Imaging Findings in the Setting of Rhabdomyolysis
Rhabdomyolysis is a syndrome characterized by the disruption of skeletal muscle leading to the release of intracellular muscle constituents, including myoglobin and creatine kinase, into the circulation and extracellular spaces. The clinical presentation is broad, ranging from asymptomatic to life-threatening.
The most significant complication is acute kidney injury, which occurs in up to 40% of patients. Other serious complications include disseminated intravascular coagulation and compartment syndrome.1 Risk factors that predict a poor outcome (eg, a need for renal replacement therapy, or death) include advanced age, female sex, initial creatine, creatinine phosphokinase, phosphate, calcium, and bicarbonate levels.2 Characteristic symptoms of rhabdomyolysis include muscle pain, weakness, and dark urine, although over half of patients do not report muscle symptoms.3 Physical findings include muscle tenderness and weakness. Muscle swelling typically occurs later in the hospital course during fluid repletion.
Creatine kinase, the most sensitive lab abnormality for muscle injury, is released into the bloodstream within 12 hours of injury. Although no specific threshold has been established for the diagnosis of rhabdomyolysis, a creatine kinase level of at least five times the upper limit of normal (ie, 1000 IU/L) is commonly used. A creatine kinase level of >5000 IU/L generally indicates a degree of muscle injury sufficient to cause acute kidney damage. Myoglobinuria is responsible for the brown to red urine of patients with this condition.
Additionally, the accumulation of myoglobin, a nephrotoxin, is responsible for the acute kidney injury of rhabdomyolysis. However, myoglobin has a short half-life and therefore may not be detected in the serum or urine of patients experiencing significant rhabdomyolysis.4,5
Imaging Considerations
Rhabdomyolysis is not diagnosed specifically with imaging, but rather through a final pathway shared by numerous etiologies resulting in muscle damage. Imaging findings depend on the underlying cause, although typically the muscles will display signs of edema and/or hemorrhage.
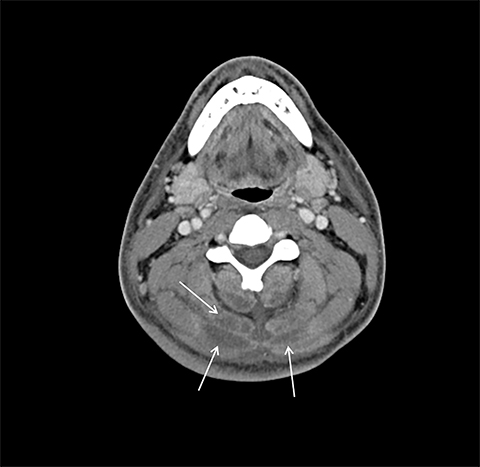
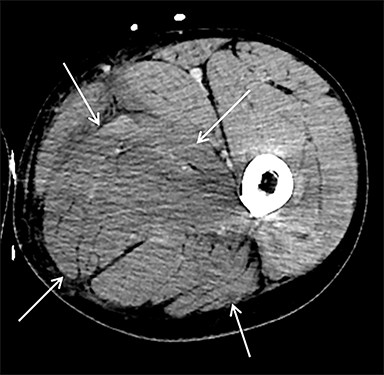
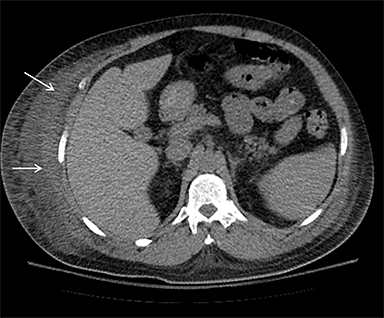
On computed tomography (CT), edematous muscles are typically hypodense and may be enlarged (Figure 1A). As muscle damage progresses, peripheral enhancement surrounding areas of infarcted or necrotic tissue may be demonstrated on postcontrast images (Figure 1B).
