Aortoenteric Fistula Following Aortobifemoral Grafting
Images


Case Summary
An adult with diabetes presented to the emergency department with a 3-week history of right-sided abdominal and right lower extremity pain. The patient was 2 years status postaortobifemoral (type) bypass graft.
Imaging Findings
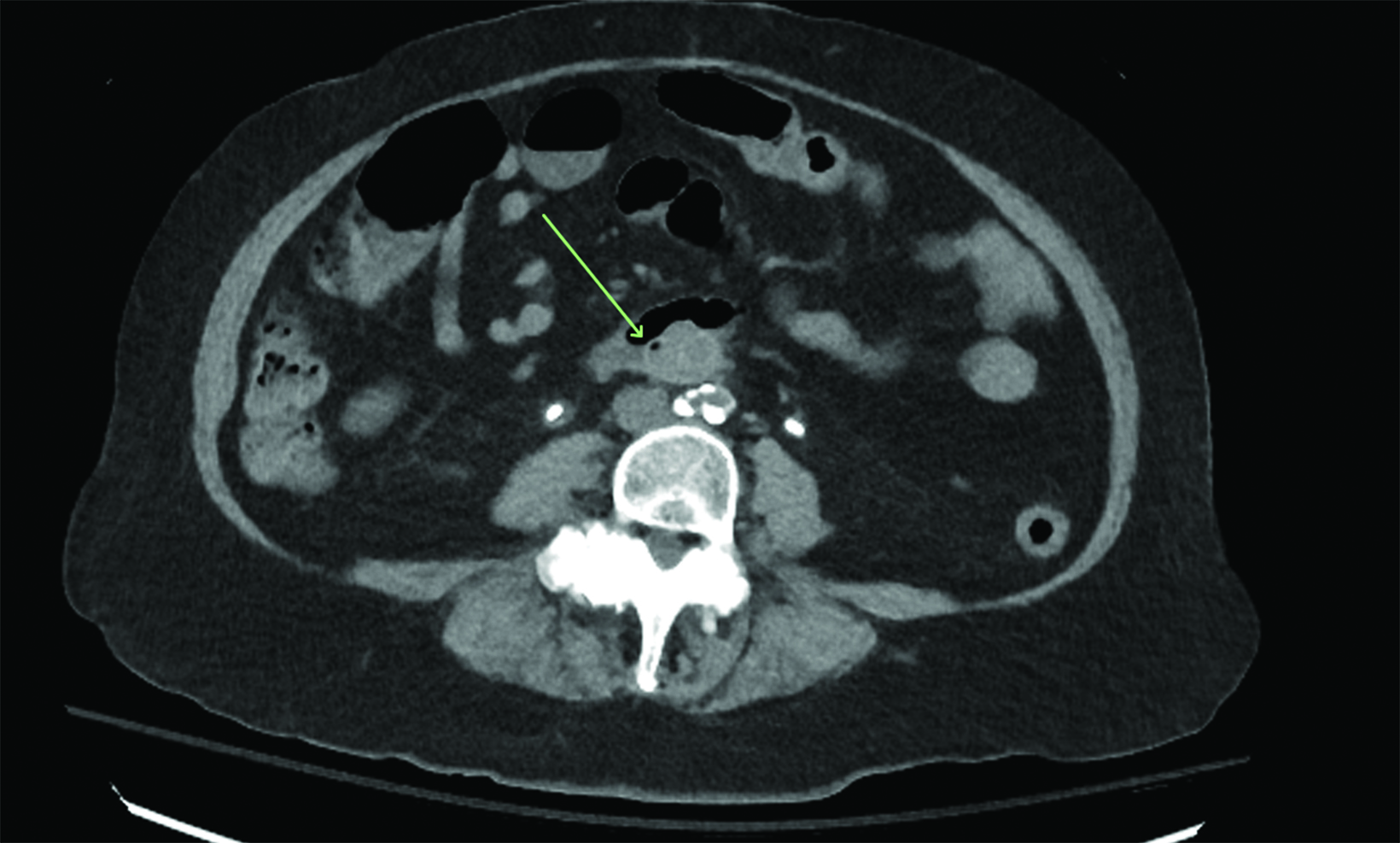
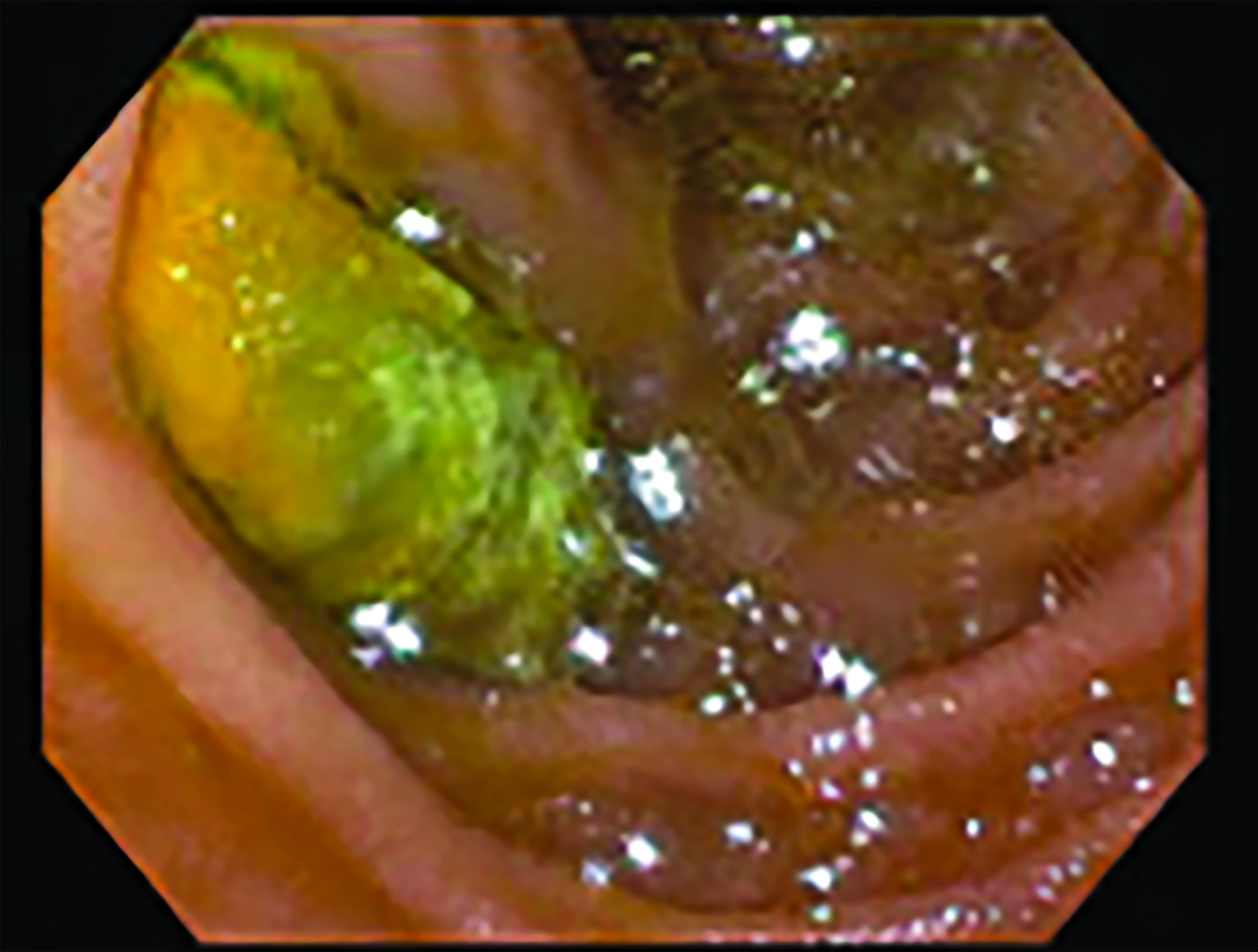
Initial noncontrast CT (Figure 1) demonstrated an aortoiliac bypass graft abutting the second portion of the duodenum without an intervening fat plane. There were several locules of gas in the proximal right iliac limb of the bypass graft concerning for abnormal fistulous communication between the duodenum and the graft. Contrast-enhanced CT corroborated this finding and further demonstrated complete occlusive thrombus of the right iliac limb of the bypass graft. Subsequent positron emission tomography (PET)/CT (Figure 2) demonstrated fluorodeoxyglucose avidity of the entire aortoiliac bypass graft, raising strong suspicion for an infected bypass graft. Following CT angiography, an upper endoscopy confirmed the suspected diagnosis of aortoenteric fistula (AEF, Figure 3.).
