Angiomyolipoma in Tuberous Sclerosis
Images


Case Summary
An adolescent presented to an outside institution with syncope, right upper quadrant abdominal pain, and pallor. The child’s medical history was significant for tuberous sclerosis, developmental delay, and an hepatic cavernous malformation. Physical exam demonstrated ash-leaf patches over the torso. Laboratory findings revealed a hemoglobin level of 9.6 g/dL, while a CT abdomen/pelvis demonstrated an acute, large, intra-abdominal hemorrhage. The patient was transferred to our emergency department and active bleeding was confirmed with a repeat hemoglobin of 6.6 g/dL. Vital signs were stable but significant for tachypnea with a respiratory rate of 40 breaths/min, tachycardia with a heart rate of 109 bpm, and normotensive with a blood pressure of 129/98 mmHg. The patient was transfused packed red blood cells while interventional radiology was consulted for embolization.
Imaging Findings
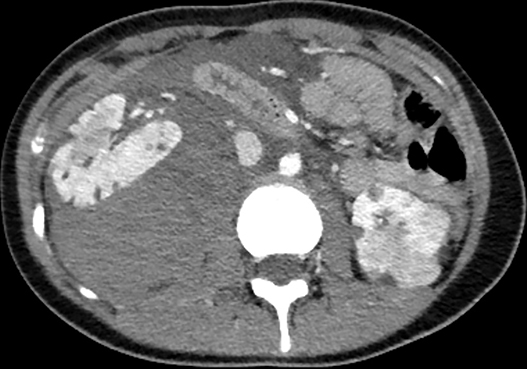
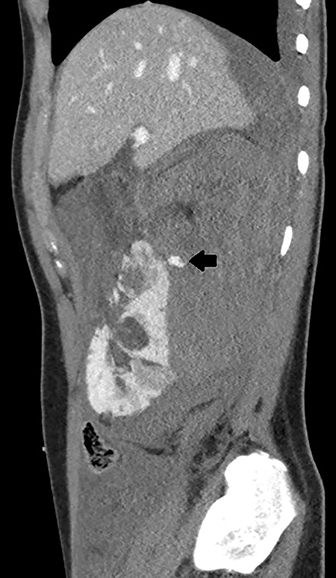
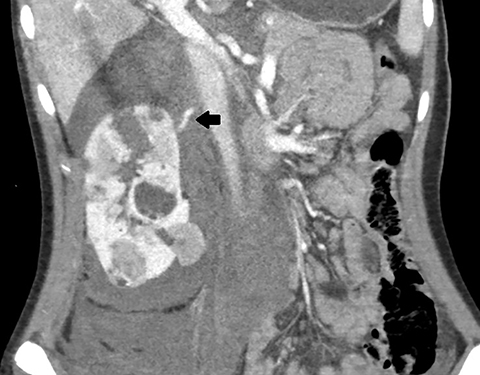
Axial, sagittal, and coronal contrast-enhanced CT of the abdomen and pelvis revealed multiple bilateral renal masses demonstrating fat-attenuation consistent with angiomyolipomas (AMLs). A large right pararenal hematoma displaced the kidney anteriorly with active contrast extravasation along the posteromedial aspect of the right upper pole (Figure 1).
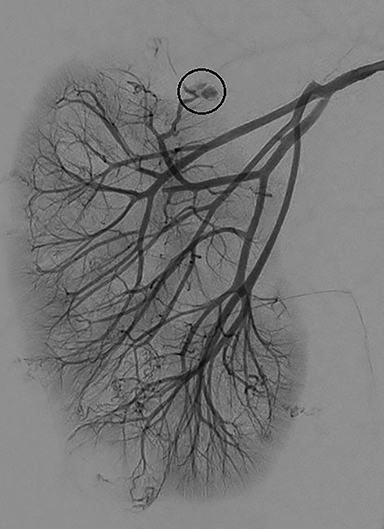
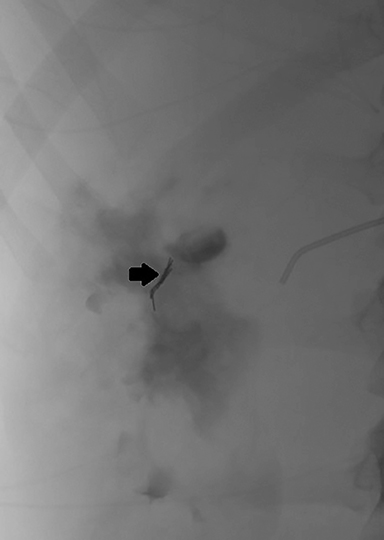
Subsequent right renal angiography revealed extravasation and pooling of contrast along the medial upper pole consistent with the site of active hemorrhage. Parenchymal phase digital subtraction angiography (DSA) image demonstrated persistence of contrast. Coil embolization was then performed with deployment immediately proximal to the site of active hemorrhage. Post-embolization arterial phase DSA demonstrated complete occlusion of the feeding artery with resolution of active hemorrhage (Figure 2).
