Alzheimer Disease Imaging in the Era of Anti-Amyloid Treatment
Images
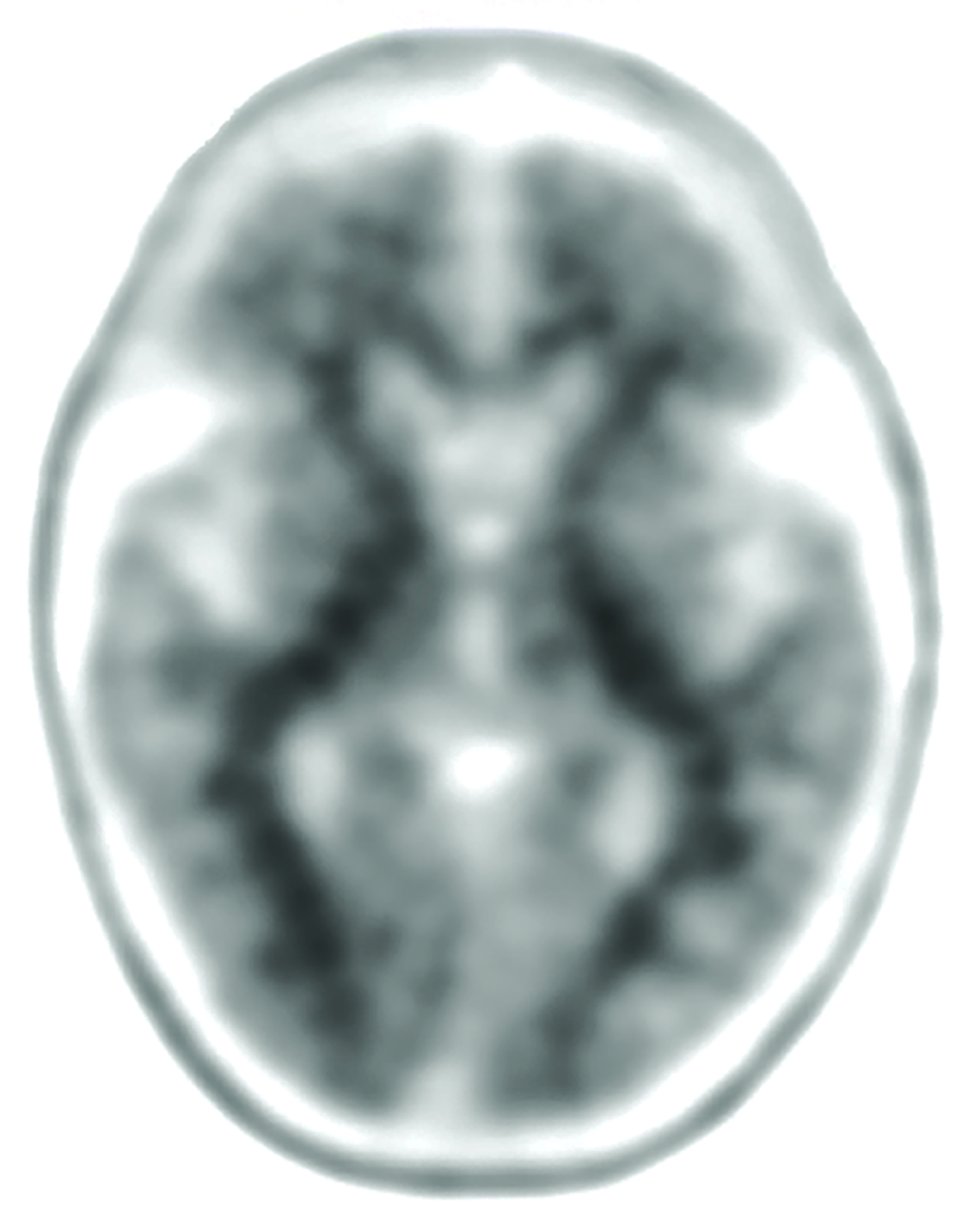
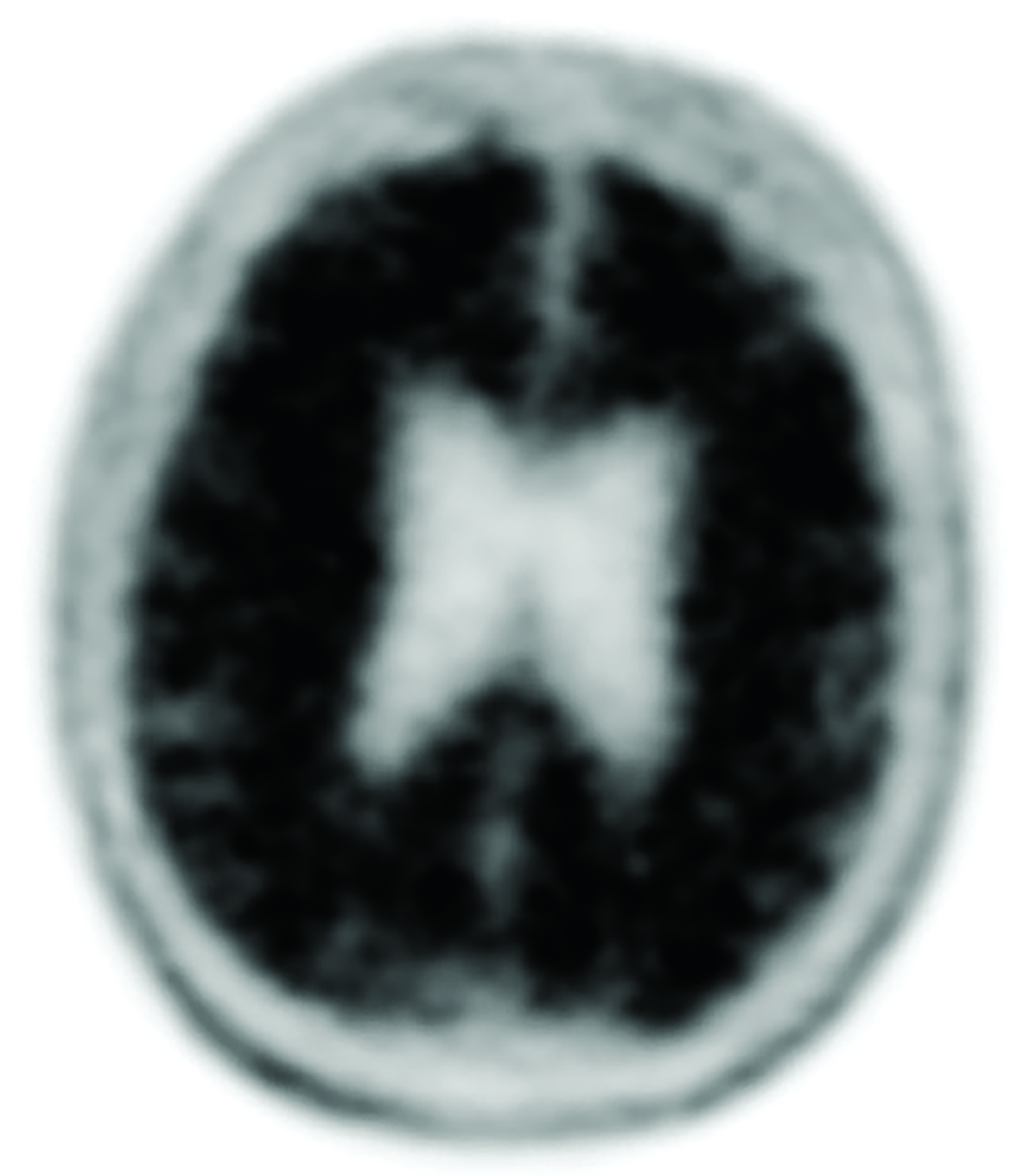
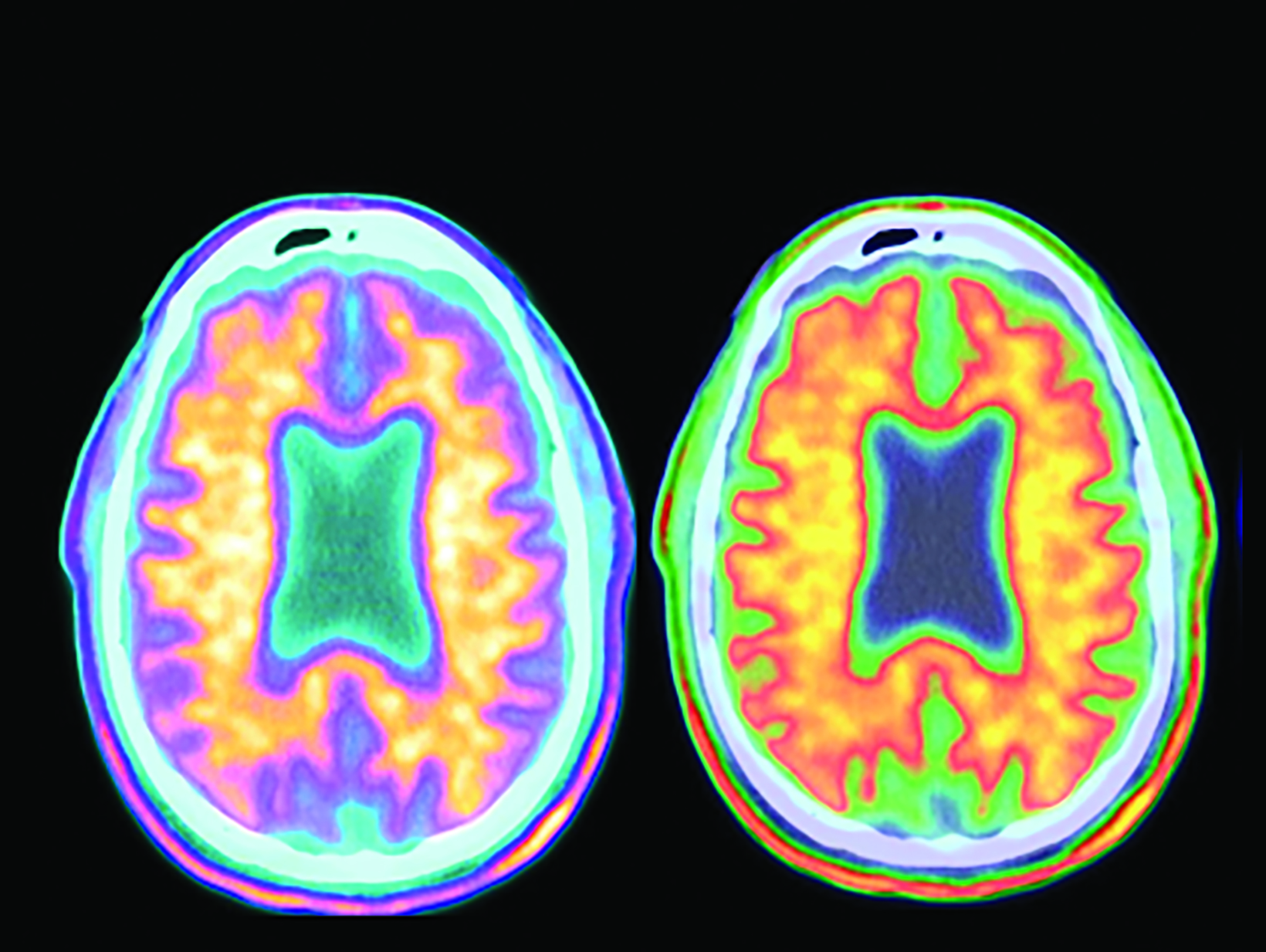
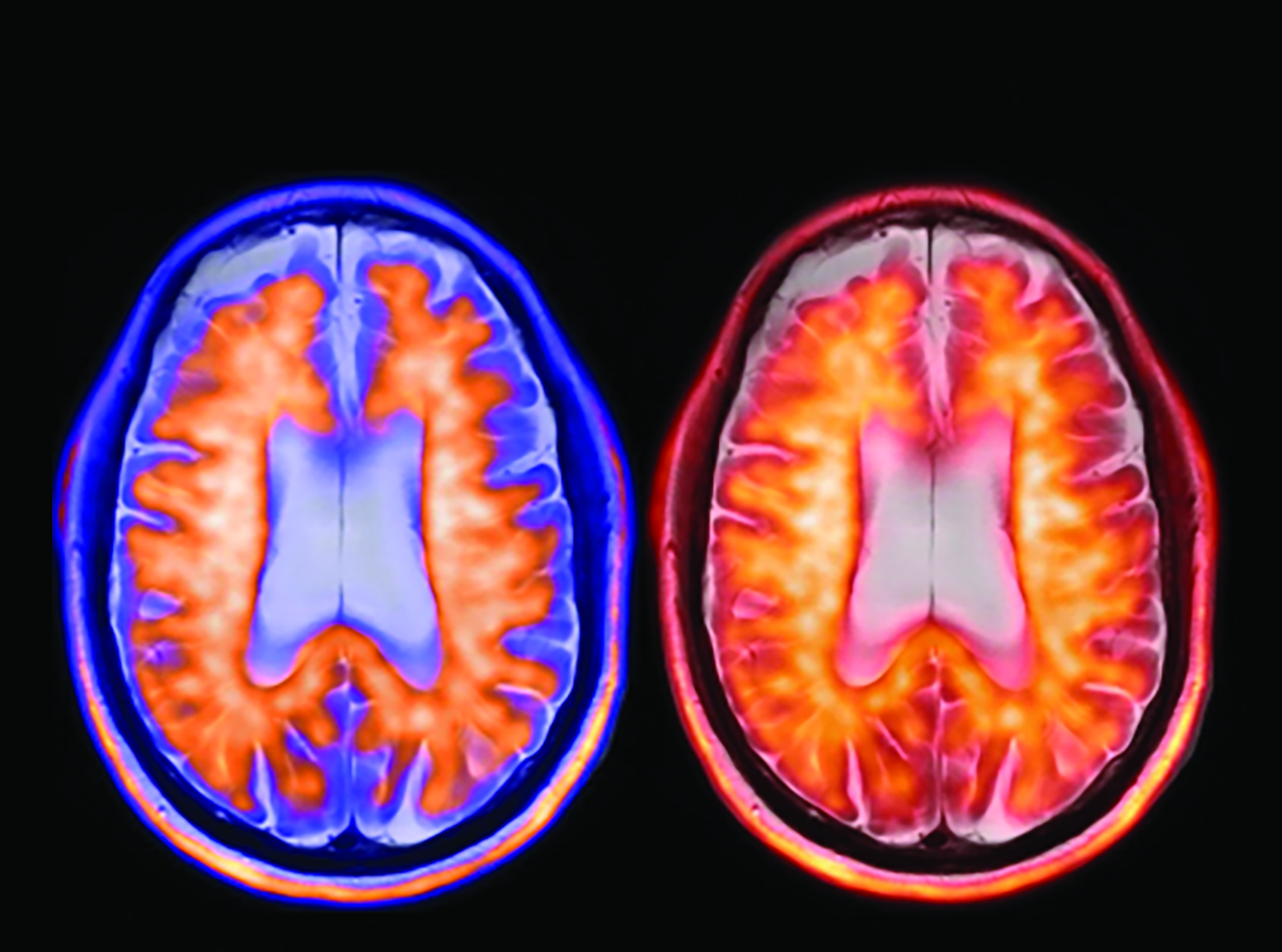
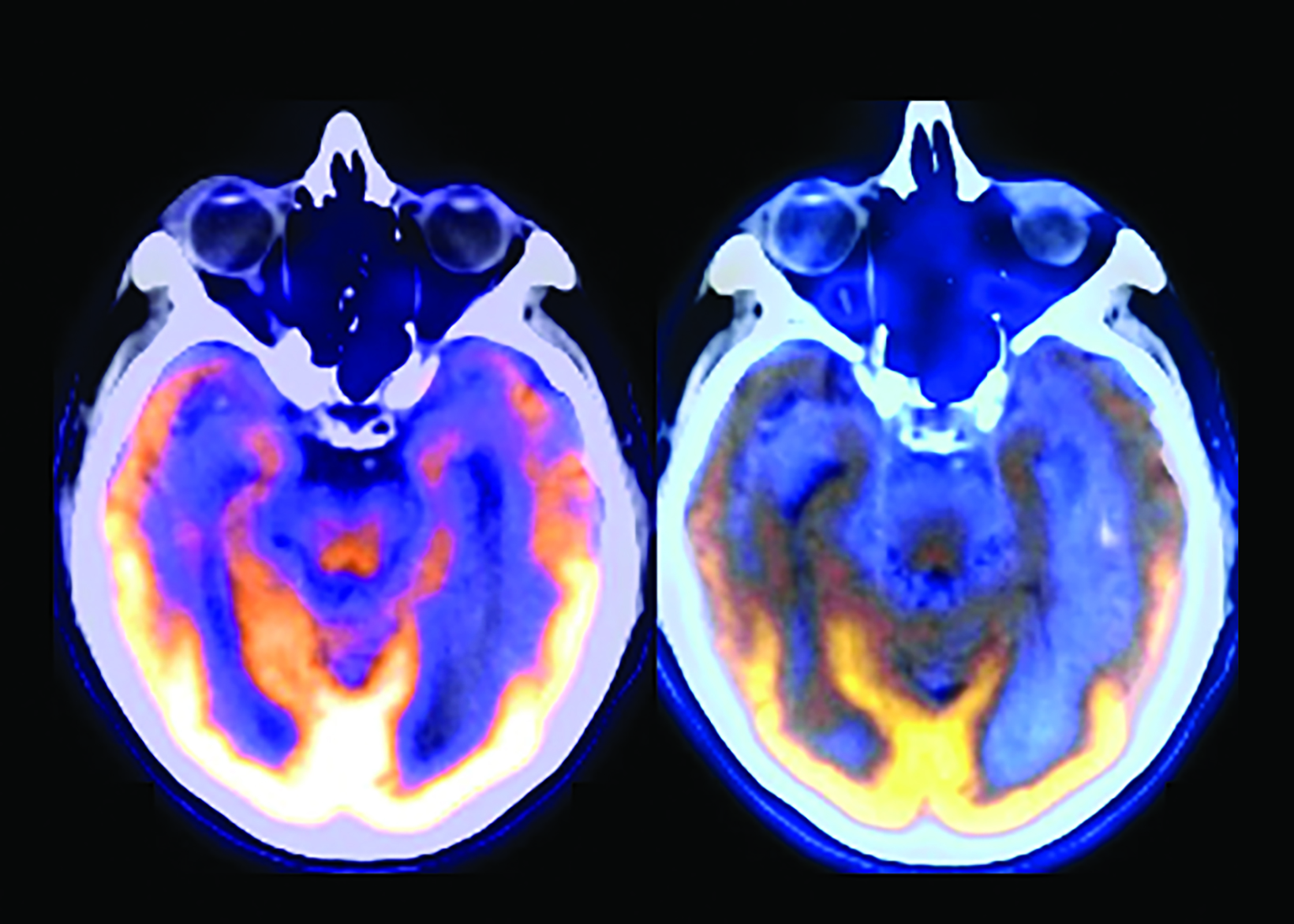
