility, cryptorchidism, and disorders of sexual differentiation. GCNIS can be found adjacent to GCTs in nearly all specimens and in 4-8% of contralateral testes,3 and 70% of patients with GCNIS develop invasive GCT within 7 years.2
Ultrasound is the standard imaging test for evaluation of scrotal pain and palpable masses.4,5 Seminomas are typically homoge- neous and hypoechoic on US, with smooth or lobulated borders. Hemorrhage, cysts, and calcification are uncommon but can be found in 10-30% of these tumors.1,6 Nonseminomatous GCTs have a more heterogeneous appearance owing to the variable tissue composition, and usually have ill-defined margins, internal cystic spaces, or calcifications.3
Testicular microlithiasis, or calcium deposition within the seminiferous tubules, is seen at US as echogenic foci in the parenchyma ranging from 1-3 mm in size. These calcifications can be seen with testicular tumors, as well as incidentally when no mass is found, and whether they serve as a risk factor or marker of neoplasm is unclear.7 The American Association of Urology does not recommend follow up for patients with isolated microlithiasis and no other risk factors for GCT.8
Traumatic testicular injuries have several US features to help distinguish them from testicular neoplasms. Acutely, intratesticular hematomas are avascular, may appear isoechoic or heterogeneous in echogenicity, and should change appearance over hours to days as the blood products liquify and become more hypoechoic. Hematomas should rapidly decrease in size but may be heterogeneous owing to necrosis or becoming abscesses. Testicular rupture is identified by irregular contour resulting from disruption of the tunica albuginea.9
Radical inguinal orchiectomy is the gold standard treatment for testicular masses. However, testis-sparing surgery has been deemed a reasonable option for tumors smaller than 2 cm in diameter and less than 50% of total testis mass. Because of the risk of seeding with a trans-scrotal approach, a partial or radical orchiectomy is performed through an inguinal approach. Patients treated with partial orchiectomy without adjuvant therapy have a greater incidence of local recurrence compared with those who receive adjuvant radiotherapy.10
The prognosis for testicular cancer is excellent, with a 5-year survival rate of about 95% for localized testicular and regionally metastatic tumors. Distant metastatic tumors have a 5-year survival rate of about 72%.6 After surgery, stage I tumors may be managed with surveillance, while stage IIA and some stage IIB tumors may be treated with radiation. Chemotherapy is used for stage IIB, IIC, and III disease.1
Conclusion
Seminoma is the most common testicular tumor and presents as a painless mass between the ages of 30 and 40 years. Ultrasound is the standard imaging test for testicular masses, and seminomas are typically homogeneous and hypoechoic, with smooth or lobulated borders. Microlithiasis may be present, although it is unclear whether this finding serves as a risk factor or marker of neoplasm. Radical inguinal orchiectomy is the gold standard treatment for testicular masses, but testis-sparing surgery is a reasonable option for small tumors. Treatment may also include surveillance, radiation, or chemotherapy, depending on tumor staging. The overall prognosis for patients with testicular cancer is excellent.
References
Citation
J H, LF A. Bilateral Germ Cell Neoplasia In Situ with Left Testicular Seminom. Appl Radiol. 2023;(5):44-46.
September 12, 2023