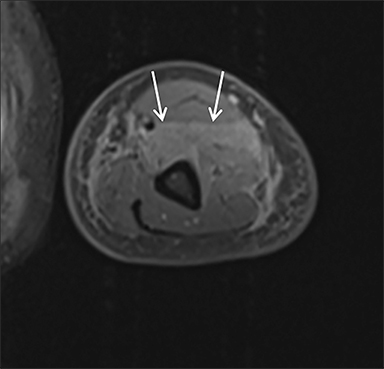
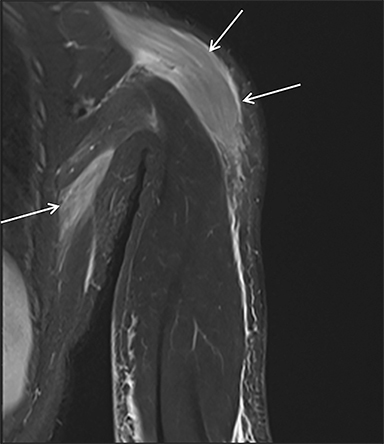
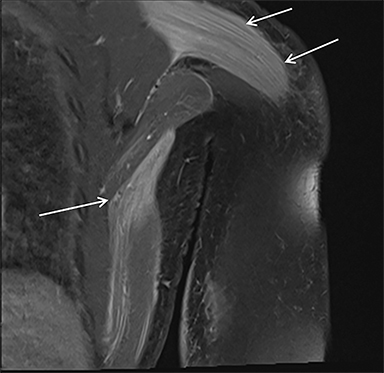
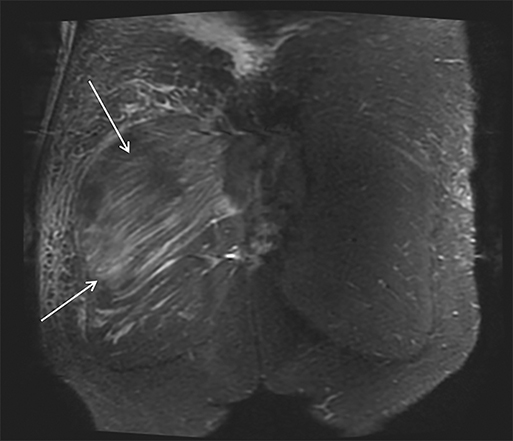
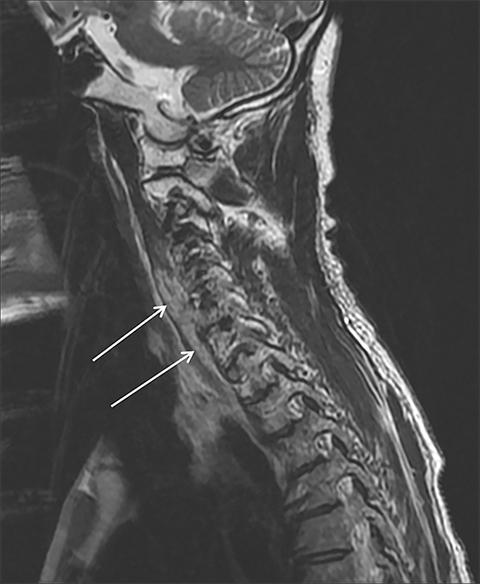
Magnetic resonance imaging (MRI) is more sensitive than CT to muscle damage.6 Involved muscle is usually isointense on T1 sequences, although mild T1 hyperintensity owing to hemorrhage or protein concentration may be demonstrated, particularly on fat-saturated sequences (Figure 2). The affected musculature typically displays high signal on T2 or short tau inversion recovery (STIR) images (Figure 2).7 The broad differential diagnosis of muscle edema can be narrowed by separating cases into symmetric or asymmetric patterns.