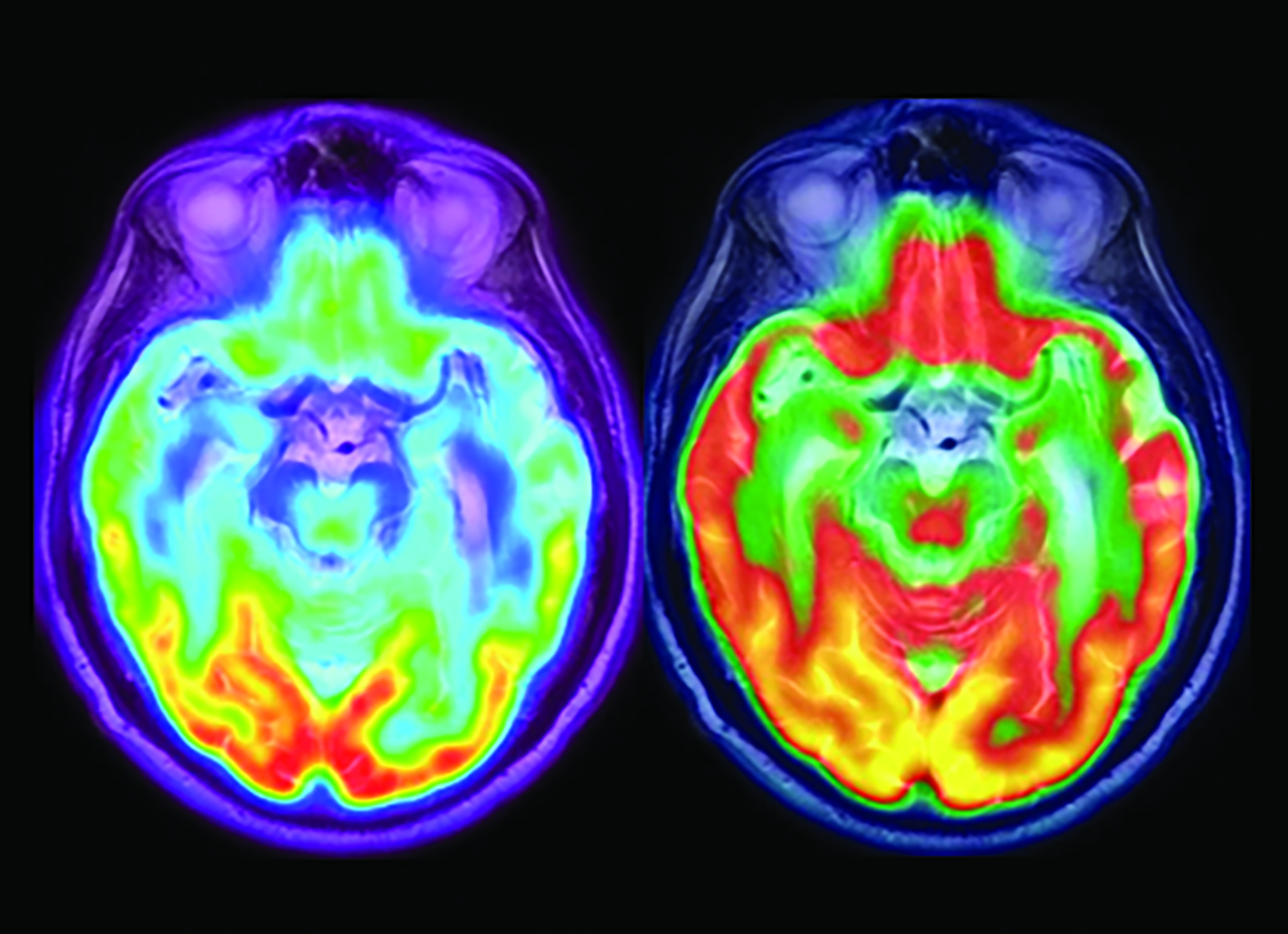
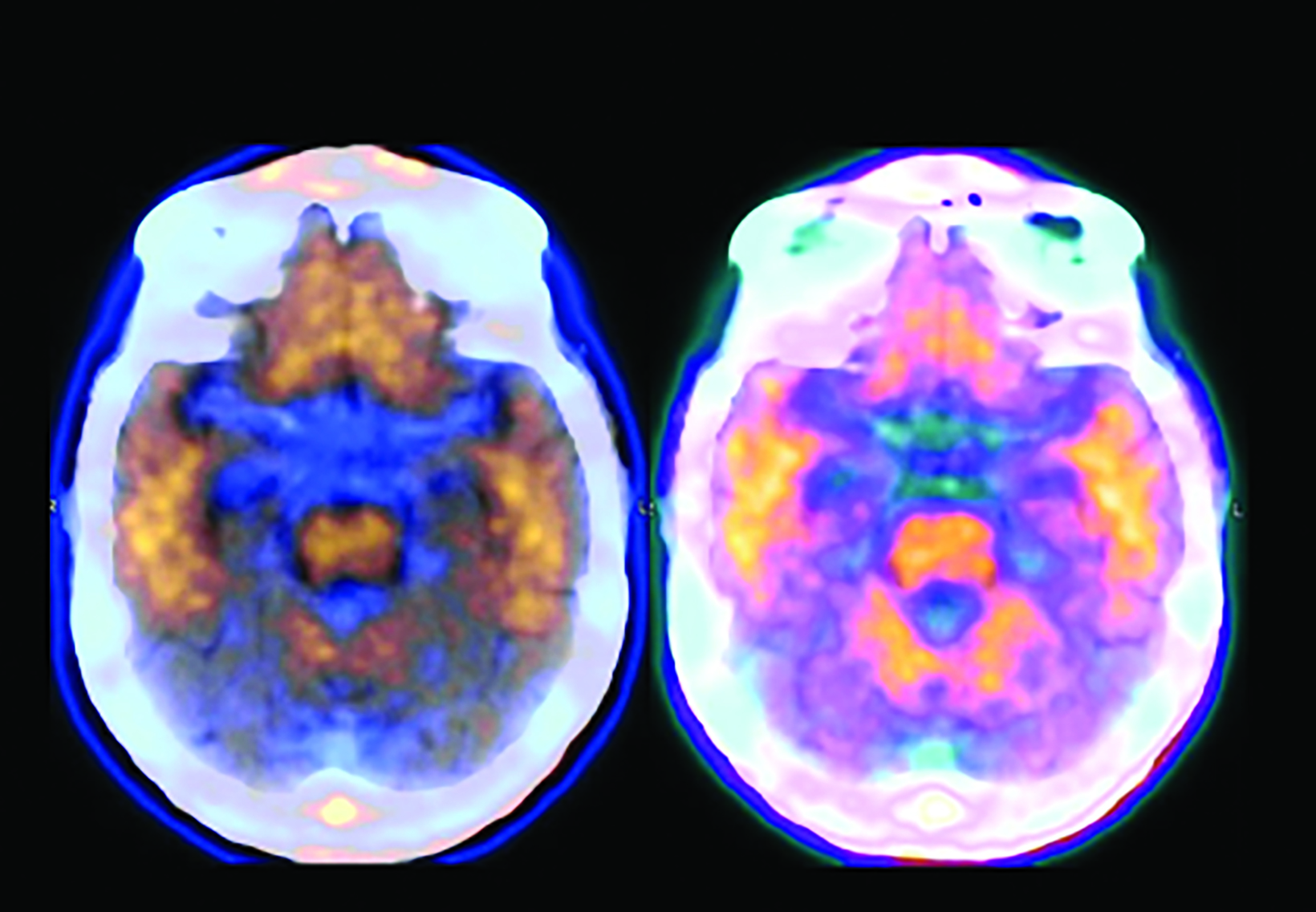
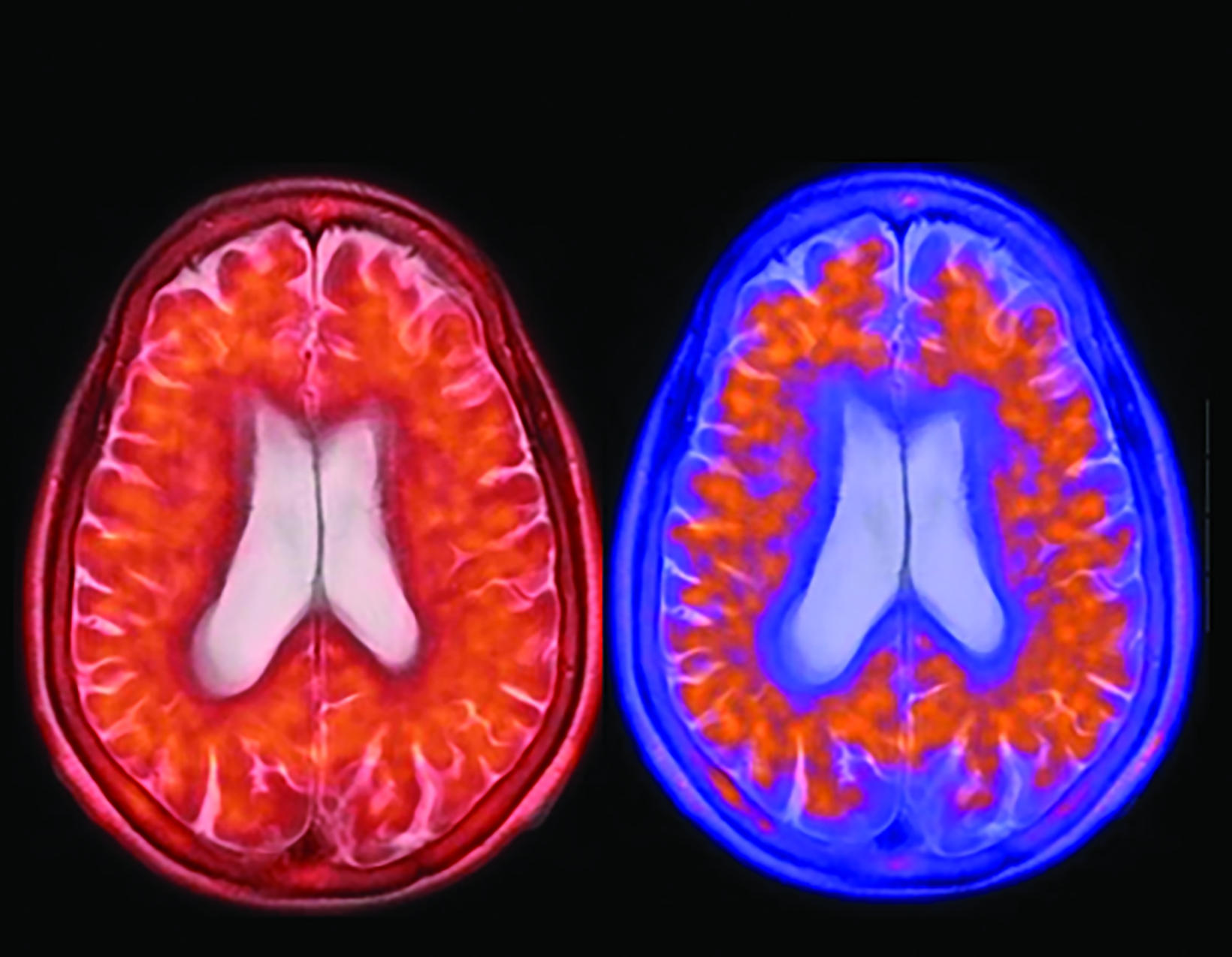
The landscape of diagnostic evaluation and treatment of Alzheimer disease (AD) is rapidly changing. While there is still no cure for AD, recent developments are bringing increased hope to the millions of Americans suffering from this progressively debilitating condition. Here we offer an overview of recent regulatory, treatment, and imaging developments that promise to significantly impact AD patients, their families, their physicians, and neuroimaging specialists.
