An Overview of Extracorporeal Membrane Oxygenation
Images







Extracorporeal membrane oxygenation (ECMO) is an advanced life support technique employed for severe respiratory, cardiac, or combined cardiopulmonary failure refractory to conventional treatments.1 The main objective of ECMO is to oxygenate systemic venous blood and remove carbon dioxide while the failing lung or heart are allowed to recover, or to serve as a bridge to longer-term life support therapies or transplantation.2
While traditionally viewed as a rescue therapy, ECMO has seen a notable surge in recent decades. Data from the international registry of the Extracorporeal Life Support Organization (ELSO) show that 16,803 ECMO procedures were conducted across 557 healthcare institutions worldwide in 2022.3
Complications related to ECMO are identified in nearly half of patients.4 The most common include hemorrhage, thromboembolic disease, renal failure, sepsis, and vascular injury.4
Noninvasive imaging plays a pivotal role in the assessment of ECMO patients, serving as a critical tool to detect complications or malpositioning of ECMO cannulas.5 Consequently, an understanding of normal and abnormal imaging appearances is imperative. Furthermore, an awareness of the hemodynamic disturbances that may arise with ECMO, necessitating specific considerations when planning contrast-enhanced studies, is essential for radiologists and imaging technologists.6 This article reviews the fundamentals of ECMO, explores the various cannulation techniques, and provides practical insights for the technical planning of contrast-enhanced CT studies in patients under ECMO support.
Fundamental Concepts
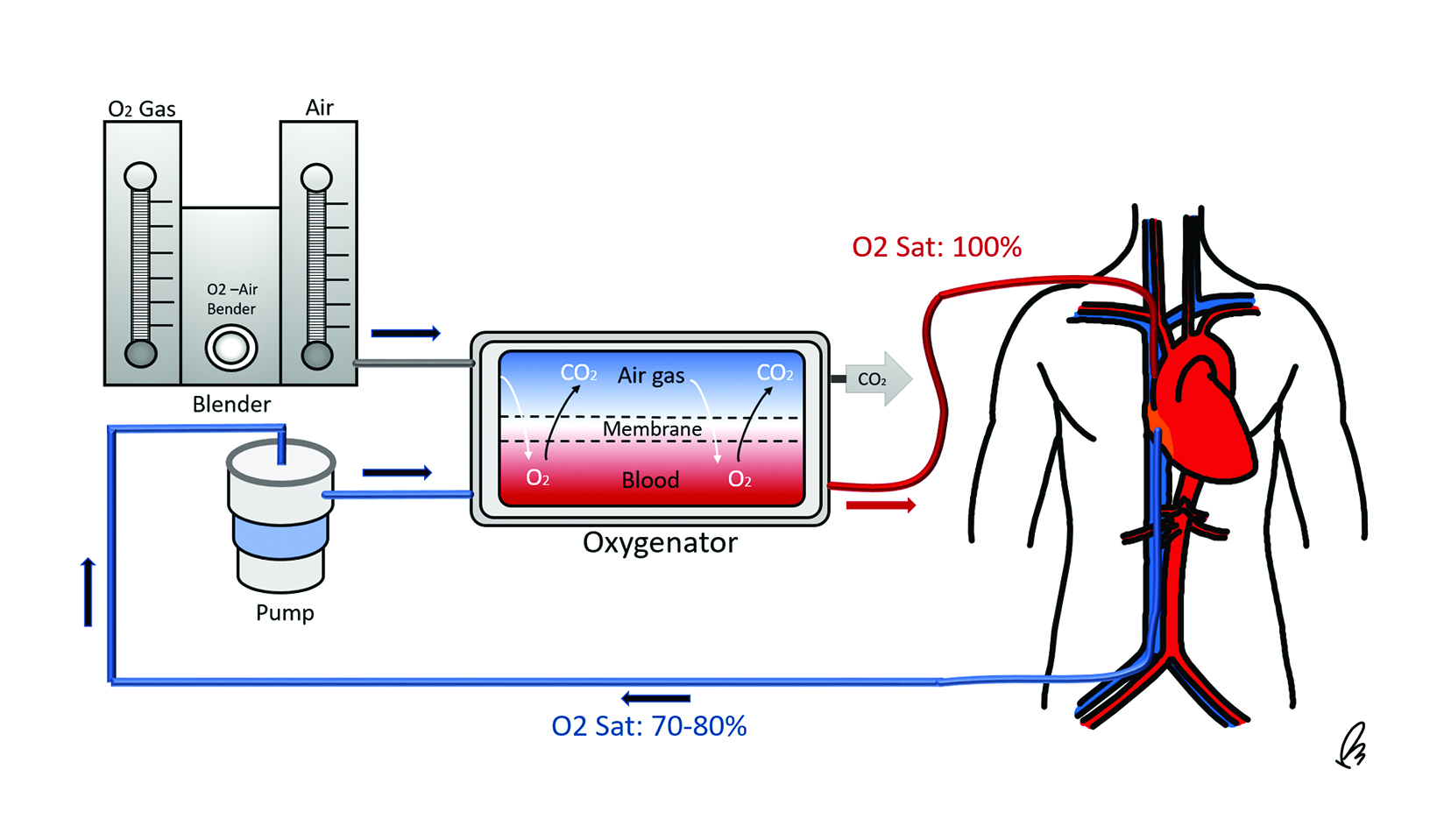
A standard ECMO circuit consists of several key components: an inflow cannula that drains deoxygenated venous blood from the body, a mechanical pump facilitating circulation of blood within the system, a membrane oxygenator that removes carbon dioxide and replenishes oxygen, a heat exchanger to warm blood as it passes through the oxygenator, and an outflow, or reinfusion, cannula that returns the oxygenated blood to circulation (Figure 1).7
There are two primary ECMO configurations, differentiated by the location and function of the return cannula: Veno-venous (VV) and Veno-arterial (VA) circuits. VV-ECMO is designed to provide respiratory support without cardiac support, as the oxygenated blood is reinfused to the systemic venous circulation. Therefore, this configuration relies on optimal cardiac function and is used only when cardiac output is adequate.