Symmetric causes of muscle edema are typically secondary to inflammatory (polymyositis, dermatomyositis, human immunodeficiency viral associated (HIV), myositis) and drug-related myopathies. Asymmetric edema is more likely to be caused by trauma, pyomyositis, radiation, myonecrosis, denervation, and compartment syndrome.8 Following gadolinium-based contrast administration, involved muscles may demonstrate homogeneous enhancement prior to liquefaction or heterogeneous enhancement (stipple sign), reflecting viable tissue intermixed with necrotic muscle (Figure 2).8 Scintigraphy is not a primary imaging modality in the setting of rhabdomyolysis, although muscle injury may be demonstrated on bone scans.
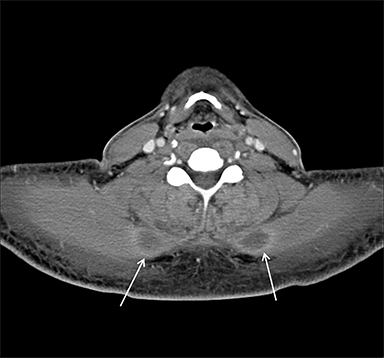
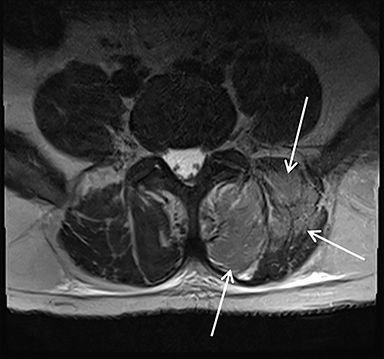
Acute compartment syndrome resulting from elevated compartmental pressure is a complication of rhabdomyolysis. The diagnosis is clinical and supported by an abnormal intracompartmental pressure measurement. Imaging plays a limited role in diagnosing acute compartment syndrome, but it may demonstrate normal or increased T1 signal within muscle, owing to hemorrhage and increased T2 signal secondary to edema. Decreased enhancement on CT or MRI within the compartment can be demonstrated on postcontrast images (Figure 3).8
Causes of Rhabdomyolysis
Any condition that leads to extensive muscle damage may theoretically result in rhabdomyolysis. A study of 425 adult patients with rhabdomyolysis found drug or alcohol abuse, medicinal drug use, and immobility to be leading causes.9 The multitude of etiologies can be organized into three main categories:
• Trauma or long-standing compression of muscle (prolonged immobilization, crush injury, electric shock, muscle tear);
• Nontraumatic (strenuous exercise, seizures, delirium tremens, muscular dystrophy, hyperthermia, heat stroke, diabetic ketoacidosis, myopathy); and,
• Muscle toxins (illicit drugs; prescription drugs, including statins; influenza, HIV, and herpes virus; bacterial infection; snake venom).1,5
Muscle Compression or Trauma
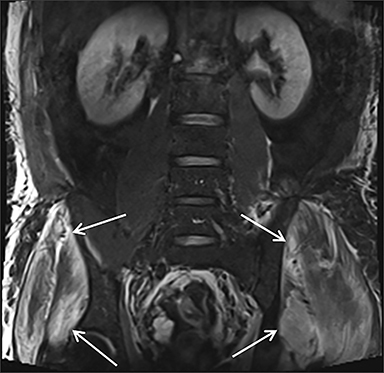
