Alzheimer Disease Imaging in the Age of Disease-Modifying Therapies
Emerging anti-amyloid, disease-modifying therapies (DMTs) promise to revolutionize the management of patients with Alzheimer disease (AD), particularly the estimated 1.5 million who are in the earliest stages of the disease.1
Neurologists will need a helping hand from their medical imaging colleagues, who will face dramatically higher demand for their services to maximize the potential of the new therapies.
“This year has brought tremendous advances in the dementia landscape, including expanded opportunities for anti-amyloid targeting therapy to decrease the rate of cognitive decline,” says Suzie Bash, MD, a neuroradiologist and medical director at RadNet, and editorial advisory board member. “The earlier AD is diagnosed, the greater the treatment benefit, so early beta-amyloid confirmation is critical.”
Alzheimer Disease Treatment: A Quick Synopsis
Historically, AD treatments aimed to manage symptoms by primarily targeting the imbalance of neurotransmitters in the brain such as acetylcholine and glutamate. In most cases, however, they only modestly improve or stabilize symptoms for a limited amount of time.
But new DMTs such as lecanemab (Leqembi) and donanemab-azbt (Kisunla) are emerging as major game changers. These drugs consist of monoclonal antibodies that focus on reducing the underlying pathology of AD, specifically the beta-amyloid plaques, to help slow progression of the disease.
Clinical trials have shown that lecanemab can slow the rate of cognitive decline by about 27% in patients with early AD (mild cognitive impairment secondary to AD or mild AD); in the Clarity-AD sub-study analysis, lecanemab performance was even more impressive, with 76% reduction in cognitive decline and 60% clinical improvement at 18 months in the low-tau group (representing patients at the earliest stage of the disease).2 Donanemab-azbt slowed cognitive and functional decline by about 35% in patients with early AD. Both lecanemab and donanemab achieved significant plaque clearance.
Although they are not a cure for AD, these treatments reflect a shift from managing symptoms to modifying disease trajectory, offering hope for improving patients’ quality of life over a longer period.
The Critical Role of Imaging
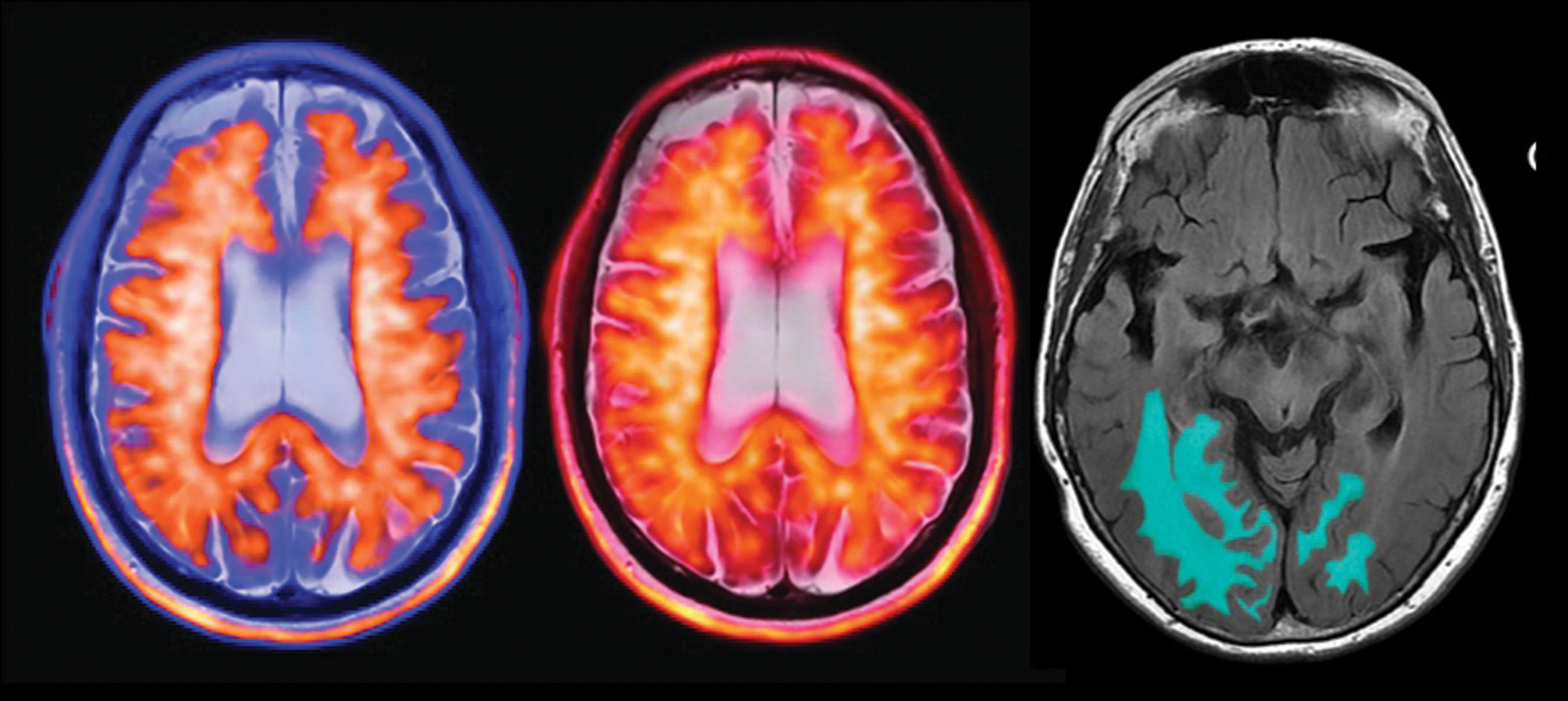
Promising as they are, before DMTs can be implemented, the presence of beta-amyloid plaque must be confirmed either through amyloid PET ( Figure 1 ) or, less commonly, through invasive cerebrospinal fluid analysis. Baseline brain MRI scans are also needed to document preexisting microhemorrhages or other findings that may preclude treatment with these drugs.