An estimated 6.7 million Americans suffer from AD, which doubles in prevalence every five years after the age of 65.1,2 One in three seniors will die of dementia.1 Since 2000, death from heart disease has decreased by 7%, but death from AD has increased by 145%.1
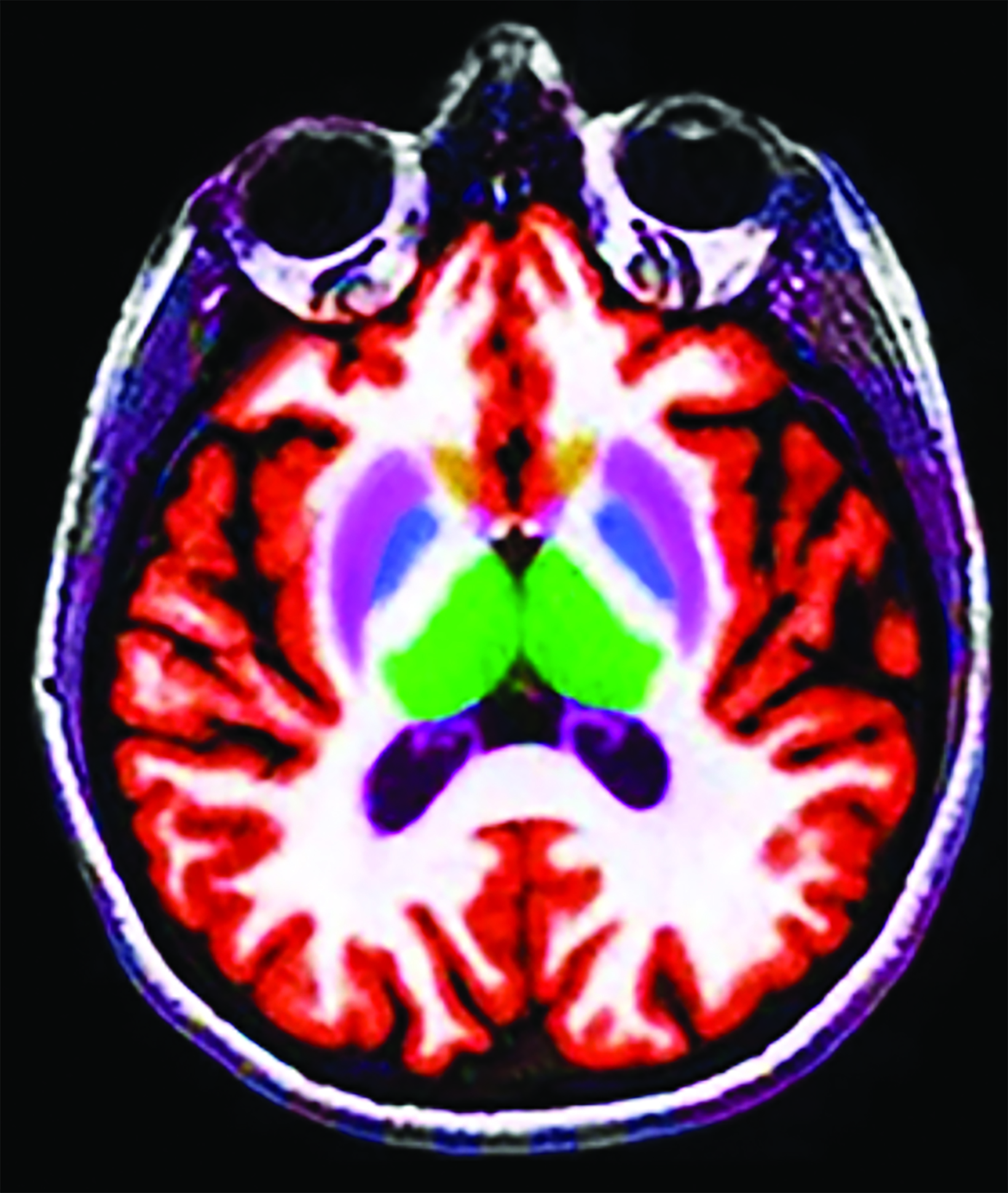
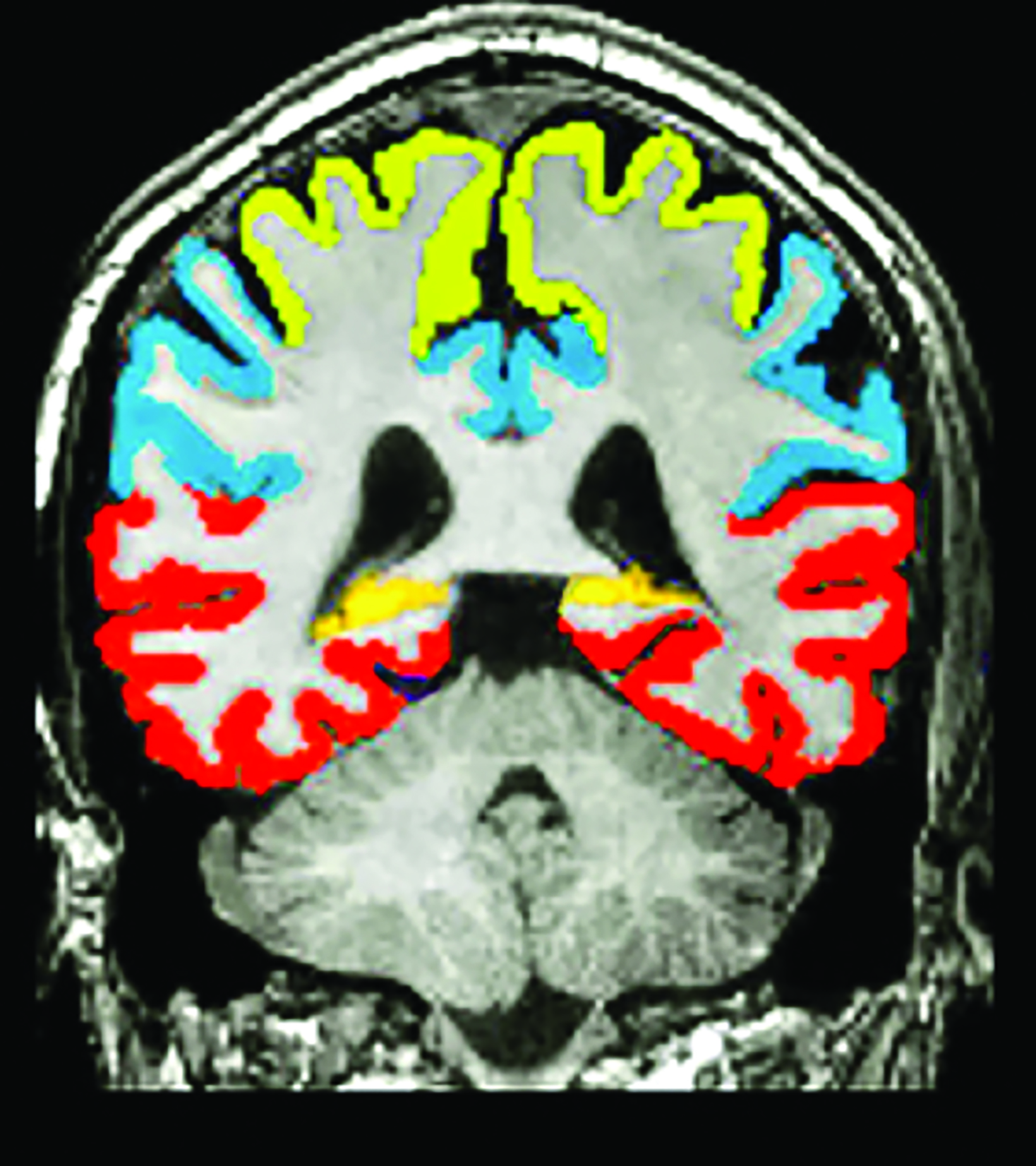
Conventional and quantitative brain MRI, as well as fluorodeoxyglucose (FDG), amyloid, and tau PET are utilized in the evaluation and clinical care pathway for dementia patients (Figures 1-4). With the recent emergence of disease-modifying therapies (DMT) for AD, neuroradiologists will play a critical role in the detection and characterization of treatment-related complications.