Conversely, VA-ECMO provides cardiac and respiratory support, bypassing the heart and lungs in a fashion similar to conventional cardiopulmonary bypass. In VA-ECMO, the return cannula is placed in the systemic arterial circulation, so the external pump aids in circulating the blood throughout the body.7,8
There are various cannulation alternatives for VV- and VA-ECMO; these are indicated based on availability and patient conditions and lead to different imaging appearances.9
Role of Imaging
Noninvasive imaging plays a pivotal role in the assessment of cannula positioning and timely detection of complications.10 Transthoracic or transesophageal echocardiography and fluoroscopy are usually used for guidance during initial cannulation.9,11 Chest and abdominal radiography is useful following initial placement to confirm adequate cannula positioning and reveal any unintended migration. Additionally, the radiographs may depict early signs of complications such as hemothorax or pneumothorax.
Ultrasound is a portable and readily available modality that may be employed to monitor complications such as insertion site hematomas, deep vein thrombosis, or distal limb hypoperfusion.
Computed tomography may be required to evaluate suspected complications that cannot be fully evaluated by radiography or ultrasound. The modality’s high spatial resolution allows for accurate assessment of cannula positioning. Additionally, administration of intravenous contrast allows for comprehensive evaluation of complications, including bleeding, vascular injury, and thromboembolic disease.
Veno-Venous ECMO: Subtypes and Normal Appearances
VV-ECMO may be considered in patients with severe, acute, and reversible respiratory failure that is refractory to standard medical management 12 or those who require a bridge to lung transplantation.13 A complete list of indications may be found in the latest ELSO guidelines.12 At present, the only absolute contraindication for initiating VV-ECMO is an expected inability of the patient to recover without a feasible plan for decannulation.12
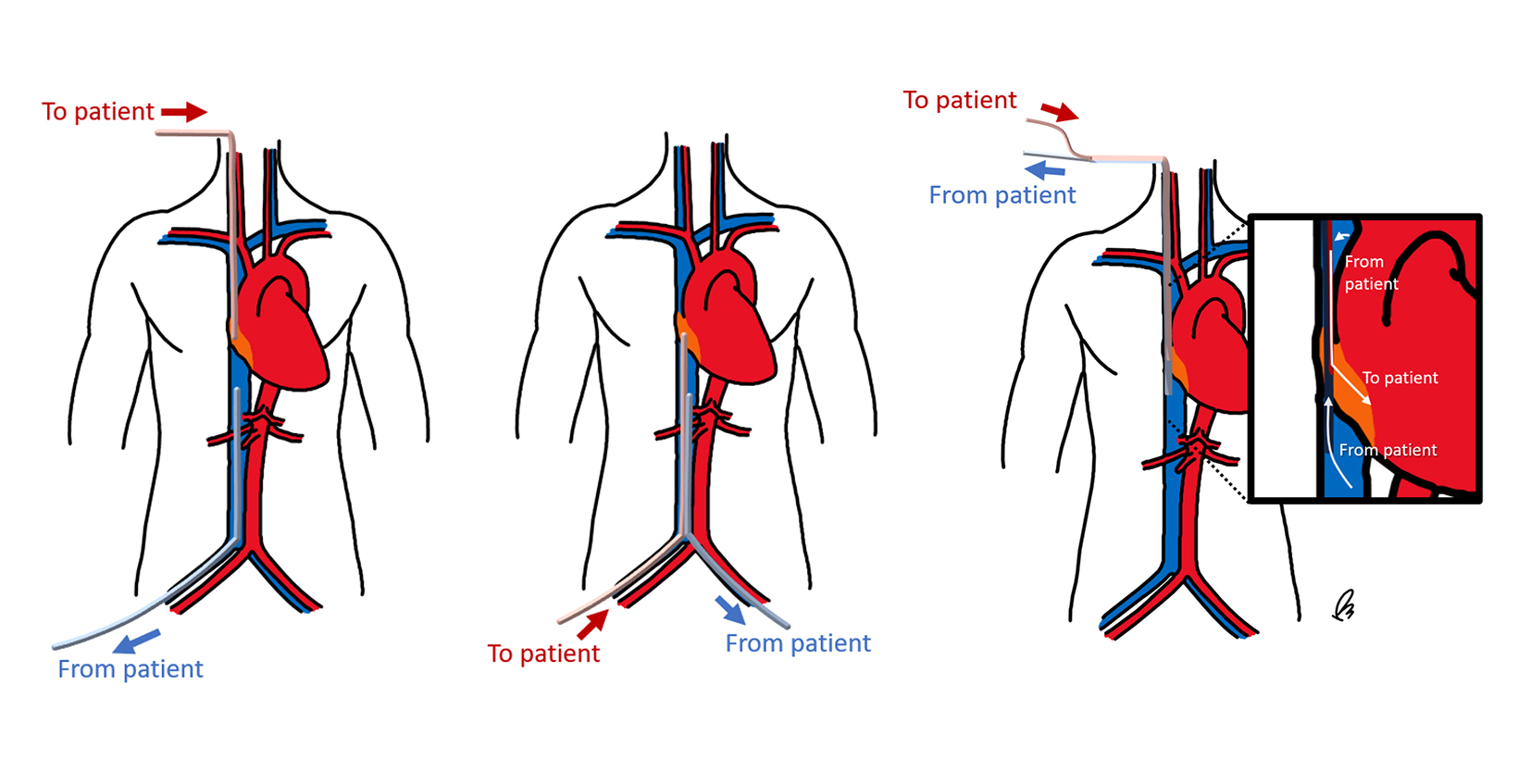
VV-ECMO can be performed either with two single-lumen cannulas or with a single dual-lumen cannula (Figure 2).14 When two single cannulas are used, they may be placed in the following locations:
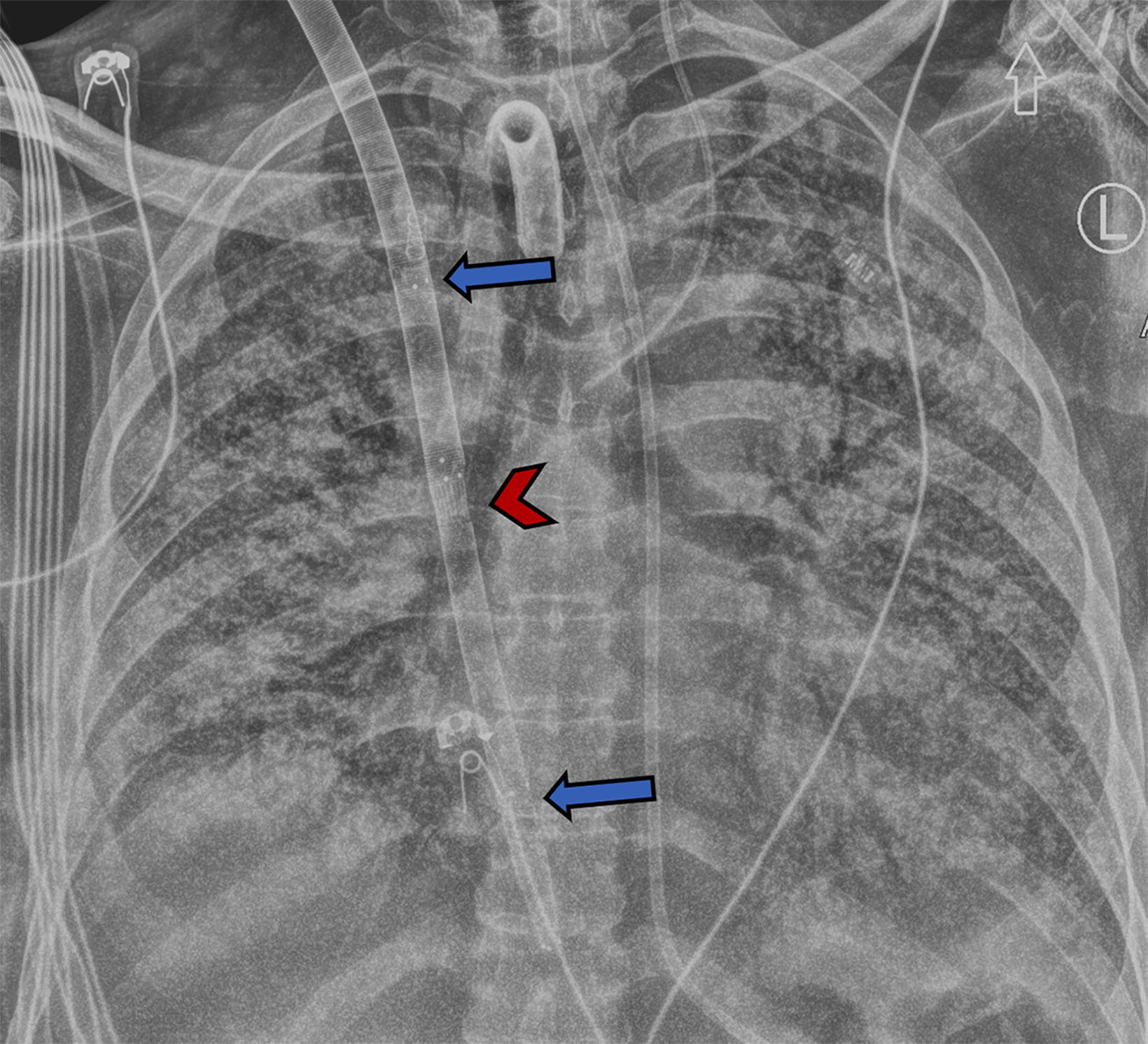
