AI Method Could Identify High-Risk Cardiac Patients in Routine CT Scans
Images
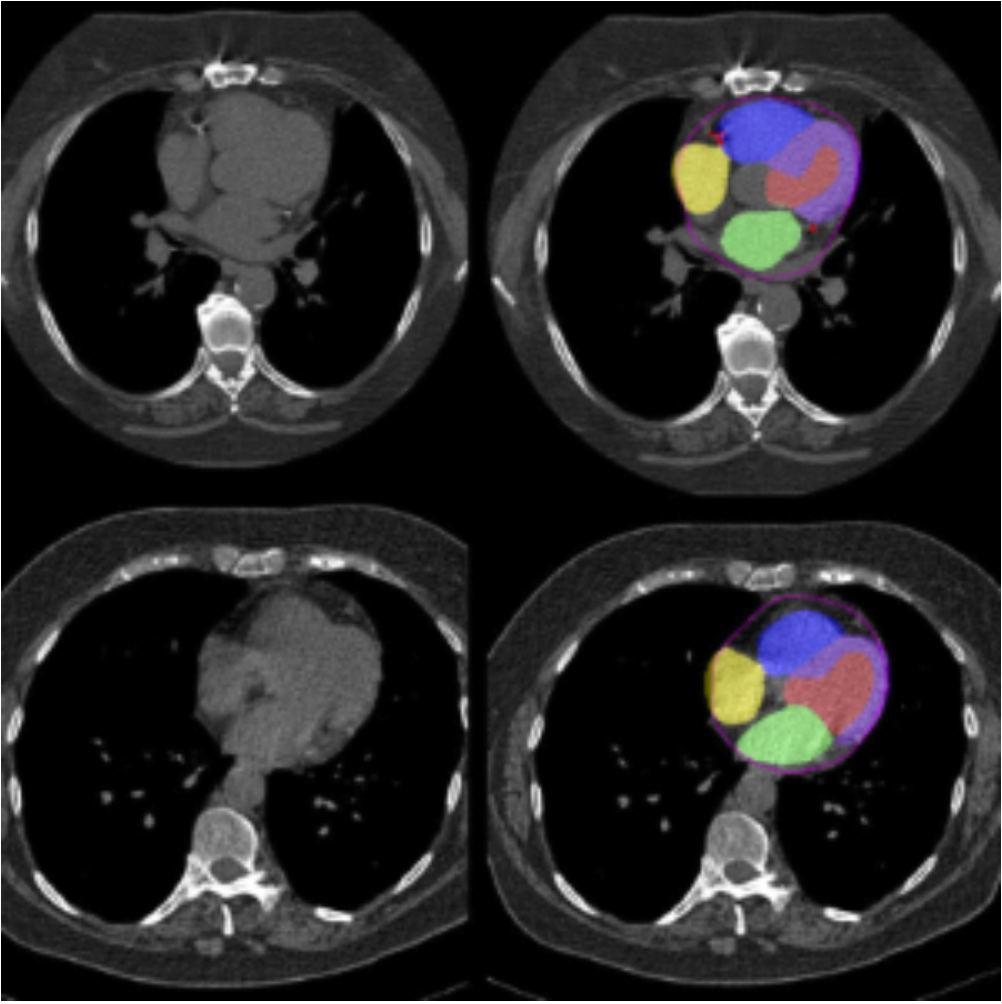
Screening for heart disease is an important step that can detect cardiac issues early, identifying patients that require further evaluation or potential treatment. While many screening tools rely on measuring factors in the blood (such as cholesterol and triglyceride levels), computed tomography (CT) scans can provide a wealth of real-time information about cardiac function. Yet quantitative, in-depth images of the heart typically require specialized equipment and dyes, and cardiac CT is therefore expensive and potentially underutilized.
Routine chest CT scans, however, are performed fairly frequently when patients have certain symptoms. These standard scans are ordered for a variety of reasons, such as suspicion of lung infection or cancer. Could these existing scans potentially be used as a screening tool for heart disease?
A collaborative team at Cedars-Sinai Medical Center in Los Angeles is using artificial intelligence (AI) to mine common chest CT scans to predict mortality. Their research identified a collection of cardiac factors that were predictive of death in a large group of patients, potentially setting the stage for improved cardiac screening. The findings were recently reported in Nature Communications.