New Regulatory Developments
In July 2023, the US Food and Drug Administration (FDA) approved Leqembi® (lecanemab), a monoclonal antibody (MAB) co-manufactured by Eisai and Biogen, for treatment of early-stage AD. This decision was the catalyst for the Centers for Medicare & Medicaid Services (CMS) to immediately affirm that the agency would cover the medication broadly. Even before this, the Veteran ’s Health Administration — the largest health system in the US — was already covering the drug.
This represents a pivotal step in the pathway to drug access for millions of Americans. While the current suggested retail price of lecanemab is high ($26,500 annually per patient), AD currently costs our nation upwards of $355 billion per year and this number is expected to increase to $1 trillion by 2050.1,3 Medicare patients will only have to pay the standard 20% coinsurance of the drug cost once they meet their Part B deductible. Referrers must enter patients in a registry that requires completion of a short, easy-to-use data submission form. Other DMTs will soon follow, but at the present time, lecanemab is the only one granted traditional FDA approval. A predecessor drug, Aduhelm® (aducanumab), Biogen ’s predecessor drug, received accelerated FDA approval but is not covered by CMS outside of clinical trials.
Confirmation of beta-amyloid (Aβ) plaque is required prior to initiation of therapy. Amyloid PET is of paramount importance as an alternative to the more invasive lumbar puncture cerebrospinal fluid analysis for evidence of the protein. In July, CMS proposed rescinding the national non-coverage determination (NCD) restricting amyloid PET coverage, permitting individual Medicare administrative contractors to make coverage determinations. When and at what rate CMS will start reliably reimbursing for amyloid PET is unclear as of this writing.
It is important to note that lecanemab is approved only for patients with early-stage cognitive impairment, including mild cognitive impairment (MCI) due to AD or mild AD. Estimates are that approximately 1.5 million Americans will be candidates for on- label therapy.1
Treatment Progress
In the phase 3 CLARITY-AD trial published in January 2023,4 lecanemab met the primary efficacy endpoint by slowing the rate of cognitive decline by 27% over 18 months based on the Clinical Dementia Rating-Sum of Boxes (CDR-SB).
In the phase 3 TRAILBLAZER-ALZ 2 trial published in July 2023,5 donanemab (Eli Lilly) met the primary efficacy endpoint by slowing the rate of cognitive decline by 35% over 18 months. Additionally, nearly half (47%) of donanemab participants had no disease progres- sion at one year, based on the CDR-SB. Patients with less advanced disease (low/medium tau burden) outperformed those with more advanced disease (high tau burden), confirming that the earlier treatment is initiated the higher the clinical benefit.
In both trials, the secondary endpoints were also met. Specifically, therapy resulted in effective clearance of Aβ plaque, a hallmark of AD, as imaged with amyloid PET.
Numerous other trials are ongoing, including disease prevention trials such as the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU). Several trials focus on pharmacological targeting and monitoring of other biomarkers for the disease such as neurofibrillary tangles, which can be imaged with tau PET. The National Institute of Aging currently funds more than 450 active clinical dementia trials.