- Inferior Vena Cava – Right atrium The drainage cannula should be positioned within the IVC through a femoral vein with the side ports at the level of the hepatic veins, and the reinfusion cannula should be positioned in the superior cavoatrial junction or right atrium through the right IJV.9,12,14 Direction of the flow may be switched, but the latter configuration is preferred.14,15 Correct positioning of the cannulas is critical to prevent recirculation of reinfused oxygenated blood into the ECMO circuit before entering the systemic circulation; however, there is no standard distance that should be maintained between the ports.12,16
- Femoral vein–Femoral vein. In this approach, both cannulas enter through different femoral veins. The drainage cannula is located within the IVC, usually lower than with the IVC-RA approach. The reinfusion cannula should be located higher than the drainage cannula, at the level of the right atrium or cavoatrial junctions. This approach poses a higher risk of oxygenated blood recirculation, as both cannulas may be positioned closer together, but the complexity of insertion is lower, and it avoids the risk of neck vessel cannulation injury.8,17
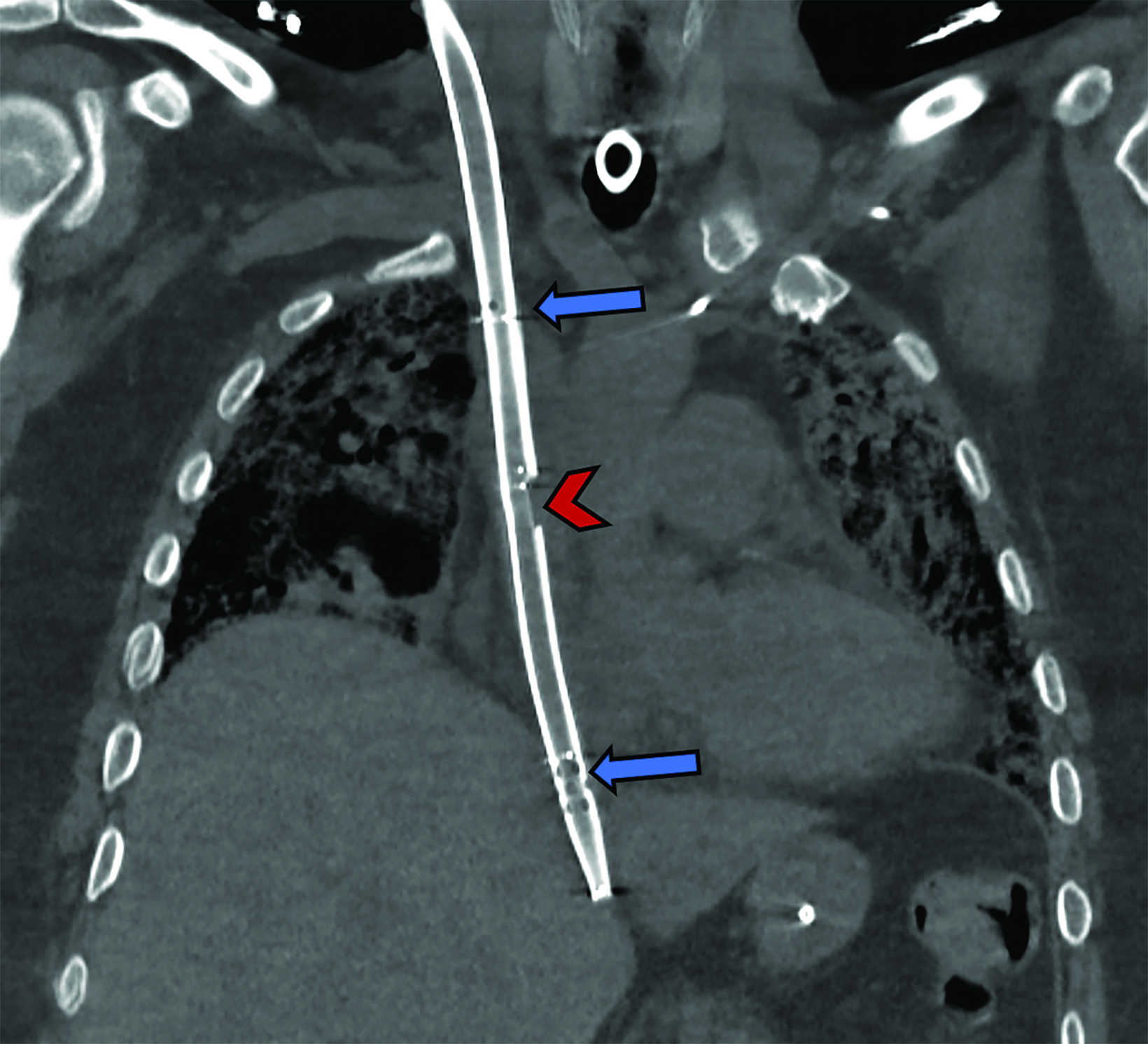
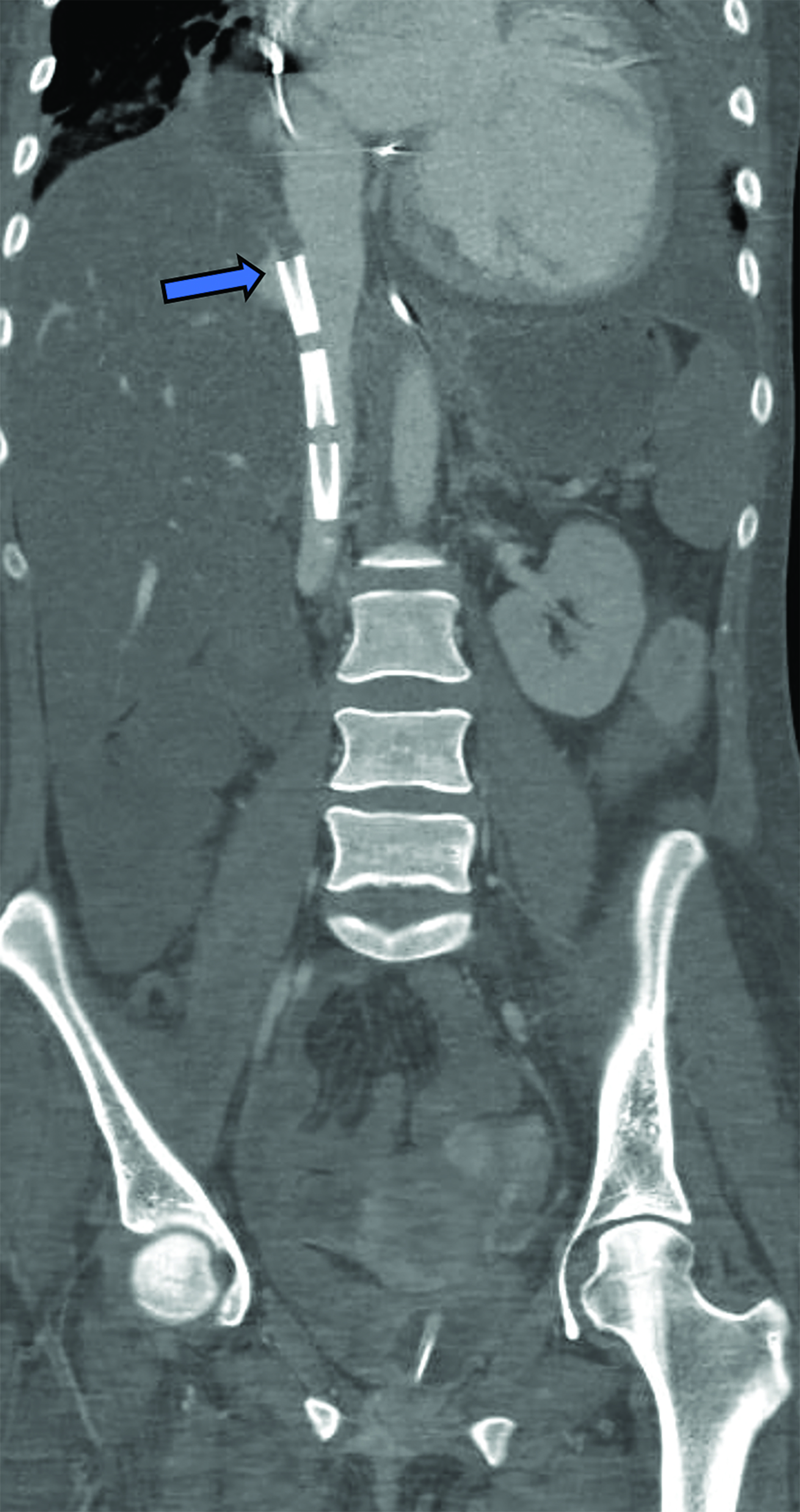
Single cannulation is achieved by inserting a double-lumen bicaval cannula with three ports through the right IJV. The proximal and distal ports drain deoxygenated blood and should be located in the superior and inferior vena cava, respectively. The mid port reinfuses oxygenated blood from the ECMO circuit and should rest in the RA, aiming towards the tricuspid valve.9,18 While dual-lumen VV-ECMO presents greater technical challenges compared to double cannulation methods, it offers advantages such as reduced recirculation rates and reduced bleeding risk due to the need for just a single vessel puncture. This is largely because most of the deoxygenated blood is drained from the proximal part of the cannula at the SVC (Figure 3).19,20
Veno-Arterial ECMO: Subtypes and Normal Appearances
VA-ECMO has evolved as a salvage strategy for patients with cardiogenic shock or cardiac arrest unresponsive to conventional treatments.21,22 However, lack of clear evidence has resulted in a low-level recommendation and no clear society-endorsed guidelines.23,24 In individuals experiencing severe yet potentially reversible cardiac injury like myocarditis, post-cardiotomy shock, or myocardial ischemia, VA-ECMO can serve as a bridge to recovery. Alternatively, for those with acute exacerbations of chronic cardiac failure or extensive myocardial infarction, VA-ECMO might be employed as a bridge to candidacy for a longer-term left ventricular assist device or heart transplantation.25,26