Diagnosis
Active hemorrhage secondary to a ruptured angiomyolipoma in patient with tuberous sclerosis
Discussion
Renal AML is a benign, mesenchymal soft-tissue tumor that arises in the cortex or medulla of the kidney. AML is considered “triphasic” in that it consists of adipose tissue, blood vessels, and smooth muscle.1 AML can arise sporadically or through an inherited disorder. AML most commonly results from tuberous sclerosis compared to sporadic development.2 The most dominant type of AML present in tuberous sclerosis is of epithelioid origin.1
Histologic fat composition of the tumor allows ready diagnosis of AML by abdominal CT or MRI.3 Most sporadic AMLs are found incidentally; however, in a patient with tuberous sclerosis, a CT scan is ordered to look for potential renal AMLs. AMLs may contain macroscopic or microscopic fat. To distinguish between the two, an MRI may be ordered to assess for fat-suppression.3 Owing to their vascular nature, AMLs are prone to aneurysm formation and rupture.
Complications such as hemorrhage can result in a symptomatic drop in hemoglobin and anemia. Up to one-third of patients can present with hypovolemic shock.4 Symptomatic AMLs can present with Lenk triad, which consists of flank pain, abdominal tenderness and internal bleeding. Bleeding typically occurs when tumors are greater than 4 cm in size. Patients are typically asymptomatic when tumors are less than 4 cm.5,6
Traditional treatments of AML have included nephrectomies or partial nephrectomies. However, these therapeutic options are associated with maximal renal tissue loss, as well as significant complications and risks.7 Today, the preferential therapy is angiography with selective arterial embolization.8
Conclusion
Renal angiomyolipomas are commonly seen in patients with tuberous sclerosis. Despite their multiplicity, they rarely are prone to aneurysm formation or rupture when measuring less than 4 cm. Small lesions can, therefore, be closely monitored via imaging. AMLs larger than 4 cm have greater propensity for acute bleeding; thus, they are amenable to angiography and embolization, as opposed to traditional surgical therapeutic options even when asymptomatic. When symptomatic, acute arterial bleeding commonly requires urgent intervention.
References
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA (2004) World Health Organization classification of tumors: pathology and genetics. Tumors of the urinary system and male genital organs. Lyon: IARC Press.
- Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology. 2008;72(5):1077–1082.
- Schieda N, Hodgdon T, El-Khodary M, Flood TA, McInnes MDF. Unenhanced CT for the diagnosis of minimal-fat renal angiomyolipoma. AJR Am J Roentgenol. 2014;203(6):1236–1241.
- Rimon U, Duvdevani M, Garniek A, Golan G, Bensaid P, Ramon J, et al. Large renal angiomyolipomas: digital subtraction angiographic grading and presentation with bleeding. Clin Radiol. 2006;61(6):520–526.
- Oesterling JE, Fishman EK, Goldman SM, Marshall FF. The management of renal angiomyolipoma. J Urol. 1986 Jun;135(6):1121–1124.
- Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002;225(1):78–82.
- Faddegon S, So A. Treatment of angiomyolipoma at a tertiary care centre: the decision between surgery and angioembolization. Can Urol Assoc J. 2011;5(6):E138–41.
- Bissler J, Cappell K, Charles H, Song X, Liu Z, Prestifilippo J, et al. Rates of interventional procedures in patients with tuberous sclerosis complex-related renal angiomyolipoma. Curr Med Res Opin. 2015;31(8):1501–1507.
References
Citation
M M, D A, R B, RB T, AJ T, DJ A,. Angiomyolipoma in Tuberous Sclerosis . Appl Radiol. 2021;(4):53-55.
July 15, 2021