Side Effects
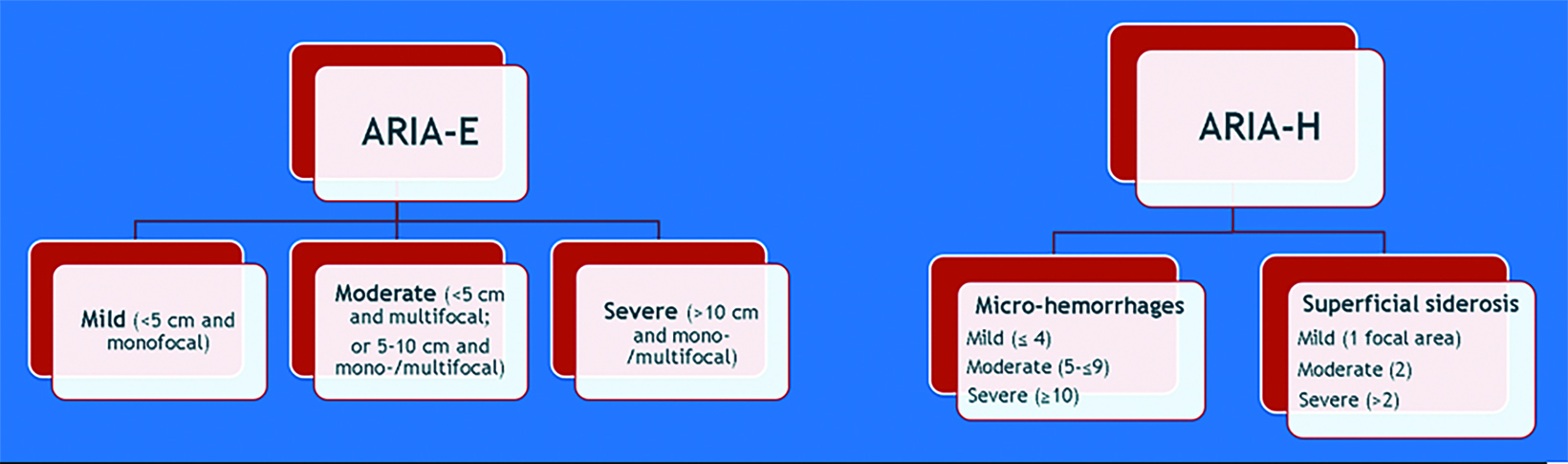
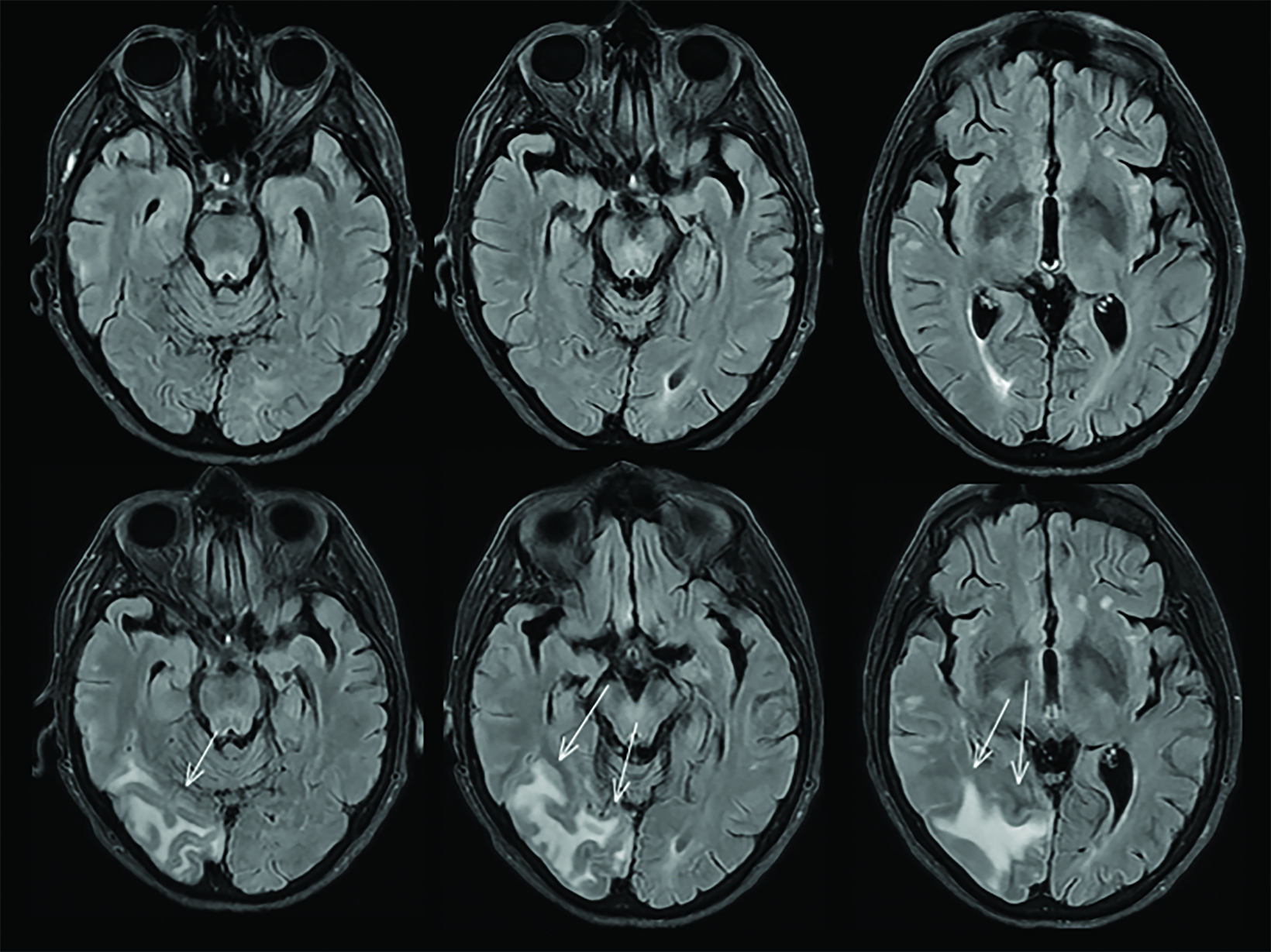
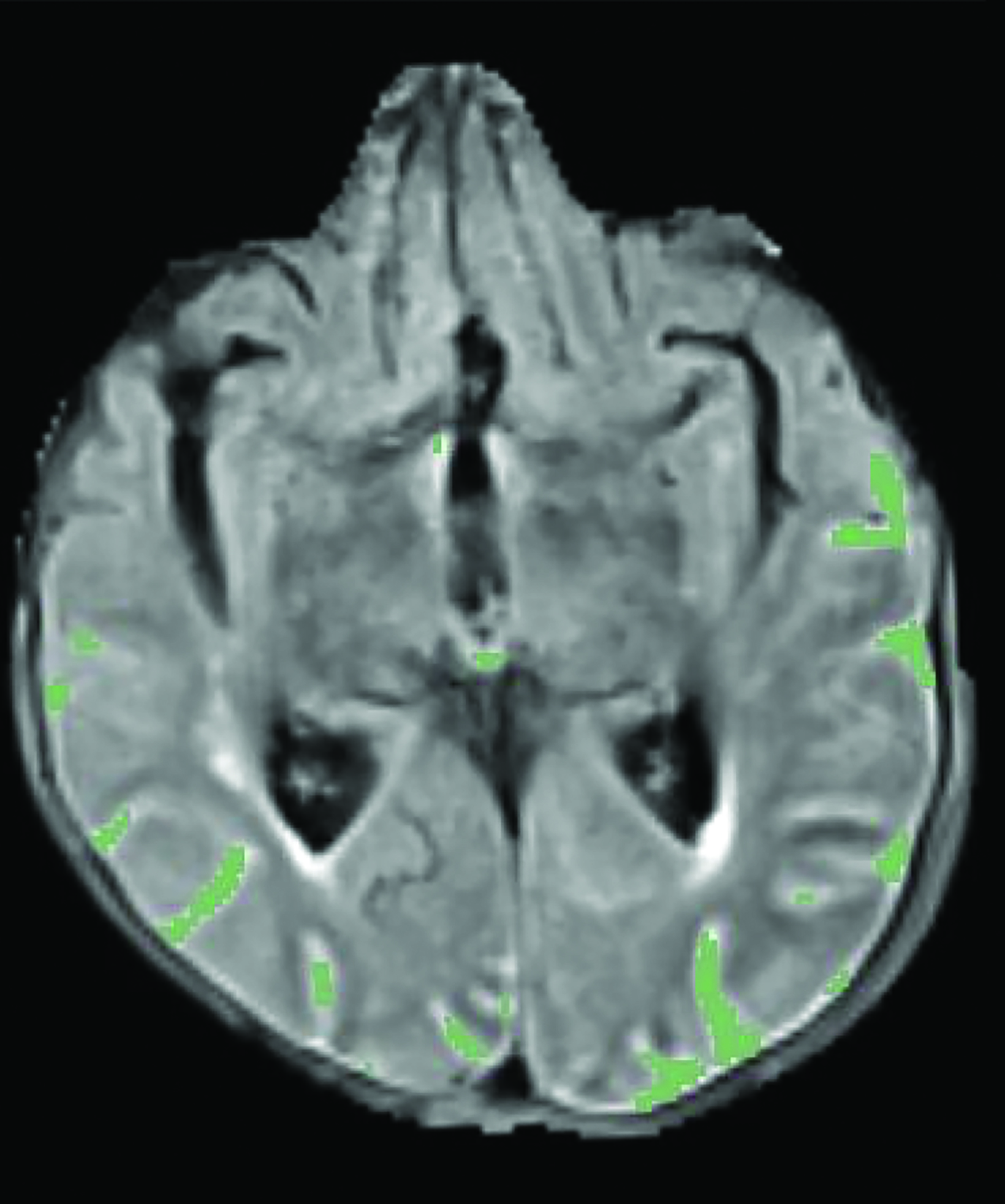
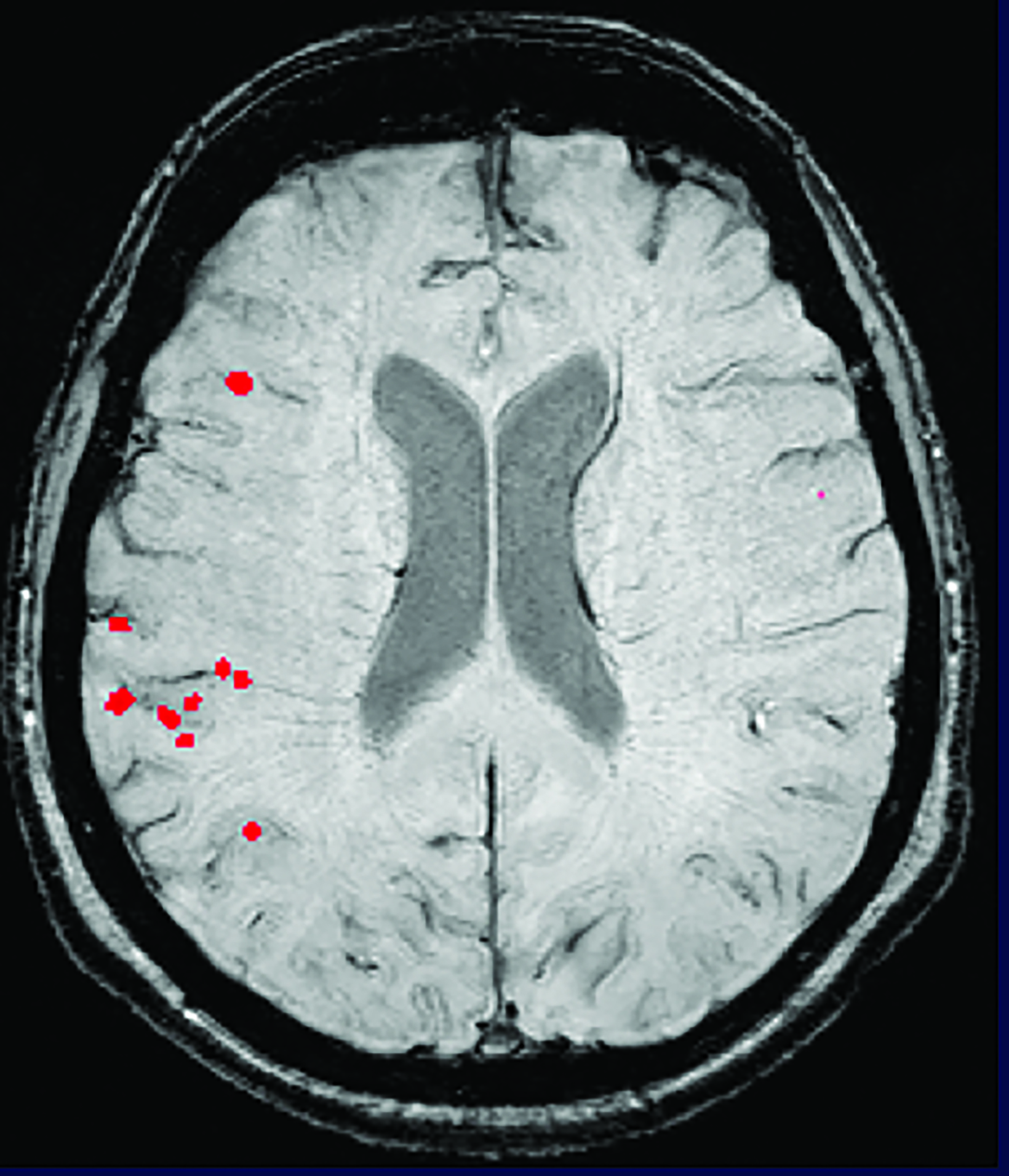
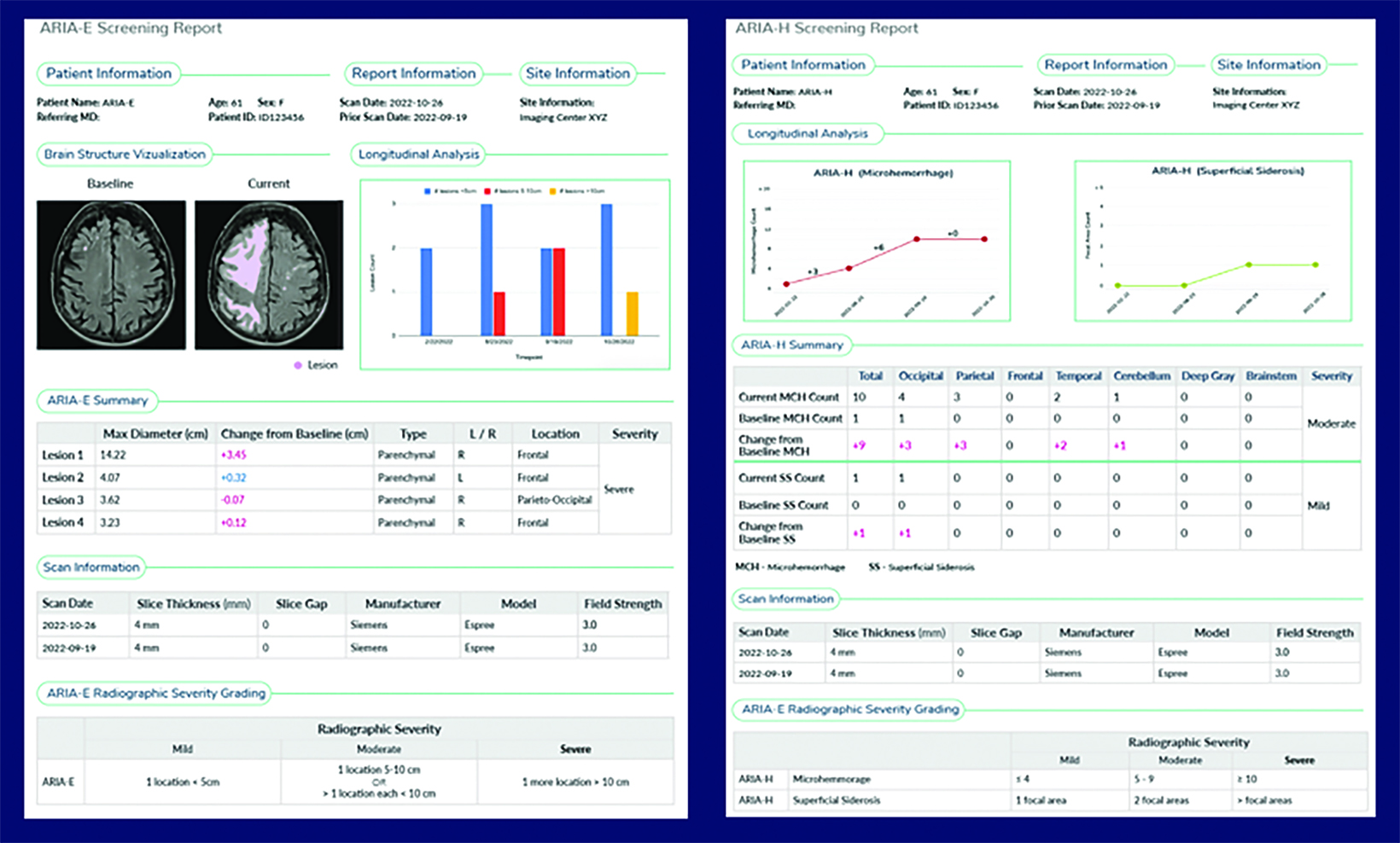
A side effect of these MAB-based therapies that target and mobilize Aβ plaque is the development of drug-induced amyloid-related imaging abnormalities (collectively known as ARIA, subdivided into ARIA-E and ARIA-H). ARIA-E is characterized by edema and/or sulcal effusions. ARIA-H is characterized by microhemorrhages and/or superficial siderosis (Figure 5).
ARIA can clinically present as headaches, confusion, nausea, or visual disturbance. ARIA risk is highest in the initial treatment period, at high dose, and in those with APOE ε4 homozygote carrier status. Caution should be exercised when considering the use of lecanemab in patients with factors that increase the risk of cerebral hemorrhage, such as concomitant anticoagulation or baseline cerebral amy- loid angiopathy.6
If the patient develops ARIA symptoms (moderate or severe symptoms for ARIA-E or any symptoms for ARIA-H) or asymptomatic radiographic moderate or severe ARIA, treatment is temporarily held until a follow-up MRI demonstrates resolution of ARIA-E and stability of ARIA-H (Figure 6). In trials, the overall incidence of ARIA was 41% for aducanumab, 37% for donanemab, and 21% for lecanemab. 4,5,7
The good news is that most patients who develop ARIA are asymptomatic. Symptomatic ARIA occurs in less than 3% of all pa- tients on lecanemab.4 Additionally, ARIA-E is temporary and typically resolves over the course of weeks to months.4,5,7 Of note, the rate of symptomatic ARIA was higher for aducanumab (26%) and donanemab (6%) than with lecanemab (<3%).4,5,7
Homozygous APOE ε4 carriers have a higher incidence of symptomatic, serious, and severe radiographic ARIA, compared to heterozygote carriers and noncarriers.6 Therefore, APOE ε4 screening is recommended (but not required) prior to initiating lecanemab treatment to inform the risk of ARIA.6 Among the general US population, 25% carry one APOE ε4 allele (heterozygous), which portends a 3-times higher risk of developing AD.8,9 An estimated 2-3% of the population carries both APOE ε4 alleles (homozygous), which portends a 12-times higher risk for the disease.8,9 About 15% of AD patients are APOE ε4 homozygotes.8,9
Infusion-related reactions occur in approximately 10% of patients, often presenting with fever and flu-like symptoms.6 Lecanemab is administered intravenously (IV) every two weeks during treatment.6 Donanemab is a once monthly IV infusion.5 Trials are underway to evaluate the efficacy of a subcutaneous injection mode of delivery for lecanemab which would enable home administration and negate the risk of infusion reactions.10 Preliminary results of subcutaneous injection trials are promising for similar efficacy with the added anticipation of reduced ARIA-E rates.10
Impact on Imaging Enterprises and Neuroradiologists
Higher MRI Volume