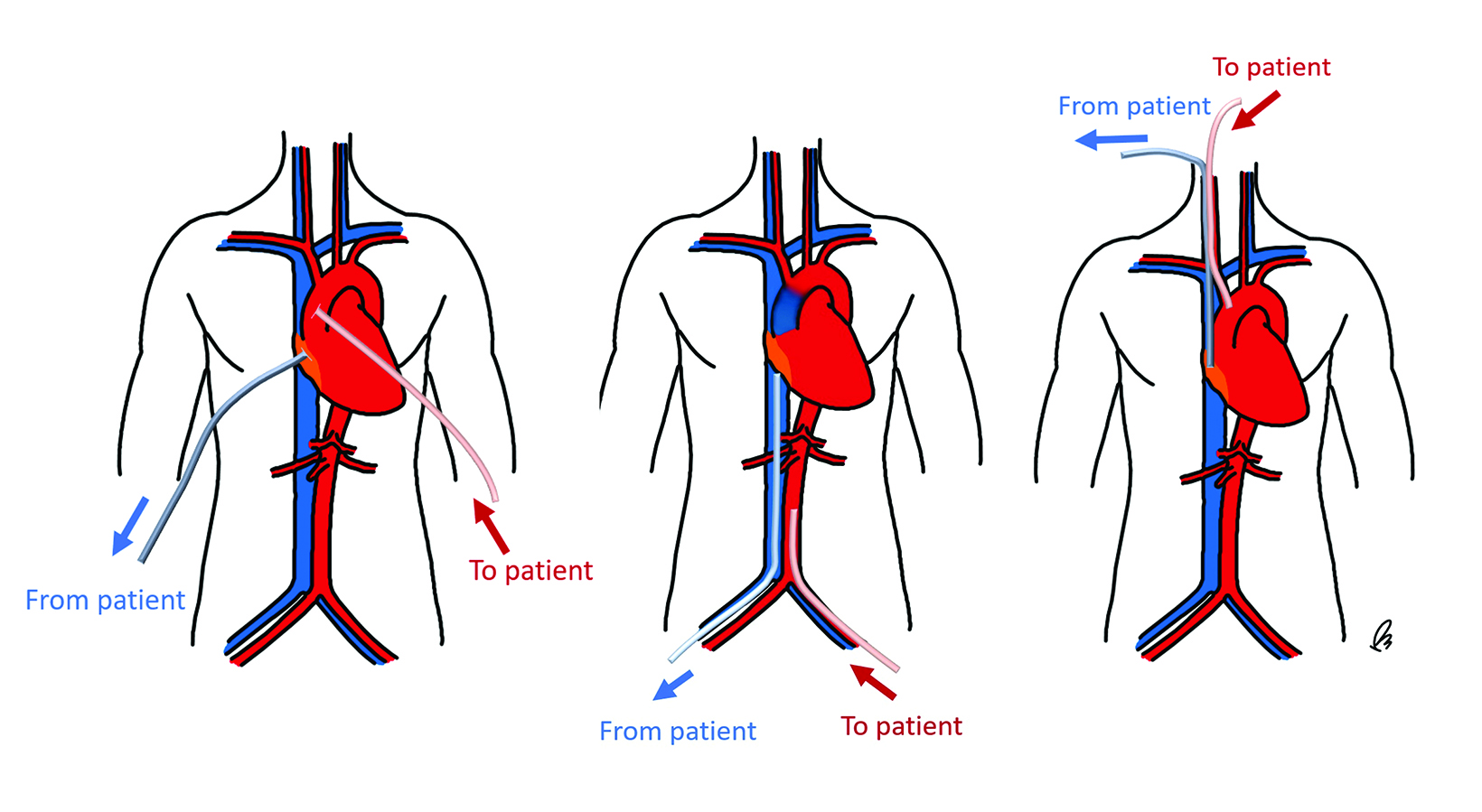
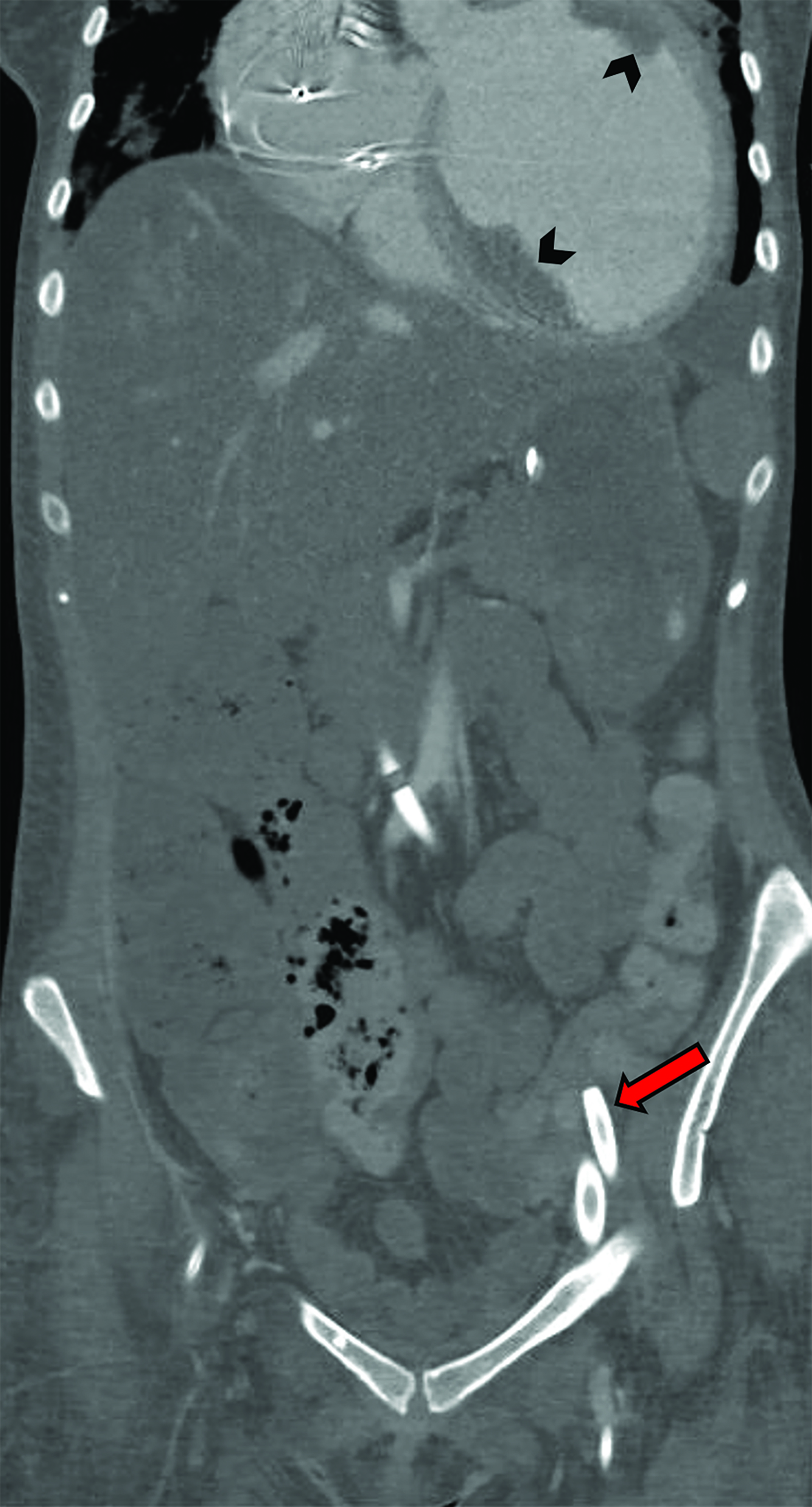
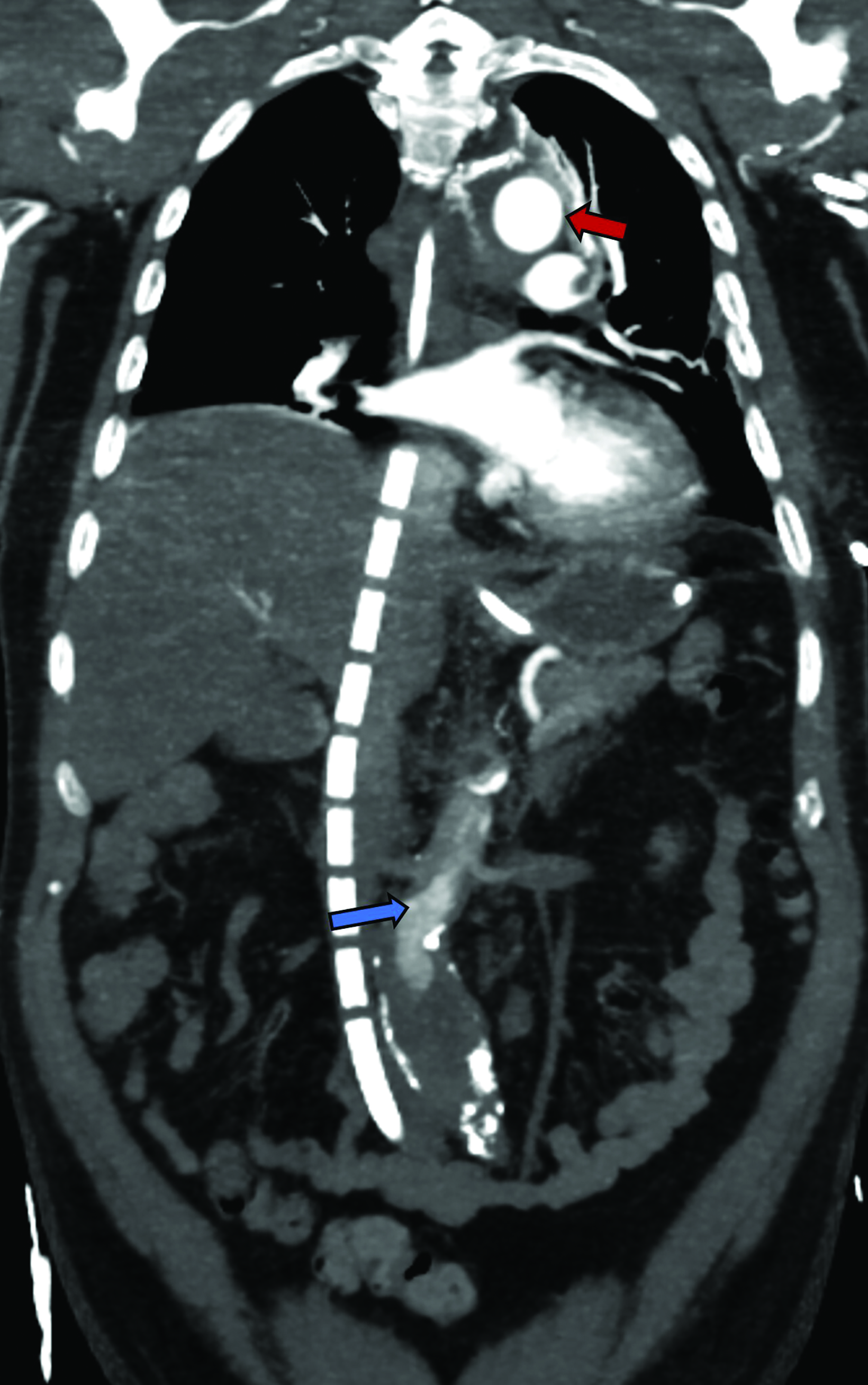
As in VV-ECMO, different circuit configurations account for distinct imaging appearances. The two main approaches are central and peripheral cannulation (Figure 4).27 Central VA-ECMO is typically initiated in the operating room for post-cardiotomy patients who cannot be weaned off cardiopulmonary bypass. The drainage and reinfusion cannulas are generally positioned directly into the RA and ascending aorta, respectively (Figure 5).28 With this configuration, the reinfused, oxygenated blood circulates in an antegrade, physiological direction. Owing to its invasive character, central VA-ECMO is primarily used during surgery when a patient is experiencing cardiogenic shock while the chest is still open.29
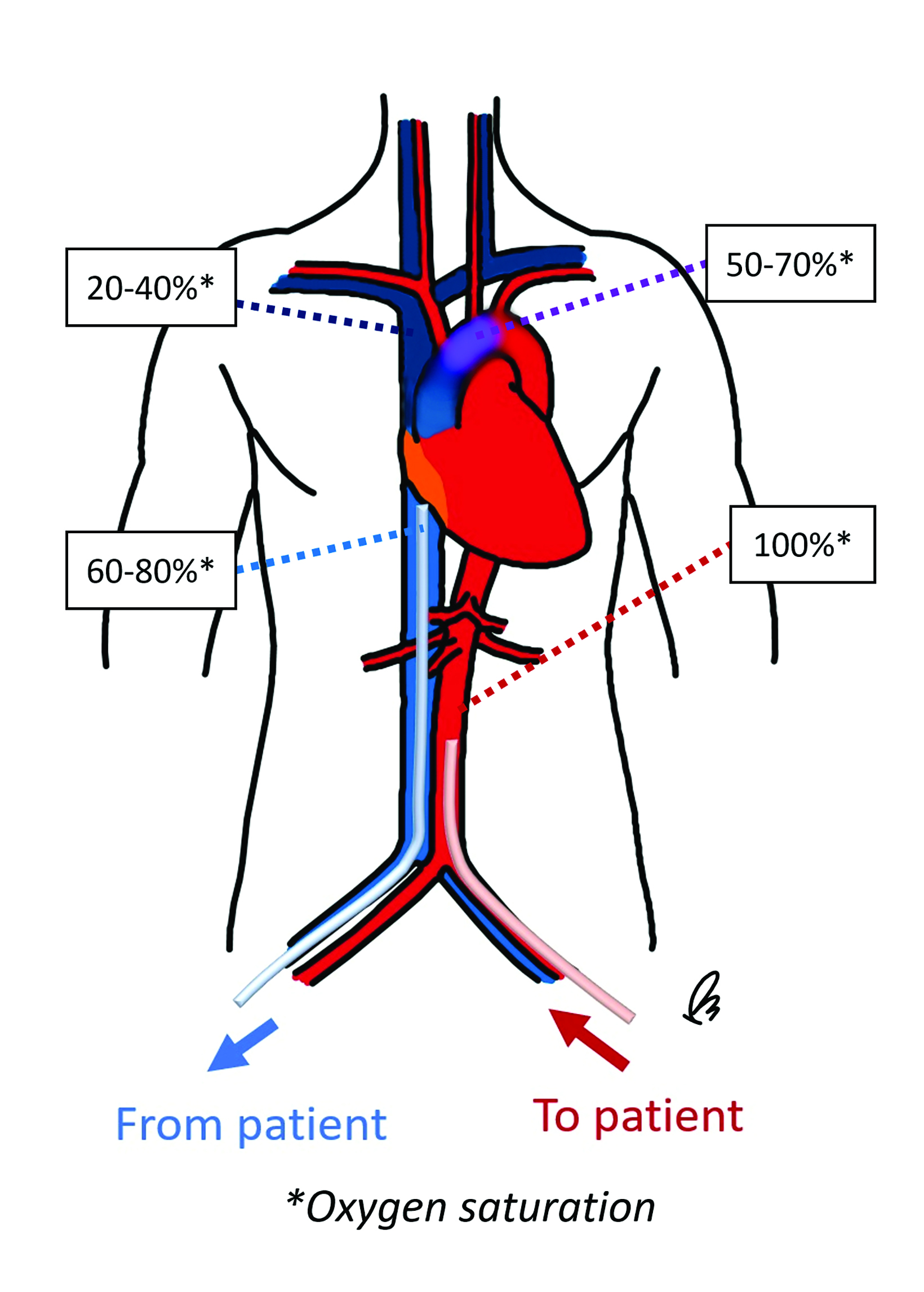
Peripheral VA-ECMO is established by cannulating a peripheral vein and artery. A key advantage of peripheral VA-ECMO is more rapid initiation; this method can be implemented outside of the operating room and even during ongoing chest compressions.30 The venous cannula may be strategically placed within the SVC, RA, or IVC with insertion through a femoral vein or, less frequently, the IJV. 31 The arterial cannula is most commonly inserted through a femoral artery, terminating in the external or common iliac arteries or within the distal abdominal aorta (Figure 6).31 Though this approach is faster and technically less complex, the reinfused oxygenated blood takes a nonphysiological route towards the upper body, contrary to the natural direction of the antegrade cardiac output.32 This can lead to a phenomenon termed “dual circulation,” also known as “Harlequin syndrome” or “North-South syndrome.” In this scenario, the lower body receives adequate oxygenation while the upper body experiences hypoxemia. The reinfused oxygenated blood supplies the lower body and then is drained by the IVC cannula. In contrast, the upper body is supplied mostly with desaturated blood from the left ventricle, which then drains to the SVC and circulates through the failing lungs without passing through the ECMO machine (Figure 7).33