“The AI pipeline described here represents an efficient, cost-effective method to potentially identify patients at high risk of heart disease who are undergoing routine CT scans for some other purpose,” said Qi Duan, PhD, a program director in the Division of Health Informatics Technologies at NIBIB. “This creative approach highlights the power of machine learning to derive meaningful information from routine medical images, paving the way for timely and improved health care.”
How can images of the heart help to predict cardiac risk? An established method is to quantify the amount of calcium that is present in the arterial plaque.
As we age, plaque can begin to build up inside the arteries of the heart. After plaque accumulates, it begins to calcify. This calcium hardens the plaque and stiffens the artery walls, making it harder for blood to flow. As such, the amount of coronary artery calcium (referred to as CAC) is strongly correlated with cardiovascular risk.
To calculate CAC, a clinician will typically order a “gated” CT scan, which uses the signal from an electrocardiogram to capture images of the heart at a specific cardiac phase. This type of advanced scan is more expensive than traditional chest CT scans and may not be covered by medical insurance.
Beyond CAC, the shape and size of the different chambers of the heart can also help predict heart disease. For example, having an enlarged left atrium has been shown to be a predictor of heart failure. But estimating the myocardial mass and volume of each heart chamber typically requires the use of a contrast agent, which is not used in all routine chest CT scans.
This is where AI has the potential to be beneficial and integrated as a screening tool. By using images from thousands of different patients, AI can begin to automatically determine prognostic features from routine chest CT scans—features that the original scan was not designed to detect. These features can be combined and analyzed to generate a prediction of cardiac-related mortality.
“There is a lot of important information that is hiding in chest CT scans,” explained senior study author Piotr Slomka, PhD, a professor at Cedars-Sinai. “By using AI to unearth and analyze key prognostic signals in these scans, we could perform opportunistic cardiac screening and potentially prompt treatments or lifestyle changes, which could ultimately save lives.”
The researchers combined two previously validated algorithms—one developed by Slomka and colleagues that measures CAC, and another that can segment cardiac chamber volumes—to analyze routine chest CT scans (i.e., low-dose scans with no contrast and no gating). They evaluated scans from 24,354 patients who had CT performed as part of the National Lung Screening Trial and identified several cardiac factors that were associated with an increased risk of death, such as a large amount of CAC and increased mass or volume of specific heart chambers. What’s more, the researchers found that combining all the factors together (including clinical data like age and medical history) was a better predictor of death than any one factor alone.
“While these scans were originally used as a screening tool for lung cancer, we were able to reanalyze them with our AI pipeline to predict both overall and cardiac-related mortality,” said Slomka. He also noted that the current clinical standard for determining cardiac risk is the identification of an abnormality by a radiologist. Their pipeline substantially improved risk classification compared to this clinical standard.
The researchers wanted to know if their method could successfully be applied to other types of chest CT scans. They next evaluated scans from 2,014 patients who received specialized CT to calculate CAC (which required gating) and from 3,319 patients who received low-dose, ungated attenuation CT scans (which are used to make nuclear medicine scans such as PET and SPECT more accurate). In both groups, they used AI to identify cardiac features predictive of death, and their models performed best when all imaging factors and clinical data were combined.
“In three separate cohorts, which used different types of CT scans for discrete purposes, we could use our combinatorial AI platform to predict mortality,” said Slomka. “These results suggest that our platform could be successfully applied in a wide array of settings.”
Slomka noted that integration of this AI pipeline into an existing clinical workflow should be simple—in fact, it has already been incorporated at Cedars-Sinai, where physicians are evaluating its utility for research purposes. “Our AI platform is currently being used to automatically evaluate routine CT scans for prognostic cardiac factors,” he said. “Radiologists that analyze cancer screening images are not trained to look for things like calcium in the arteries or enlargement of cardiac chambers—having our method mine these images in the background can flag at-risk patients for follow-up scans and potentially treatment.”
An important limitation of this study is that race and ethnicity were not incorporated into the analyses. Further, more than 90% of the patients evaluated from the National Lung Screening Trial (the largest cohort in this study) were white. Future work should require including more diverse populations to ensure that this AI pipeline can perform equally for all people.