Imaging centers around the country are gearing up for an anticipated steep increase in the number of brain MRI requests. Magnetic resonance imaging is required at baseline and prior to the 5th, 7th, and 14th infusions because of ARIA risk.6 The baseline MRI must be recent to accurately screen for exclusionary findings and microhemorrhages. Appropriate use recommendations for lecanemab published by the Alzheimer ’s Disease and Related Disorders Therapeutics Work Groups also suggest an additional MRI prior to the 26th infusion (week 52).11 Patients on treatment who develop neurologic symptoms which could portend ARIA will require an additional brain MRI that may be requested on an urgent basis. If ARIA is present, follow-up MRI scans are recommended every two to four months until ARIA-E has resolved, and ARIA-H has stabilized.6
The anticipated increase in scan volume is expected to widely impact outpatient and inpatient imaging enterprises.12,13 Organizations can estimate this increase by evaluating their regional demographics and number of patients with MCI or mild AD being treated locally. One example is cited in a white paper from the Alzheimer/ARIA leadership group of the American Society of Neuroradiology (ASNR) (in press — Cogswell, P, Benzinger T, et al): The local Olmsted County, Minnesota, population is approximately 160,000; per latest census data, about 30% (48,000) are 55 years or older. Assuming 15% have MCI (7,200) and a 50% rate of presentation for evaluation, one could estimate 3,600 additional patients presenting for evaluation at some level of the regional health system. MRI equipment manufacturers anticipate increased new system orders to accommodate this demand.
Higher Amyloid PET Volume
With the recent proposal to remove the CMS NCD restricting amyloid PET coverage, imaging practices can expect to see an uptick in amyloid PET studies to confirm Aβ. In the clinical trials, amyloid PET scans were used to confirm therapeutic candidates and perform longitudinal analysis of Aβ clearance.4,5,7 In clinical practice, neurologists may choose to order additional amyloid PET studies beyond baseline to assess disease progression and drug efficacy. Medical enterprises should ensure sufficient scanner availability and enough radiologists familiar with amyloid PET to interpret these studies.
New Imaging Protocols
Standardized, appropriate protocols for dementia therapeutic imaging are critically important.13,14 It is essential for local neurologists to include DMT information in imaging requests in a way that alerts schedulers and neuroradiologists, perhaps by offering “AD therapeutic baseline” or “AD therapeutic monitoring” as the study indication. Both 1.5T and 3T scanners can be used for imaging, but in challenging patient populations open 1.2T systems may also be employed in practice. Ideally, patients should be imaged on the same field-strength scanner throughout surveillance. If quantitative imaging tools are being used for analysis, imaging may best be performed on the same scanner, although this may not always be practical.
Minimum imaging protocols for screening and surveillance should include GRE, FLAIR, and DWI, but most radiologists will simply employ their routine brain protocol. A 3D T1 sequence should be included if quantitative analysis of brain volume is desired.
A conventional 2D GRE scan is mandatory, as it was the standard method to assess microhemorrhages in the trials, 4,5,7 but some may choose to add 3D susceptibility weighted imaging (SWI) for greater sensitivity to blood products. The ASNR has partnered with equipment manufacturers to present optimized AD therapeutic imaging protocols, which are posted on the ASNR website at www.asnr.org.