Muscle compression during prolonged unconsciousness, rehabilitation, or surgery may lead to muscle oxygen deprivation, eventually resulting in muscle necrosis and rhabdomyolysis. Muscle compression resulting in ischemia can develop after drug or alcohol intoxication in which the patient has been lying or sitting in one position for a prolonged period of time (Figures 4,5). Crush injuries and blunt trauma are also common etiologies of rhabdomyolysis, particularly in the elderly. Amongst 167 patients with rhabdomyolysis, all of whom were over 65 years, 56.9% of cases were caused by falls.10 In the setting of a crush syndrome, such as from a building collapse or automobile crash, rhabdomyolysis occurs once the muscle compression is relieved. Injuries related to high-voltage electricity (electrocution, lightning strikes) cause rhabdomyolysis in up to 10% of survivors.1
Nontraumatic Muscle Damage
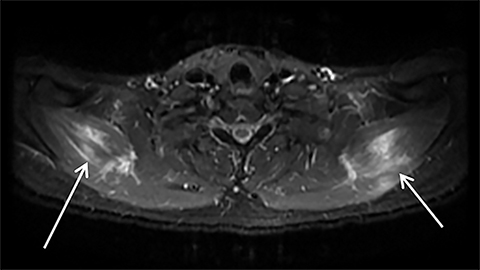
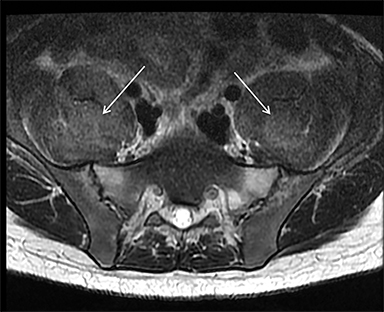
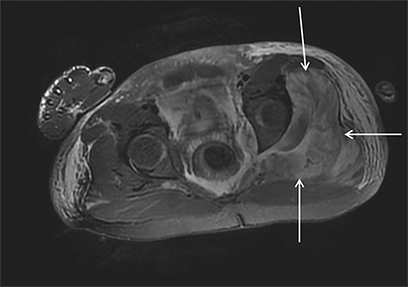
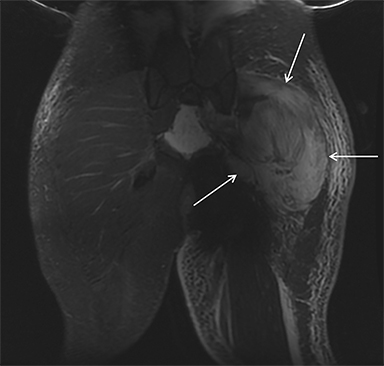
Rhabdomyolysis may occur when the metabolic demands of normal muscle exceed the energy supply. Although serum creatine kinase naturally rises after strenuous exercise, rhabdomyolysis typically occurs when exertion generates levels of pain and fatigue beyond that which would normally compel an individual to stop exercising. Factors such as competitive athletic or military training, extreme sports, and peer pressure during group exercise may predispose individuals to this level of exertion (Figures 2,6). Compounding risk factors include insufficient physical training and exertion in hot and humid conditions. Additionally, pathologic muscle exertion during delirium tremens, psychotic agitation, and seizures (Figure 7) may result in rhabdomyolysis.3 Other causes of nontraumatic muscle damage include malignant hyperthermia, neuroleptic malignant syndrome, and heat stroke.1,3
Muscle Toxins
Prescription medications and abused substances may produce a myopathy leading to rhabdomyolysis. Statin medications are a well-known cause of muscle toxicity and rhabdomyolysis (Figure 8), especially in patients with predisposing conditions such as hypothyroidism, those on high doses of these medications, advanced age, and female sex.1,12,13 Other classes of drugs that can cause rhabdomyolysis include psychiatric agents, non-statin lipid-lowering agents, and antihistamines.