Less frequently, the arterial cannula may be inserted through the axillary, common carotid, innominate, or subclavian arteries toward the ascending aorta.34 This approach provides a more physiological anterograde flow of deoxygenated blood, facilitates ambulation, diminishes the risk of limb ischemia, and may increase cerebral oxygen saturation levels. However, it is associated with an increased risk of bleeding and requires surgical placement.35
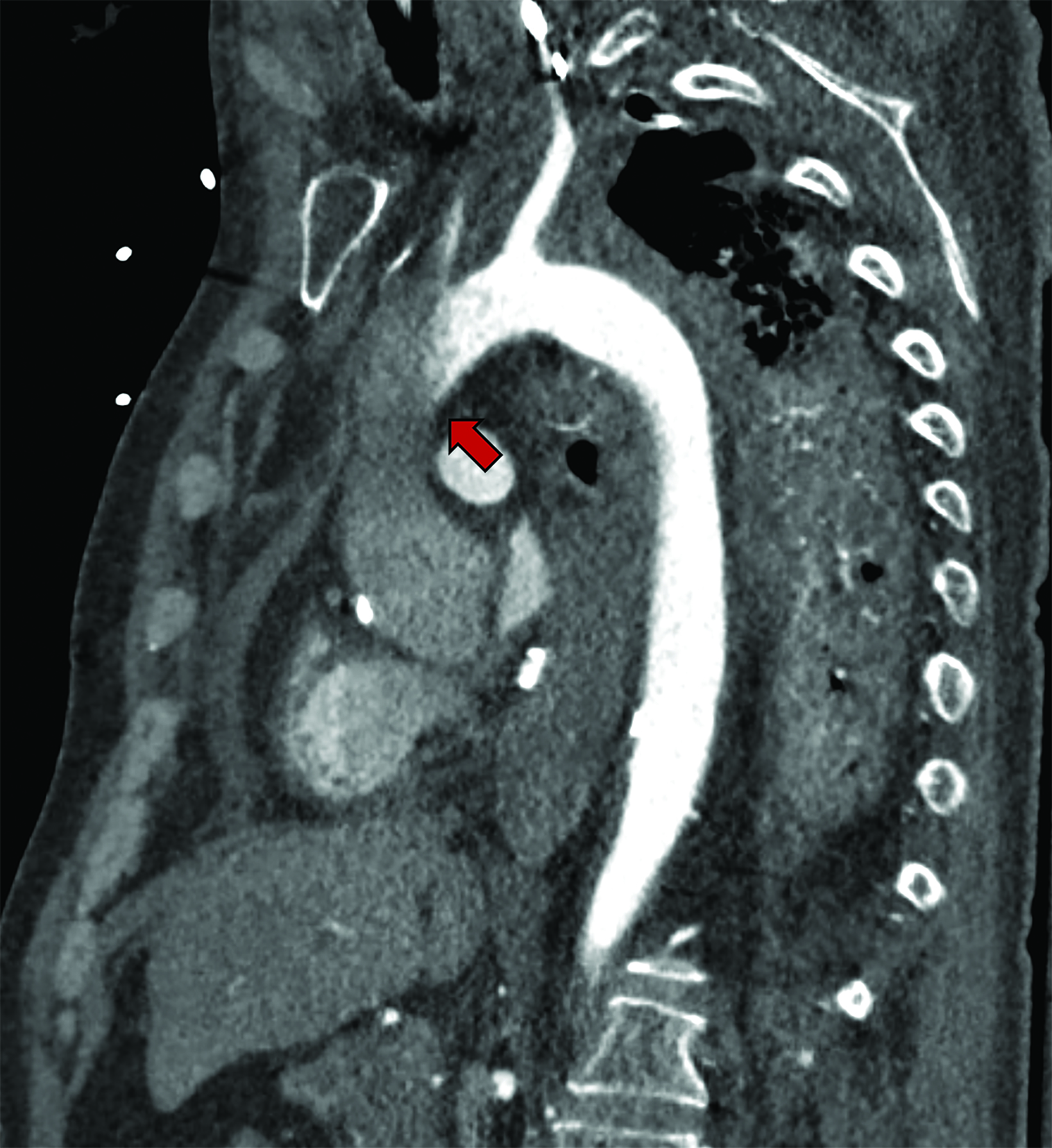
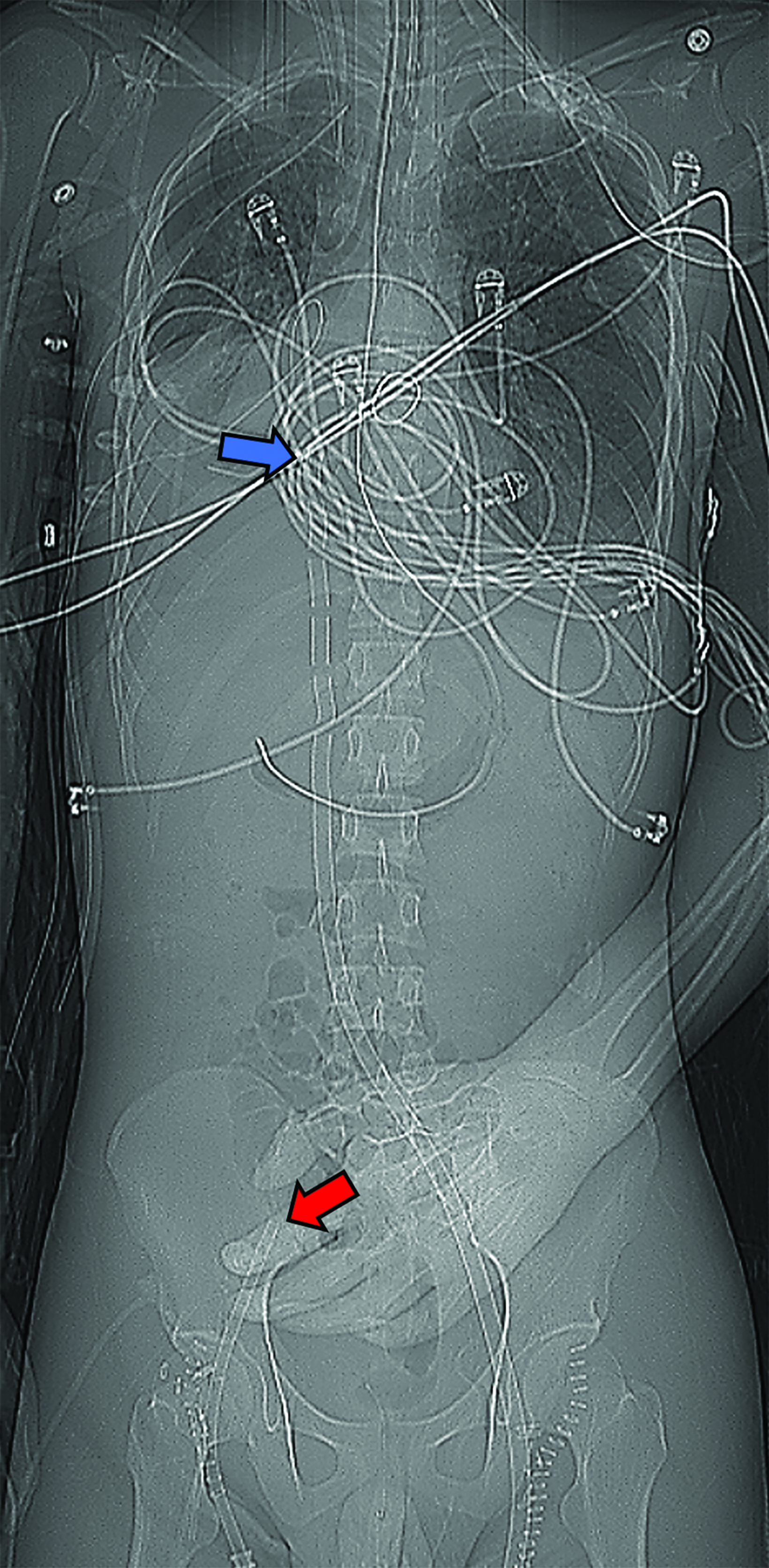
When VA-ECMO is used in the setting of severe left ventricular dysfunction, left-heart, end-diastolic pressures can increase significantly and cause pulmonary congestion and edema. Left-heart unloading or venting can be achieved by various interventions, including placement of a transeptal cannula connected to the venous ECMO circuit within the left atrium,36 which should not be misinterpreted as an abnormally positioned cannula (Figure 8). Additional temporary devices such as a heart pump or an intra-aortic balloon pump may be used in conjunction with VA-ECMO to help further unload the left ventricle.37-39
Contrast-enhanced Computed Tomography
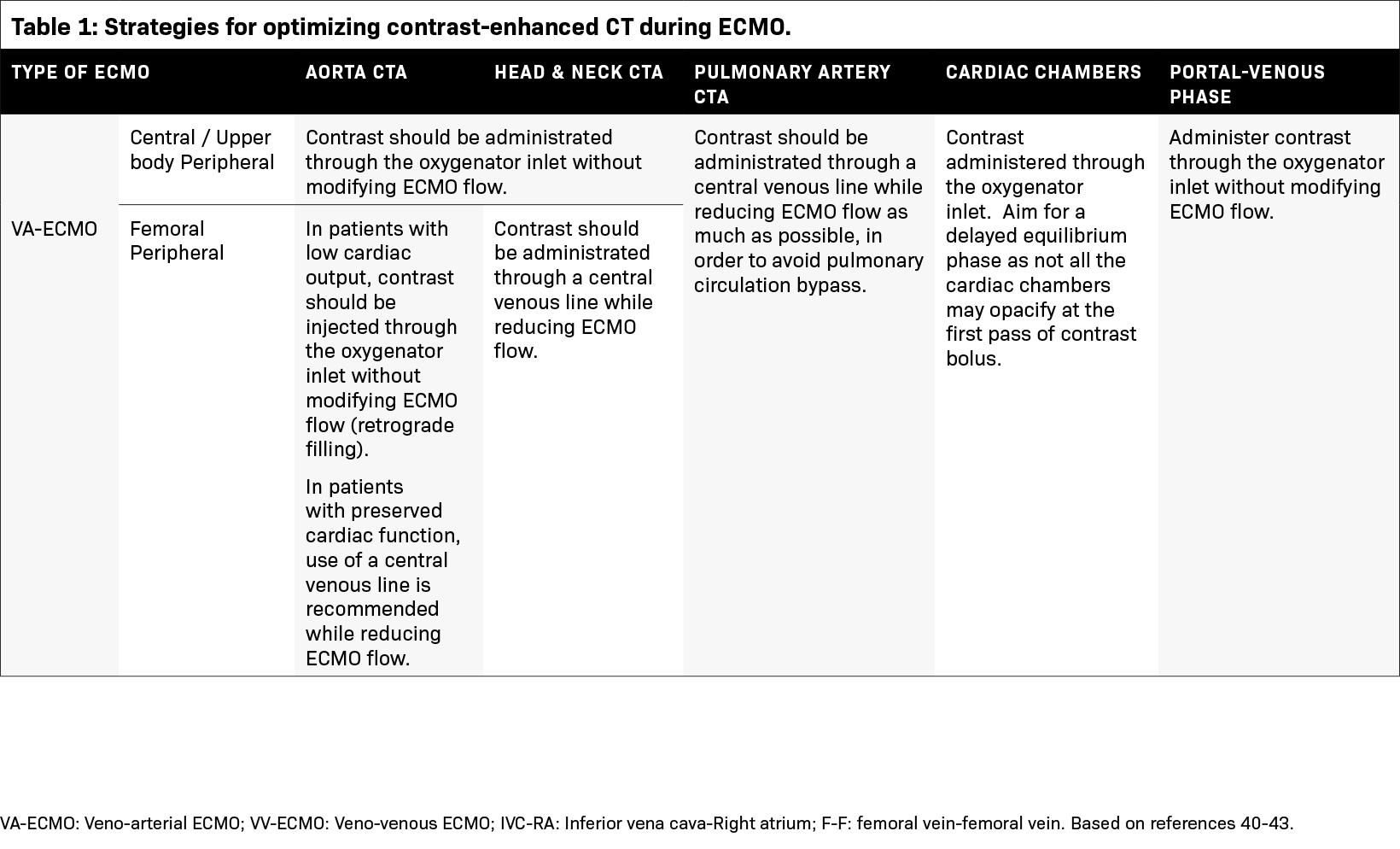
Several factors must be considered to ensure the success of contrast-enhanced CT studies for patients undergoing ECMO circulation (Table 1, Figure 9). The contrast distribution patterns can differ from those with normal physiology and are determined by the unique hemodynamic changes induced by the ECMO circuit.40
Patients with VV-ECMO typically have normal cardiac output and similar hemodynamics to patients without extracorporeal support. For such patients, intravenous contrast should be administrated through the oxygenator inlet within the ECMO machine. While a fraction of the contrast might recirculate through the ECMO circuit before entering the systemic arterial circulation, the amount is generally not significant and ECMO flow can be maintained during the examination. Recirculation of IV contrast is higher when a venous line is used for injection. In these instances, it is advisable to reduce ECMO flow during injection.41