The positive amyloid PET image with color PET-MR fusion on a T2-weighted MR sequence is of a patient with early Alzheimer disease (AD) (left and middle images). A quantitative MRI artificial intelligence-assist tool with automated amyloid-related imaging abnormality segmentation on a FLAIR MR sequence (right image) shows a patient with early AD who was on disease-modifying therapy (image courtesy of Dr Suzie Bash).
As a result, the demand for imaging is growing, says Lawrence Tanenbaum, MD, editorial advisory board member and former chief technology officer at RadNet.
“There is great impact on the imaging enterprise as a result of this patient population coming in for clinical assessment and consideration for DMT,” Dr Tanenbaum says. “Amyloid PET volumes across the U.S. are skyrocketing, going from the occasional case to many cases per day, per facility, because of its key role. Brain MRI is also increasing as it is used for pre-treatment screening baseline analysis and ARIA [amyloid-related imaging abnormality] surveillance.”
Debbie Gibbons, senior director of sales, PETNET Solutions, agrees, noting that because of the new treatment options, the Centers for Medicare & Medicaid Services last October began approving reimbursement for amyloid PET—a development certain to accelerate demand for imaging.
“Now that there is a pathway to treat with multiple options and it’s covered [via reimbursement], more people can get the answer as to whether they have AD versus a different dementia,” says Gibbons.
Imaging Protocols for DMT
Amyloid PET imaging can help determine whether a patient has AD. Brain MRI is required prior to starting treatment, as well as for amyloid-related imaging abnormality (ARIA) surveillance during treatment.
“The non-ionizing nature of MRI and its versatility makes it ideal for longitudinal tracking of brain changes,” says Suchandrima Banerjee, senior global director of Neuro MR for GE HealthCare, “as well as identifying preexisting hemorrhage and managing surveillance.”
With lecanemab, which requires twice-a-month infusions, MRI scans are performed at baseline and prior to the 5th, 7th, and 14th doses. Donanemab, meanwhile, is administered every 4 weeks and requires MRI scans just before the second, third, fourth, and seventh doses. If ARIA signs or symptoms are present, treating physicians often request additional MRI scans.
As pathophysiological changes that result from mobilization of amyloid in the brain, ARIA causes inflammation in the blood vessel walls. MRI can identify associated edema and/or microhemorrhages or superficial siderosis to help physicians decide whether to pause or discontinue therapy, explains Dr Tanenbaum.
“ARIA complications are generally mild and self-limiting, but serious events have occurred,” he says.