Substance abuse can result in metabolic acidosis and induce rhabdomyolysis. Alcohol, cocaine, heroin, and amphetamines have all been implicated. Myopathy secondary to systemic drug use typically affects the buttocks, quadriceps, adductors, and calf muscles in a symmetric distribution.8 Toxins, including poisons and venoms, may directly interfere with muscle energy production, cause electrolyte abnormalities, or degrade the muscle cell.14,15
A vast number of bacterial and viral infections (eg, influenza, HIV, herpes simplex) can cause muscle damage. Infections that invade the muscle cells may lead to muscle necrosis. Some infections may generate toxins that inhibit metabolic activity, inducing rhabdomyolysis.1 MRI of pyomyositis-induced rhabdomyolysis demonstrates swelling of involved muscles on T1 images. T2 images display increased muscle signal owing to pus and edema (Figure 9). Following contrast administration, small fluid collections representing myonecrosis or larger, non-enhancing abscesses may be demonstrated.8
Rhabdomyolysis Treatment
The specific cause of muscle damage, such as drugs, toxins, or infection, should be identified and, where possible, eliminated.15 Intravenous fluid replacement to achieve a urine output of 300 mL/h is important to prevent acute kidney injury. Similarly, bicarbonate administration can prevent acute kidney injury by increasing urine pH, which helps mitigate myoglobin toxicity.15,16 Closely monitoring patients for fluid and electrolyte abnormalities is essential for preventing metabolic disturbances. Patients with severe kidney damage may require dialysis;17 those with suspected acute compartment syndrome require urgent surgical consultation.
Conclusion
Rhabdomyolysis is a complex syndrome characterized by significant muscle damage and subsequent release of muscle components into the bloodstream and extracellular spaces. The most significant complication is acute kidney injury. The numerous causes can be categorized into three main groups: Muscle compression or trauma, nontraumatic/exertional muscle damage, and toxins. Rhabdomyolysis is a clinical diagnosis and imaging reflects the underlying cause and extent of muscle damage. Involved muscles typically display enlargement, edema, and hemorrhage.
References
- Torres PA, Helmsetter JA, Kaye AM, Kaye AD, Kaye AD. Rhabdomyolysis: Pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15: 58-69.
- McMahon G, Zeng X, Waikar S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2016;173:1 821-1828.
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore). 1982;61:141-152.
- Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Critical Care. 2005;9:158.
- Miller, M. Causes of rhabdomyolysis. UptoDate. 2020. https://www.uptodate.com/contents/causes-of-rhabdomyolysis.
- Lamminen A, Hekali P, Tula E, et al. Acute rhabdomyolysis: evaluation with magnetic resonance imaging compared with computed tomography and ultrasonography. Br J Radiol. 1989;62:326-331.
- Moratella MB, Braun P, Fornas GM. Importance of MRI in the diagnosis and treatment of rhabdomyolysis. Euro J Radiol. 2008;65:311-315.
- Smitaman E, Flores DV, Gomez CK, Pathria MN. MR imaging of atraumatic muscle disorders. Radiographics. 2018;38:500-522.
- Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore). 2005;84:377.
- Wongrakpanich S, Kallis C, Prasad P, et al. The study of rhabdomyolysis in the elderly: An epidemiological study and single-center experience. Aging Dis. 2018;9:1-7.
- Landau ME, Kenny K, Deuster P, Campbell W. Exertional rhabdomyolysis: A clinical review with a focus on genetic Influences. J Clin Neuromuscul Dis. 2012;13:122-136.
- Antons, KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of stain-induced rhabdomyolysis. Am J Med. 2008;119:400.
- Kiernan TJ, Rochford M, McDermott JH. Simvastatin-induced rhabdomyolysis and an important clinical link with hypothyroidism. Int J Cardiol. 2007;119:374.
- Prendergast BD, George CF. Drug-induced rhabdomyolysis---mechanisms and management. Postgrad Med J. 1993;69:333.
- Criddle LM. Rhabdomyolysis: Pathophysiology, recognition, and management. Crit Care Nurse. 2003;23:14.
- Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: A systematic review of rhabdomyolysis for clinical practice. Critical Care. 2016;20:135.
- Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553.
Affiliations: Butler University, Indianapolis, Indiana (Emily Neal); Department of Radiology, Mt Carmel St Ann’s Hospital, Westerville, OH, and Radiology Incorporated, Dublin, OH. (Dr Burky). Disclaimers: None. Prior publication/presentation: No portion of this manuscript has been previously published.