Different strategies are required for VA-ECMO contingent upon the imaging target, arterial cannulation strategy, and the patient’s cardiac output.42 When systemic arterial enhancement is required, the oxygenator pathway is recommended for patients with either central or upper body peripheral cannulation. For those undergoing femoral cannulation, the contrast delivery method hinges on the interplay between cardiac output and ECMO flow rates. In patients with low cardiac output, oxygenator injection with retrograde filling of the aorta is favored while maintaining the ECMO flow. Conversely, injection through a venous line is preferred for patients with higher cardiac outputs while ECMO flow should be decreased, if safe to do so, during the acquisition phase.41-43
Pulmonary artery evaluation is challenging for all VA-ECMO configurations, as blood bypasses the pulmonary arteries. Contrast should be administered through a venous line while reducing the pump flow rate as much as possible.42
If parenchymal enhancement is required (ie, a portal-venous phase), contrast should be injected through the oxygenator, and ECMO flow can be maintained (applicable for all cannulation strategies).41
To ensure patient safety, it is recommended that contrast-enhanced CT studies be performed with a perfusionist present, particularly when adjustments to ECMO flow are required.
A Range of Potential Complications
The overall benefit, incidence of adverse events, and mortality associated with ECMO remain topics of ongoing debate. Various meta-analyses and registries report overall in-hospital survival rates for patients with ECMO in the range of 38-43%.4,44-47
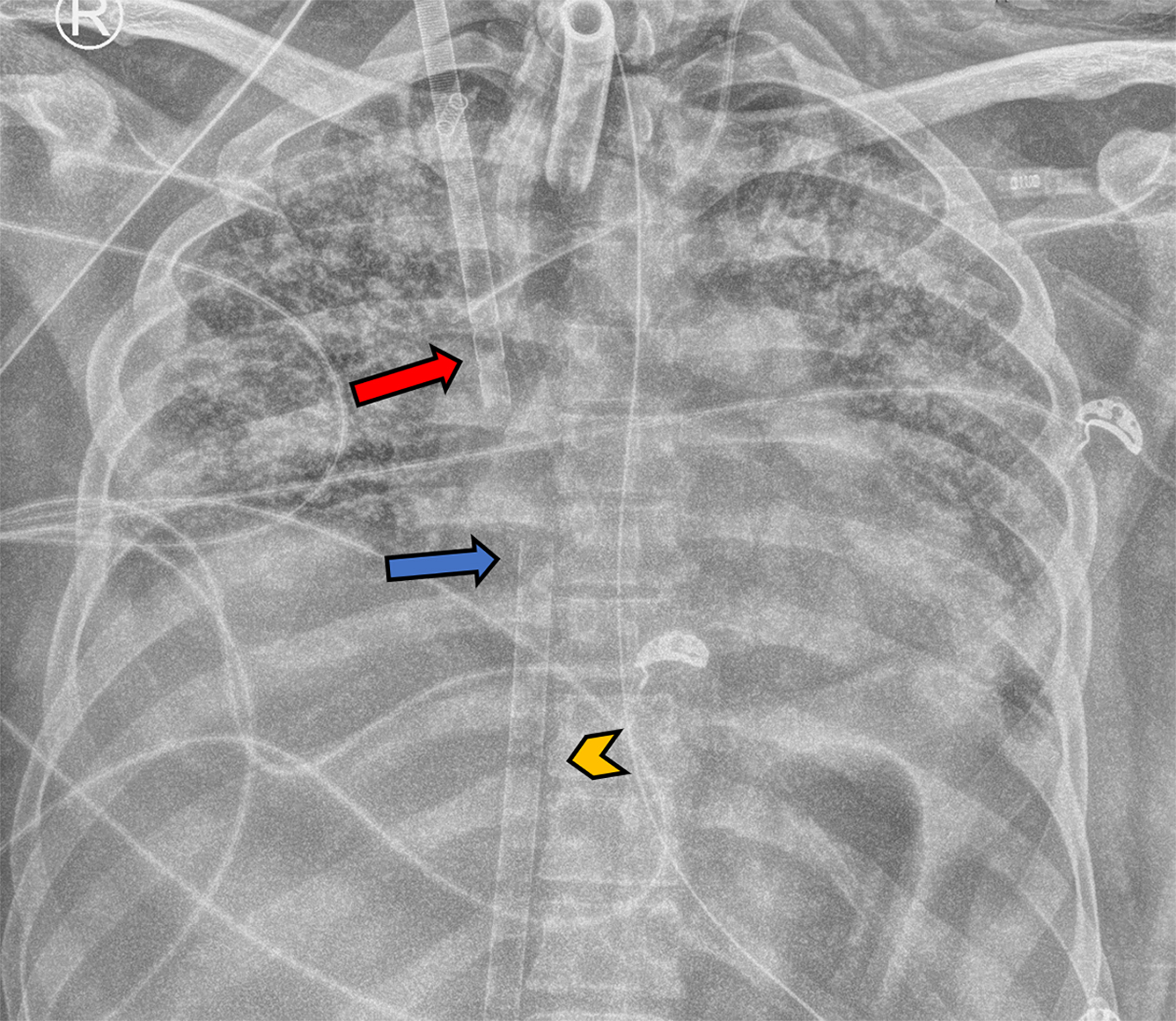
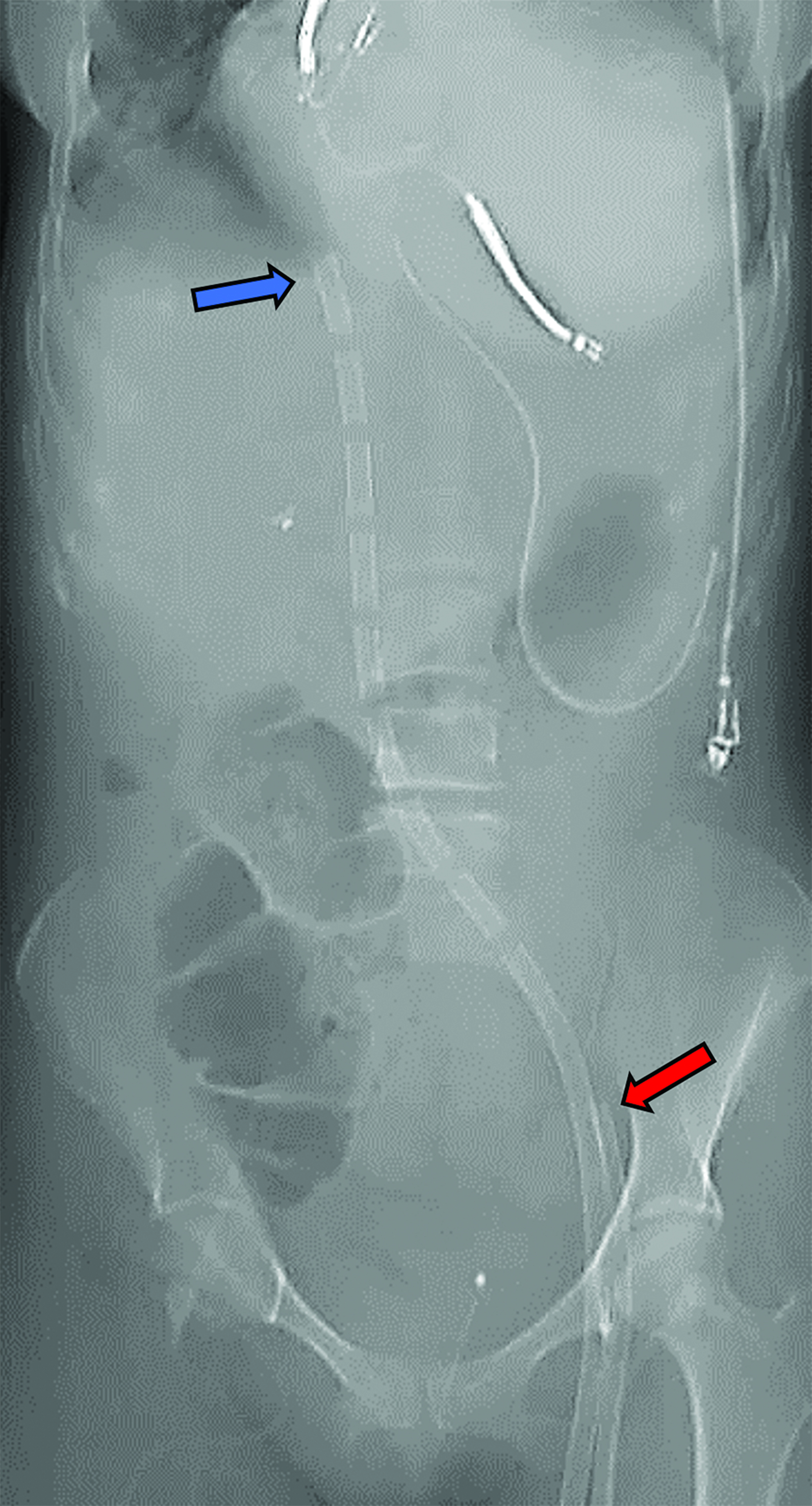
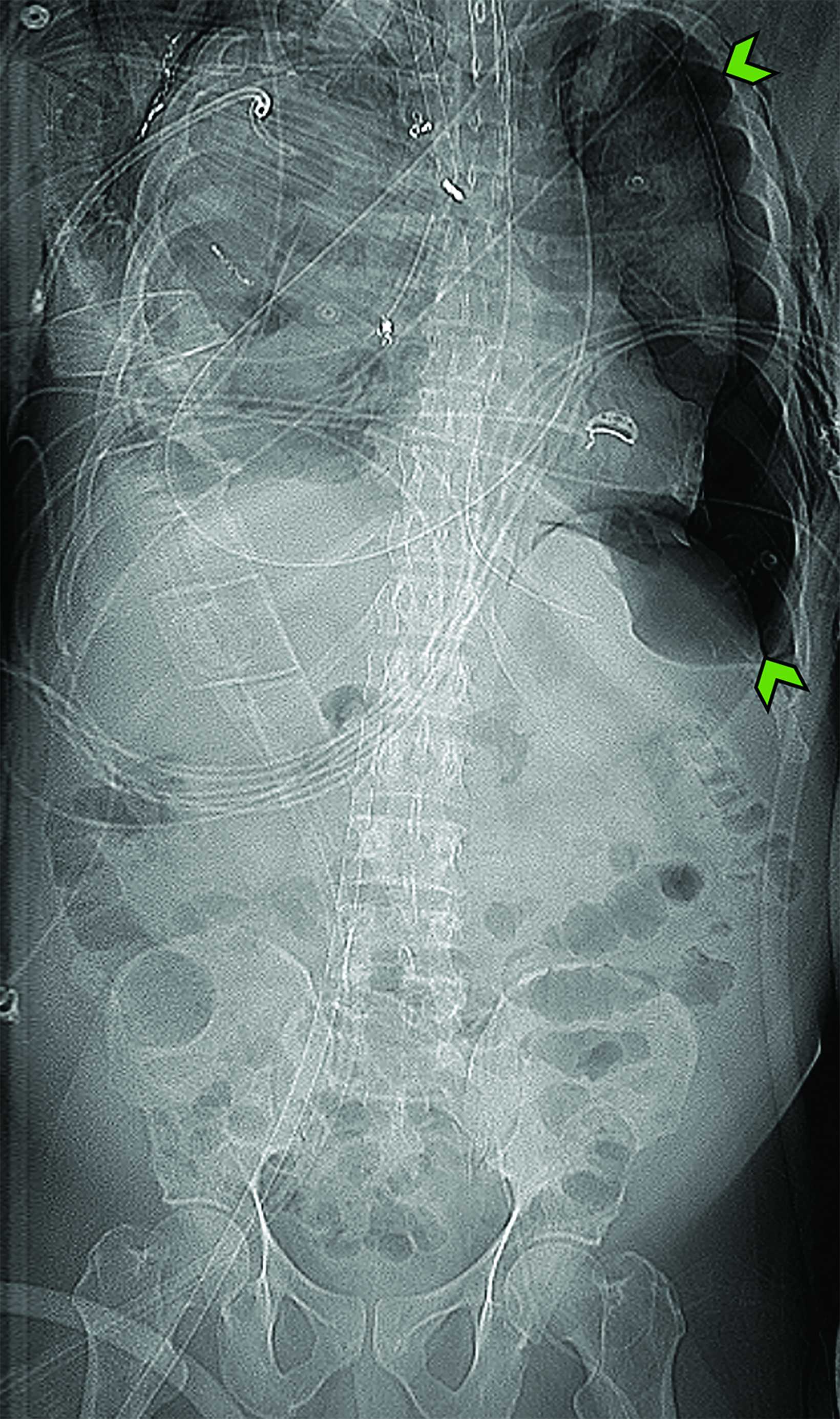
Patients with ECMO are at increased risk of bleeding, given their need for anticoagulation and higher prevalence of coagulopathies compared to the general population.48 Nearly half of patients experience hemorrhage, with the cannulation and surgical areas as the most common sites of bleeding, followed by hemothoraces and hemopericardium.44,48 Chest radiography is useful for identifying hemothoraces and hemopericardium as new pleural collections or enlargement of the cardiopericardial silhouette, respectively, which can be confirmed by CT (Figure 10).
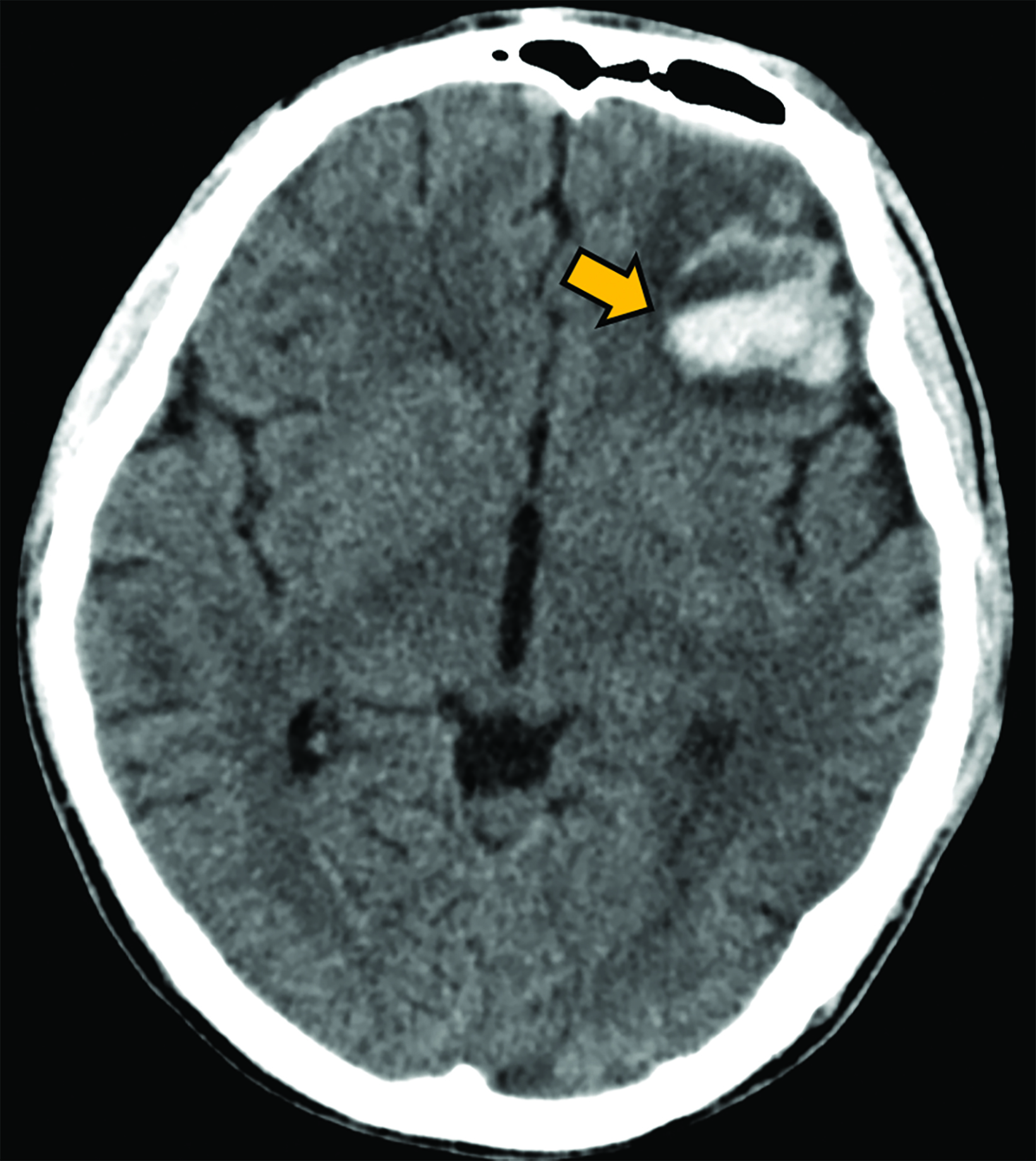
Neurological complications, while less frequent, are associated with significant morbidity and mortality. Intracranial hemorrhage and ischemic stroke may be observed in approximately 3% and 1.4% of patients, respectively, although they carry a mortality rate as high as 80% (Figure 11).49
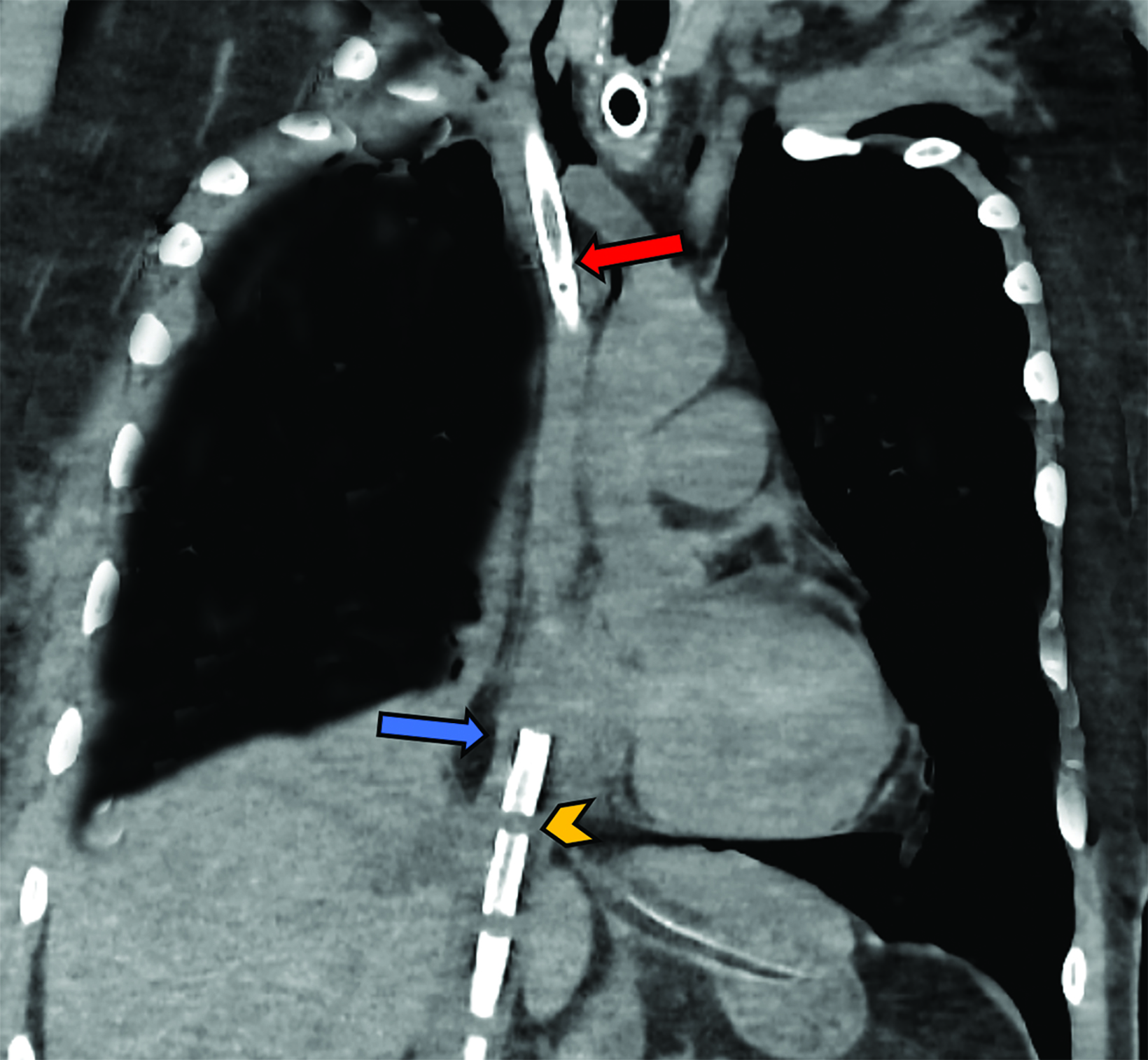