Throughput Considerations
As more patients undergo treatment with DMTs, PET and MRI volumes are rising, bringing challenges to imaging enterprises, such as timely distribution of amyloid PET tracers, nationwide shortages of PET technologists, and reimbursement delays, according to Dr Bash.
“Growth has been tremendous for all amyloid imaging agents that have been filed to Medicare,” Gibbons observes, while noting that many imaging providers do not have the bandwidth to accommodate the new patients.
“We’re seeing bottlenecks on the imaging side,” she says, adding that some imaging practices are using mobile units to scan patients 1 or 2 days a week, while some neurology practices want to run their own PET scanner or mobile set-up.
Similar impacts face MRI centers, says Dr Tanenbaum. Patients with AD require at least 4 or 5 scans annually, and potentially as many as 10 in the event of higher-risk genetics or treatment-related complications.
“Imaging enterprises are already gearing up for this significant boost in MR volume,” he says.
Technology Support
Dr Tanenbaum and other experts envision greater demand for additional MRI scanners and other tools, including artificial intelligence (AI), to optimize throughput across imaging enterprises.
“Our AI reconstruction technology can help reduce scan times, in some cases, approximately a 70% reduction for brain imaging,” says Saurabh Sharma, business development manager, MRI, for Siemens Healthineers. Sharma adds that biometric technology will address biovariability in patients and automation will reduce technologist burden to make imaging centers more efficient.
“We have prioritized AI-driven innovations that not only reduce scan times, but also make scans more consistent and less susceptible to [variation], allowing radiologists to have greater confidence,” says Banerjee. GE’s deep-learning-based landmark identification technique can ensure a patient’s images are acquired with the same angulation at each exam.
Quantitative assessment of brain volume changes with AI-driven segmentation can be helpful in early diagnosis and may be used to augment interpretation over time, Dr Tanenbaum says.
A recent study shows that AI-based assistive software may enhance the diagnostic accuracy of monitoring ARIA for patients receiving amyloid-beta-directed antibody therapies.3 Quantitative AI-powered tools can improve the quality and consistency of ARIA surveillance through automated quantification and radiographic grading. One AI-assist tool recently received US Food and Drug Administration 510(k) clearance,4 and an additional tool is approaching the market and aspiring for approval under the CADx designation.3 In a recent poll by the American Society of Neuroradiology, the majority of respondents indicated that they were interested in utilizing an AI solution designed to enhance ARIA surveillance.5
“Brain MRI and quantitative MRI [QMRI] volumes have dramatically increased,” says Dr Bash. “Dedicated vendor-neutral QMRI CPT III codes are starting to get reimbursed by Medicare administrative contractors, paving the way for continued increased utilization benefits.”
A Collaborative Future
Radiologists must be educated on the unique requirements of AD imaging, particularly the need to identify the MRI findings of ARIA, which can be “subtle and unfamiliar,” says Dr Tanenbaum, who notes that the findings of ARIA are frequently missed.3
“There’s the continued need for all neuroradiologists to receive ARIA training, since accurate and timely interpretation of ARIA surveillance MRIs directly impacts therapeutic decisions,” Dr Bash agrees. “The neuroradiology community has stepped up by initiating dedicated ARIA training opportunities, and original equipment manufacturers have worked collaboratively to make dedicated dementia protocols available.”
Radiologists will focus on establishing appropriate scanning protocols and optimal workflows, from scheduling through exam execution, as well as providing clear reports to treating physicians. Communication among all, particularly with ordering physicians, is also key to effective diagnosis and treatment, Dr Tanenbaum says.
Dr Bash notes that the medical community is responding well to the challenges and opportunities of DMTs in “remarkably collaborative and creative ways by employing measures to streamline communication, optimize workflow, overcome capacity barriers, train staff, and provide necessary services and resources to allow imaging and treatment access for all eligible patients.”
“As patient volumes continue to increase, providers will further adapt their practices to accommodate, because patient-centric care always remains the highest priority,” Dr Bash concludes.
References
Citation
Reeves, K. Alzheimer Disease Imaging in the Age of Disease-Modifying Therapies. Appl Radiol. 2024;(6):28 - 31.
doi:10.37549/AR-D-24-0040
December 1, 2024