Neuroradiology Educational Initiatives
ARIA can be subtle and the requirements in reporting are specific to the needs of treatment. An educational initiative is ramping up throughout the neuroradiology community, as radiologists and imaging enterprises are poised to play a critical role in therapeutic dementia imaging. Educational resources and ARIA training are available through multiple sources, including the ASNR website, AlzImaging.com, expert forums,13,14 and recent publications.15
Artificial Intelligence Tools
Artificial intelligence (AI) solutions like deep learning-based image reconstruction can maximize scanning efficiency by enabling 50-70% faster acquisition, which will help to accommodate increased MRI scan volumes. The faster scan times are also advantageous for motion-prone dementia patients, who may also be suffering from confusion and anxiety.16,17
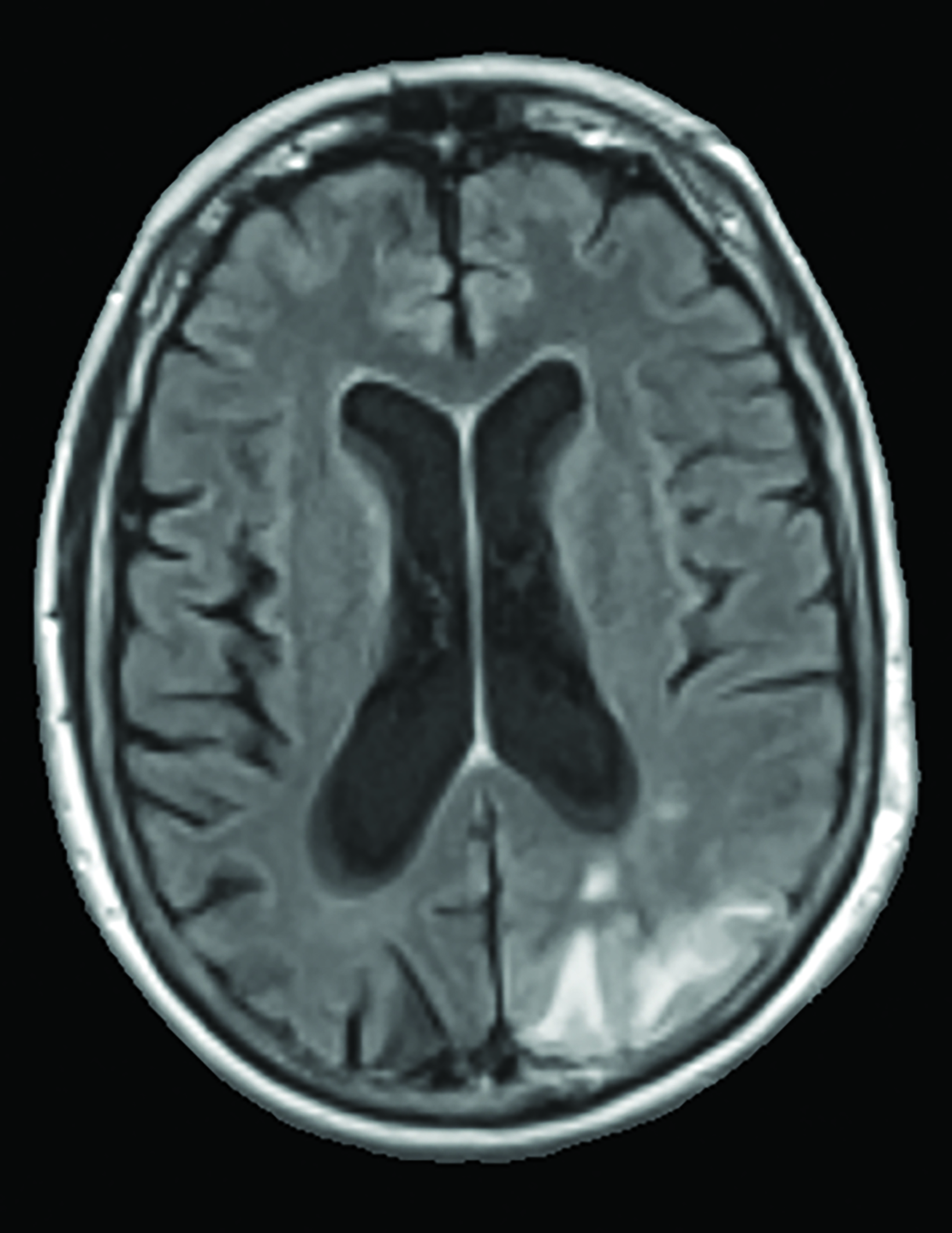
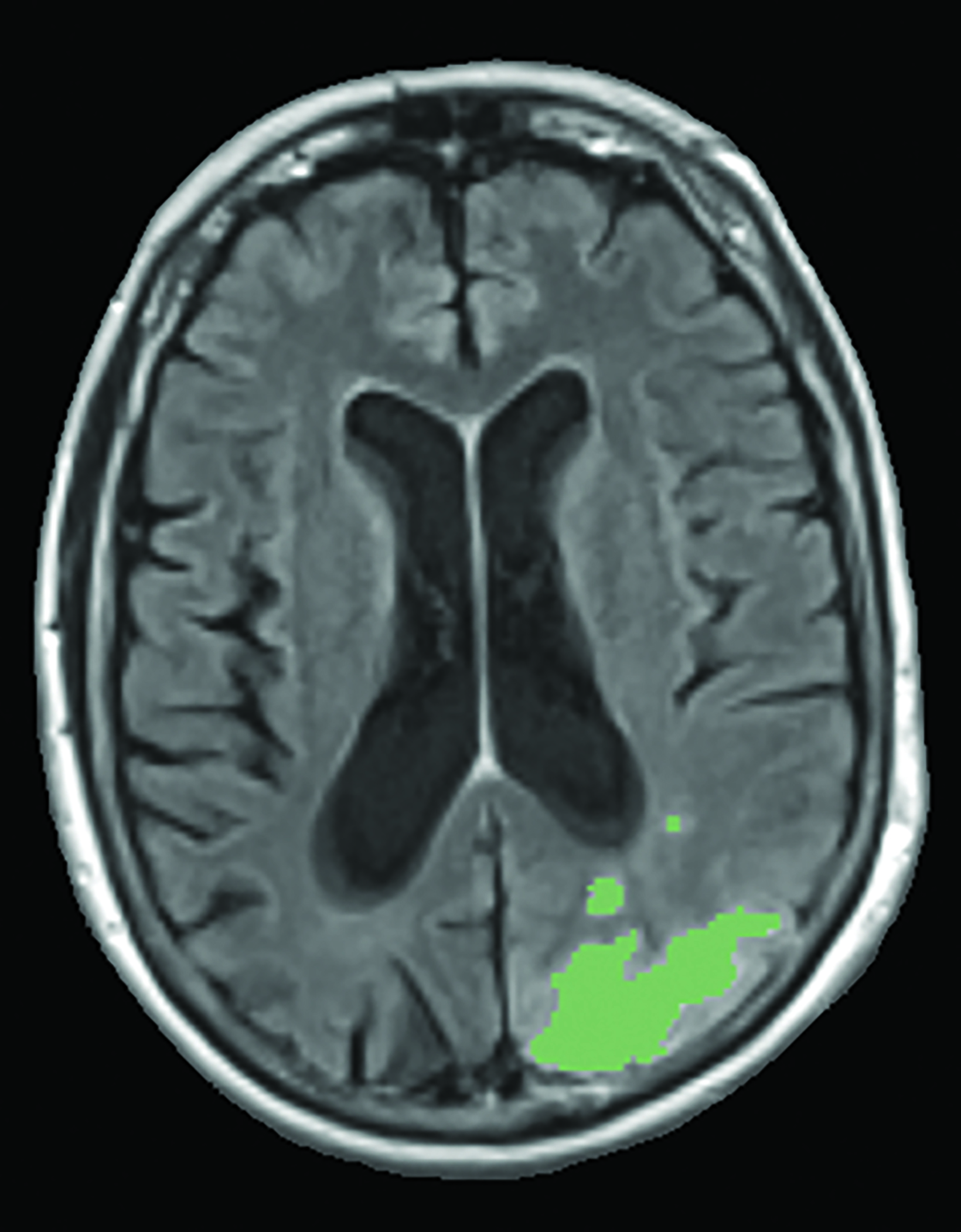
A recent survey sent to the 2,700 members of ASNR revealed that 63% of responding neuroradiologists are interested in utilizing an AI-based quantitative tool to aid in ARIA detection and longitudinal follow-up. Multiple vendors are working to develop quantitative ARIA screening solutions to aid neuroradiologists in efficient and accurate ARIA segmentation (Figures 7-9) and reporting (Figure 10).12 These products should detect and quantify ARIA-H and measure the volume of ARIA-E, as well as highlight changes that occur during surveillance.12 It is anticipated that the tools will provide standardized classification and automated ARIA grading, eg, mild, moderate, or severe.
The TRAILBLAZER-ALZ 2 trial found better preservation of brain volumetry in the treatment limb than in the placebo limb,5 so quantitative assessment of brain volumes (Figure 11) may also be beneficial as a quantitative structural biomarker in determining longitudinal treatment efficacy.18 In the interest of enhancing workflow, prepopulated quantitative MRI (QMRI) reporting templates may facilitate standardization and efficiency.18
New CPT Codes for QMRI
The American Medical Association issued new CPT Category III codes, 0865T and 0866T, for QMRI analysis of the brain in July 2023, representing a significant milestone in enhancing AD diagnosis and, more importantly, improving patient care.19 These vendor-neutral, standardized temporary codes, which take effect Jan. 1, 2024, will hopefully create a path to reimbursement for comprehensive QMRI analysis for lesion identification, characterization, and quantification, as well as for brain volumetry. As is typically the case with novel codes, payments are expected to be inconsistent initially. Billing studies correctly under these codes, rather than employing common workarounds, is important for driving conversion to Category I and universal payment.
Agility Required
It is becoming increasingly apparent that the dynamic landscape of AD diagnosis and treatment is profoundly impacting healthcare. Neuroradiologists will play a key role in clinical decision making regarding patient eligibility for and continuance of DMT. Radiologists and their enterprises will require the agility to quickly adapt to changing demands and opportunities at this pivotal time in AD diagnosis and treatment.
References
Citation
S B, LN T.Alzheimer Disease Imaging in the Era of Anti-Amyloid Treatment. Appl Radiol. 2023; (5):16-23.
September 12, 2023