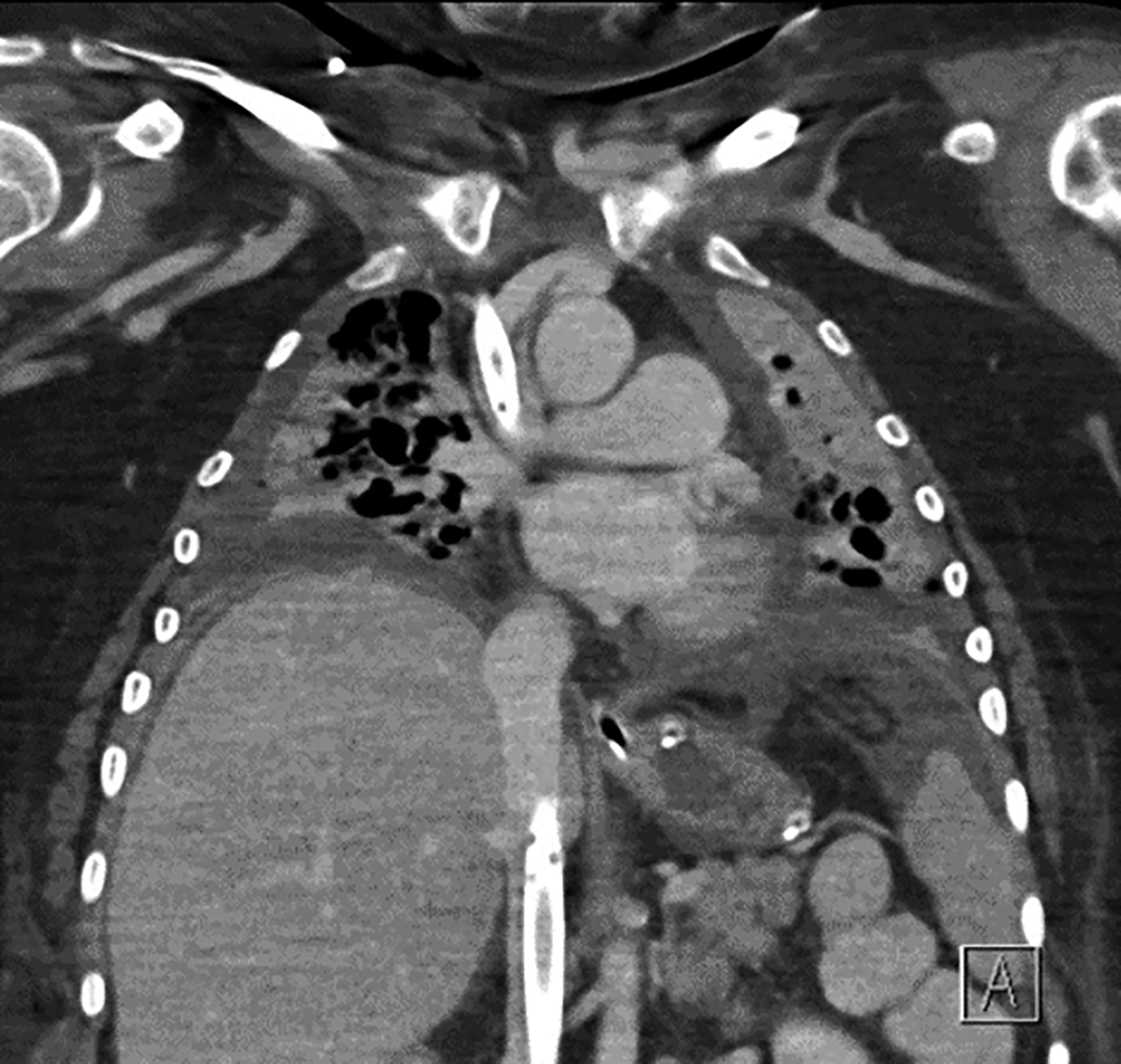
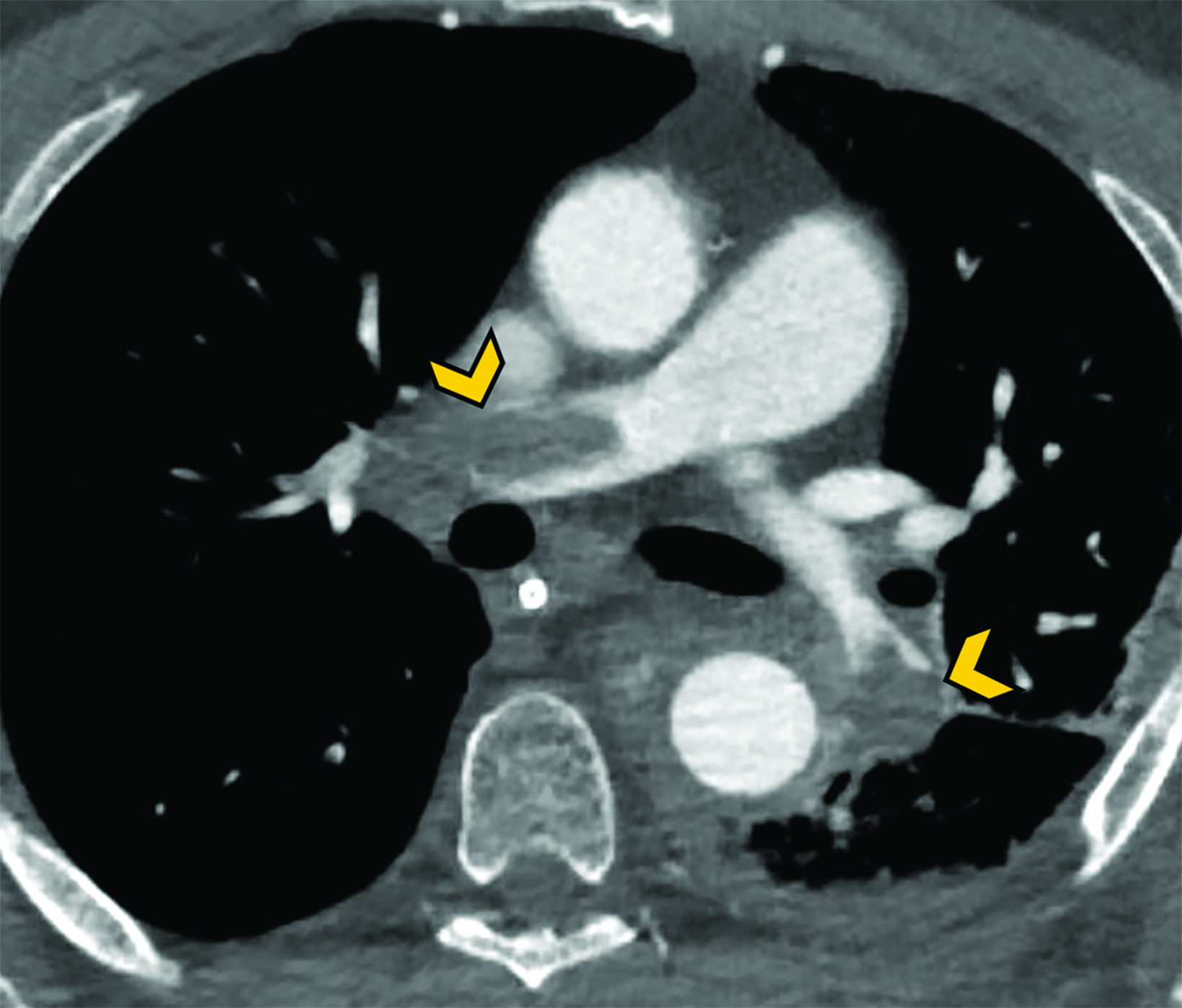
Thromboembolic disease is frequent in patients connected to extracorporeal life support. The incidence of deep vein thrombosis is estimated at 53% and is more prevalent in VV-ECMO than in VA-ECMO.50 Hemocompatibility within the ECMO circuit may trigger an inflammatory response and initiate the clotting cascade. Moreover, the mechanical stress may affect the arrangement of coagulation factors, posing a significant challenge for anticoagulation (Figure 12).51
Vascular complications, including access vessel obstruction with resultant limb ischemia, traumatic arterial dissection, and transection can occur in up to 30% of patients, are more frequent with VA-ECMO,52 and are associated with increased mortality.53 Other complications include renal failure, superimposed infection, sepsis, disseminated intravascular coagulation, and ECMO circuit component clots.44
Conclusion
As ECMO utilization increases in the treatment of severe cardiopulmonary failure, familiarity with the different types of ECMO circuits, expected locations of cannulas, optimal CT imaging protocols and possible complications is essential for radiologists to ensure accurate imaging interpretation and diagnosis.
References
- von Bahr V, Hultman J, Eksborg S, Frenckner B, Kalzén H. Long-Term Survival in Adults Treated With Extracorporeal Membrane Oxygenation for Respiratory Failure and Sepsis. Crit Care Med. Feb 2017;45(2):164-170. doi: 10.1097/ccm.0000000000002078.
- Gajkowski EF, Herrera G, Hatton L, Velia Antonini M, Vercaemst L, Cooley E. ELSO Guidelines for Adult and Pediatric Extracorporeal Membrane Oxygen- ation Circuits. Asaio j. Feb 1 2022;68(2):133-152. doi: 10.1097/mat.0000000000001630.
- Extracorporeal Life Support Organization (ELSO) ECLS International Summary of Statistics. website. https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx. Published 2023. Updated April 18, 2023. Accessed September 26, 2023.
- Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. Sep 2013;15(3):172-178.
- Gaddikeri R, Febbo J, Shah P. Imaging Adult ECMO. Current Problems in Diagnostic Radiology. 2021/11/01/ 2021;50(6):884-898. doi: https://doi.org/10.1067/j. cpradiol.2020.10.018.
- Shen J, Tse JR, Chan F, Fleischmann D. CT Angiography of Venoarterial Extracorporeal Membrane Oxygenation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2022/01/01 2021;42(1):23-37. doi: 10.1148/rg.210079.