Diagnosis
Aortoenteric fistula
Discussion
Primary AEFs can be caused by abdominal aortic aneurysms, which have mass effect upon gastrointestinal (GI) structures and can erode into the bowel in the presence of inflammation. Primary AEFs have an incidence of 0.04-0.07%, and are typically associated with septic aortitis, cancer, and autoimmune diseases.1< Secondary AEFs usually result from aortic prosthetic graft erosion after open repair and affect the third portion of the duodenum because of this portion’s retroperitoneal fixation and proximity to the aorta. The overall incidence of secondary AEFs is 0.36-1.6%, and they are rarely observed after endovascular aneurysm repair.2
Aortoenteric fistulae typically present with minor “herald” GI bleeding followed by catastrophic life-threatening hemorrhage, making early diagnosis and treatment crucial for improved clinical outcomes.3 In addition to recurrent septicemia from enteric pathogens and abdominal pain, some patients may present with a pulsatile abdominal mass that indicates an aneurysm.4 Management of AEFs involves surgical intervention, including repair or excision of the affected aortic segment, as well as GI reconstruction if necessary. Although no imaging modality provides high sensitivity and specificity to diagnose AEFs, CT is the preferred for emergency evaluation, owing to its speed and availability. The accuracy of CT in diagnosing AEFs varies widely, with sensitivity ranging from 40% to 90% and specificity ranging from 33% to 100%.5
The development of secondary aortoenteric fistula involves various mechanisms, including bacterial contamination of a prosthetic graft leading to infection, anastomotic failure, and perigraft infection.3 Other factors contributing to secondary AEF pathogenesis include bowel damage, ischemia, mechanical injury, inflammation, and erosion. Pseudoaneurysms or perigraft abscesses can compress or invade the intestinal lumen, while mechanical erosion of the intestinal wall can be caused by graft-induced inflammation and infection. In this case, the placement of the graft near the small bowel may have resulted in repetitive microtrauma from pulsatile motion, leading to bacterial seeding and graft infection and ultimately resulting in aortic graft thrombosis. The breakdown of the graft wall resulted in the formation of a fistulous connection.
The cardinal manifestations of secondary AEF primarily consist of GI bleeding and severe hemorrhagic shock.6 Frequently, patients with AEF may experience graft infection, which can manifest with fever and sepsis. Our patient had right lower extremity pain secondary to aortoiliac occlusive disease stemming from the thrombosed graft. This thrombus can be protective against active arterial extravasation into the GI tract.
This patient exhibited marked leukocytosis without fever, raising concerns of possible graft infection, a suspicion that was confirmed by the PET scan findings. PET scanning can help determine the extent of infection in a graft, which can help guide the extent of graft explant. The surgical approaches in these cases may be open or endovascular and should be individualized for each patient.
Conclusion
Secondary AEF is a rare and life-threatening complication of abdominal aortic aneurysm repair. Diagnosis of secondary AEF can be challenging and relies on a high index of clinical suspicion in patients with prior aortic intervention. Distinctive CT findings associated with AEF include ectopic gas within the aortic wall, thickening of the local bowel near the aorta, and rupture of the aortic wall. This case highlights the importance of recognizing the potential for mechanical factors to contribute to the development of secondary aortoenteric fistula.
References
Citation
G PPW, S L, B C, P K. Aortoenteric Fistula Following Aortobifemoral Grafting. Appl Radiol. 2024;(2):46-47.
March 5, 2024