- Squiers JJ, Lima B, DiMaio JM. Contemporary extracorporeal membrane oxygenation therapy in adults: Fundamental principles and systematic review of the evidence. The Journal of Thoracic and Cardiovascular Surgery. 2016;152(1):20-32. doi: 10.1016/j.jtcvs.2016.02.067.
- Wrisinger WC, Thompson SL. Basics of Extracorporeal Membrane Oxygenation. Surg Clin North Am. Feb 2022;102(1):23-35. doi: 10.1016/j.suc.2021.09.001.
- Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med. Feb 2017;5(4):70. doi: 10.21037/ atm.2016.11.47.
- Lee S, Chaturvedi A. Imaging adults on extracorporeal membrane oxygenation (ECMO). Insights into imaging. Dec 2014;5(6):731-742. doi: 10.1007/ s13244-014-0357-x.
- Calcaterra D, Heather B, Kohl LP, Erickson HL, Prekker ME. Bedside veno-venous ECMO cannulation: A pertinent strategy during the COVID-19 pandemic. J Card Surg. Jun 2020;35(6):1180-1185. doi: 10.1111/jocs.14641.
- Tonna JE, Abrams D, Brodie D, et al. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). Asaio j. Jun 1 2021;67(6):601-610. doi: 10.1097/mat.0000000000001432.
- Kim K, Leem AY, Kim SY, et al. Complications related to extracorporeal membrane oxygenation support as a bridge to lung transplantation and their clinical significance. Heart Lung. Nov-Dec 2022;56:148-153. doi: 10.1016/j.hrtlng.2022.07.008.
- Frenckner B, Broman M, Broomé M. Position of draining venous cannula in extracorporeal membrane oxygenation for respiratory and respiratory/circulatory support in adult patients. Crit Care. Jun 15 2018;22(1):163. doi: 10.1186/s13054-018-2083-0.
- Ling SK-h. Comparison of atrio-femoral and femoro-atrial venovenous extracorporeal membrane oxygenation in adult. Perfusion. 2022;37(1):14-18. doi: 10.1177/0267659120969020.
- Conrad SA, Wang D. Evaluation of Recirculation During Venovenous Extracorporeal Membrane Oxygenation Using Computational Fluid Dynamics Incorporating Fluid-Structure Interaction. Asaio j. Aug 1 2021;67(8):943-953. doi: 10.1097/mat.0000000000001314.
- Burrell AJC, Ihle JF, Pellegrino VA, Sheldrake J, Nixon PT. Cannulation technique: femoro-femoral. Journal of thoracic disease. Mar 2018;10(Suppl 5):S616-s623. doi: 10.21037/jtd.2018.03.83.
- Shaheen A, Tanaka D, Cavarocchi NC, Hirose H. Veno-Venous Extracorporeal Membrane Oxygenation (VV ECMO): Indications, Preprocedural Considerations, and Technique. J Card Surg. Apr 2016;31(4):248-252. doi: 10.1111/jocs.12690.
- Parker LP, Svensson Marcial A, Brismar TB, Broman LM, Prahl Wittberg L. Hemodynamic and recirculation performance of dual lumen cannulas for veno- venous extracorporeal membrane oxygenation. Scientific Reports. 2023/05/08 2023;13(1):7472. doi: 10.1038/s41598-023-34655-1.
- Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. Asaio j. Mar-Apr 2015;61(2):115-121. doi: 10.1097/mat.0000000000000179.
- Lorusso R, Centofanti P, Gelsomino S, et al. Venoarterial Extracorporeal Membrane Oxygenation for Acute Fulminant Myocarditis in Adult Patients: A 5-Year Multi-Institutional Experience. The Annals of thoracic surgery. Mar 2016;101(3):919-926. doi: 10.1016/j.athoracsur.2015.08.014.
- Kagawa E, Inoue I, Kawagoe T, et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation. Aug 2010;81(8):968-973. doi: 10.1016/j.resuscitation.2010.03.037.
- Richardson AC, Tonna JE, Nanjayya V, et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO Journal. 2021;67(3):221-228. doi: 10.1097/mat.0000000000001344.
- Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366-S468. doi: doi:10.1161/CIR.0000000000000916.
- Pavasini R, Cirillo C, Campo G, et al. Extracorporeal Circulatory Support in Acute Coronary Syndromes: A Systematic Review and Meta-Analysis. Crit Care Med. Nov 2017;45(11):e1173-e1183. doi: 10.1097/ccm.0000000000002692.
- de Waha S, Fuernau G, Eitel I, Desch S, Thiele H. Long-term prognosis after extracorporeal life support in refractory cardiogenic shock — results from a real-world cohort. EuroIntervention. Jun 20 2016;12(3):414. doi: 10.4244/eijv12i3a71.
- Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circulation: Heart Failure. 2018;11(9):e004905. doi: doi:10.1161/CIRCHEARTFAILURE.118.004905.
- Biancari F, Perrotti A, Dalén M, et al. Meta-Analysis of the Outcome After Postcardiotomy Venoarterial Extracorporeal Membrane Oxygenation in Adult Patients. J Cardiothorac Vasc Anesth. Jun 2018;32(3):1175-1182. doi: 10.1053/j.jvca.2017.08.048.
- Rubino A, Costanzo D, Stanszus D, et al. Central Veno-Arterial Extracorporeal Membrane Oxygenation (C-VA-ECMO) After Cardiothoracic Surgery: A Single-Center Experience. Journal of Cardiothoracic and Vascular Anesthesia. 2018;32(3):1169-1174. doi: 10.1053/j.jvca.2017.12.003.
- Camp PC, Jr. Short-Term Mechanical Circulatory Support. Operative Techniques in Thoracic and Cardiovascular Surgery. 2013;18(3):239-251. doi: 10.1053/j. optechstcvs.2013.11.004.
- Jayaraman AL, Cormican D, Shah P, Ramakrishna H. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: Techniques, limitations, and special considerations. Ann Card Anaesth. Jan 2017;20(Supplement):S11-s18. doi: 10.4103/0971-9784.197791.
- Sorokin V, MacLaren G, Vidanapathirana PC, Delnoij T, Lorusso R. Choosing the appropriate configuration and cannulation strategies for extracorporeal membrane oxygenation: the potential dynamic process of organ support and importance of hybrid modes. Eur J Heart Fail. May 2017;19 Suppl 2:75-83. doi: 10.1002/ejhf.849.
- Contento C, Battisti A, Agrò B, et al. A novel veno-arteriovenous extracorporeal membrane oxygenation with double pump for the treatment of Harlequin syndrome. Perfusion. 2020;35(1_suppl):65-72. doi: 10.1177/0267659120908409.
- Biscotti M, Bacchetta M. The “Sport Model”: Extracorporeal Membrane Oxygenation Using the Subclavian Artery. The Annals of thoracic surgery. 2014;98(4):1487-1489. doi: 10.1016/j.athoracsur.2014.02.069.
- Chicotka S, Rosenzweig EB, Brodie D, Bacchetta M. The “Central Sport Model”: Extracorporeal Membrane Oxygenation Using the Innominate Artery for Smaller Patients as Bridge to Lung Transplantation. Asaio j. Jul/Aug 2017;63(4):e39-e44. doi: 10.1097/mat.0000000000000427.
- Bernhardt AM, Hillebrand M, Yildirim Y, et al. Percutaneous left atrial unloading to prevent pulmonary oedema and to facilitate ventricular recovery under extracorporeal membrane oxygenation therapy. Interact Cardiovasc Thorac Surg. Jan 1 2018;26(1):4-7. doi: 10.1093/icvts/ivx266.
- Takeda K, Garan AR, Topkara VK, et al. Novel minimally invasive surgical approach using an external ventricular assist device and extracorporeal membrane oxygenation in refractory cardiogenic shock. Eur J Cardiothorac Surg. Mar 1 2017;51(3):591-596. doi: 10.1093/ejcts/ezw349.
- Rao P, Mosier J, Malo J, et al. Peripheral VA-ECMO with direct biventricular decompression for refractory cardiogenic shock. Perfusion. Sep 2018;33(6):493-495. doi: 10.1177/0267659118761558.
- Cappannoli L, Galli M, Zito A, et al. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) with vs. without left ventricular unloading by Impella: a systematic review and meta-analysis. European Heart Journal — Quality of Care and Clinical Outcomes. 2022;9(4):358-366. doi: 10.1093/ehjqcco/qcac076.
- Liu K-L, Wang Y-F, Chang Y-C, et al. Multislice CT Scans in Patients on Extracorporeal Membrane Oxygenation: Emphasis on Hemodynamic Changes and Imaging Pitfalls. Korean J Radiol. 6/ 2014;15(3):322-329. https://doi.org/10.3348/kjr.2014.15.3.322.
- Gullberg Lidegran M, Gordon Murkes L, Andersson Lindholm J, Frenckner B. Optimizing Contrast-Enhanced Thoracoabdominal CT in Patients During Extracorporeal Membrane Oxygenation. Academic Radiology. 2021;28(1):58-67. doi: 10.1016/j.acra.2020.01.029.
- Shen J, Tse JR, Chan F, Fleischmann D. CT Angiography of Venoarterial Extracorporeal Membrane Oxygenation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2022;42(1):23-37. doi: 10.1148/rg.210079.
- Lambert L, Grus T, Balik M, Fichtl J, Kavan J, Belohlavek J. Hemodynamic changes in patients with extracorporeal membrane oxygenation (ECMO) demonstrated by contrast-enhanced CT examinations - implications for image acquisition technique. Perfusion. 2017;32(3):220-225. doi: 10.1177/0267659116677308.
- Rajsic S, Treml B, Jadzic D, et al. Extracorporeal membrane oxygenation for cardiogenic shock: a meta-analysis of mortality and complications. Ann Intensive Care. Oct 5 2022;12(1):93. doi: 10.1186/s13613-022-01067-9.
- Xie A, Phan K, Tsai YC, Yan TD, Forrest P. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a meta-analysis. J Cardiothorac Vasc Anesth. 2015;29(3):637-645. doi: 10.1053/j.jvca.2014.09.005.
- Wilson-Smith AR, Bogdanova Y, Roydhouse S, et al. Outcomes of venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock: systematic review and meta-analysis. Ann Cardiothorac Surg. Jan 2019;8(1):1-8. doi: 10.21037/acs.2018.11.09.
- Supady A, Taccone FS, Lepper PM, et al. Survival after extracorporeal membrane oxygenation in severe COVID-19 ARDS: results from an international multicenter registry. Critical Care. 2021/03/01 2021;25(1):90. doi: 10.1186/s13054-021-03486-9.
- Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. Dec 2016;6(1):97. doi: 10.1186/s13613-016-0196-7.
- Lorusso R, Gelsomino S, Parise O, et al. Neurologic Injury in Adults Supported With Veno-Venous Extracorporeal Membrane Oxygenation for Respiratory Failure: Findings From the Extracorporeal Life Support Organization Database. Crit Care Med. Aug 2017;45(8):1389-1397. doi: 10.1097/ccm.0000000000002502.
- Iannattone PA, Yang SS, Koolian M, Wong EG, Lipes J. Incidence of Venous Thromboembolism in Adults Receiving Extracorporeal Membrane Oxygenation: A Systematic Review. Asaio j. Dec 1 2022;68(12):1523-1528. doi: 10.1097/mat.0000000000001694.
- Abruzzo A, Gorantla V, Thomas SE. Venous thromboembolic events in the setting of extracorporeal membrane oxygenation support in adults: A systematic review. Thrombosis Research. 2022/04/01/ 2022;212:58-71. doi: https://doi.org/10.1016/j.thromres.2022.02.015.
- Jia D, Yang IX, Ling RR, et al. Vascular Complications of Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Regression Analysis. Crit Care Med. Dec 2020;48(12):e1269-e1277. doi: 10.1097/ccm.0000000000004688.
- Tanaka D, Hirose H, Cavarocchi N, Entwistle JW. The Impact of Vascular Complications on Survival of Patients on Venoarterial Extracorporeal Membrane Oxygenation. The Annals of thoracic surgery. May 2016;101(5):1729-1734. doi: 10.1016/j.athoracsur.2015.10.095.
Citation
F ST, A F, M AG, S ZB, GR K, E N, Y M, K H.An Overview of Extracorporeal Membrane Oxygenation. Appl Radiol. 2024; (1):22-34.
January 24, 2024