Advances in prenatal diagnosis
Images
Dr. Goldman is an Assistant Clinical Professor, Department of Obstetrics & Gynecology, Columbia University Medical Center, New York, NY. Dr. Malone is Professor and Chair, Department of Obstetrics and Gynaecology, Royal College of Surgeons in Ireland, Rotunda Hospital, Dublin, Ireland.
Prenatal diagnosis has revolutionized prenatal care from the perspective of both the patient and the physician. For the patient, prenatal diagnosis provides genetic, anatomic, and physiologic information about the fetus or fetuses that can be used to make informed and individualized decisions regarding the pregnancy. For the physician, prenatal diagnosis provides vital information that can be utilized for better antepartum management. Information regarding specific anatomic anomalies affords the physician the opportunity to offer the patient sophisticated prenatal procedures, such as fetal surgery or selective fetal reduction in multiple gestations. Likewise, prenatal knowledge about genetic, physiologic, and/or anatomic abnormalities enables the physician to tailor or manage the timing and mode of delivery for optimal maternal and fetal outcomes. Prenatal diagnosis also allows the neonatal and pediatric specialists to be adequately prepared for a potentially ill neonate at delivery.
Recent progress in the fields of maternal fetal medicine, radiology, and genetics has resulted in great advances in prenatal diagnosis. The following article will review the recent major advances in this rapidly progressing field.
Advances in the detection of fetal aneuploidy
In recent years, there have been significant advances in antenatal screening for fetal aneuploidy. Fetal aneuploidy is defined as an abnormal number of chromosomes instead of the usual diploid complement of 46 chromosomes. One single additional chromosome is termed trisomy and is an important cause of congenital malformations as well as of congenital mental retardation. Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Patau syndrome (trisomy 13) are the most common trisomies. Trisomies 18 and 13 are lethal and affect approximately 1 in 3000 and 1 in 5000 live births, respectively. The majority of infants with trisomy 18 die by 1 year of age, while the majority of infants with trisomy 13 die by 3 months of age. Trisomy 21 is compatible with life and affects approximately 1 in 800 to 1 in 900 live births. It is associated with congenital heart defects, most commonly the atrio-ventricular canal defect.
Initially, prenatal screening for Down syndrome constituted offering amniocentesis to women of advanced maternal age (age ≥35 at estimated date of delivery) and to women with a previously affected pregnancy. In the mid 1980s, it was noted that women in the second trimester with decreased levels of maternal serum alpha fetoprotein (AFP) were at increased risk for their pregnancy to be complicated by Down syndrome. As a result, noninvasive second-trimester Down syndrome screening for the general obstetric population became feasible, and a multiple-marker serum panel was established for this purpose. 1 Since >90% of all structural and chromosomal abnormalities derive from pregnancies without risk factors, it is reasonable that universal screening be provided. 2
Second-trimester maternal serum screening currently includes a combination of 4 markers and is known as the "quad test." These markers include AFP, human chorionic gonadotropin (hCG), unconjugated estriol (uE3), and inhibin A. 1 For screening purposes, sonographic dating should be used instead of menstrual dating, as it optimizes the performance of the test. While screening references are available between 15 and 21 weeks' gestation, the optimal time to perform this test is between 15 and 16 weeks' gestation. In addition, laboratories adjust for factors that may affect values, such as maternal weight, race, multiple gestation, and history of diabetes. It is important to note that with regard to twins, the sensitivity for Down syndrome using the quad test is only 47%, with a 5% false-positive rate. 3 Maternal serum screening in multiples has been limited because of the potential for discordance between fetuses and because of the impact of the various placentas on analytes. 4
In patients with a pregnancy affected by Down syndrome, the AFP and uE3 levels are lower, while the hCG and inhibin A levels are higher, than in unaffected pregnancies. In pregnancies affected by trisomy 18, the AFP, uE3, and hCG levels are all low. The quad test can detect approximately 75% cases of Down syndrome, with a 5% screen-positive rate. 5 Meanwhile, the quad test detects 85% to 95% of cases in women ≥35 years of age at estimated date of delivery, with a 25% screen-positive rate. 6,7 The disadvantages of this screening method include its relatively late performance in the second trimester, resulting in definitive diagnosis by amniocentesis being provided even later in gestation. In addition, many cases of Down syndrome will not be detected with this test, and the 5% screen-positive rate indicates that many amniocenteses will be performed for every 1 case of Down syndrome identified. 8
First-trimester screening
Due to the limitations of the quad test, great efforts have been put forth to devise more efficient screening protocols. Because of its ability to provide results early in pregnancy, first-trimester screening is becoming increasingly more important. First-trimester screening provides the opportunity for early risk assessment and early diagnosis of fetal aneuploidy via chorionic villus sampling (CVS). Early diagnosis allows for pregnancy termination earlier in gestation, if the patient so desires, affording the patient greater privacy. In addition, earlier pregnancy termination is associated with decreased maternal morbidity. 7
First-trimester ultrasound is one of greatest contributors to the recent advances in prenatal diagnosis and is rapidly becoming the cornerstone of screening for fetal aneuploidy. Techniques used to evaluate risk for Down syndrome during the first trimester include nuchal translucency (NT) sonography, assay of first-trimester maternal serum markers, nasal bone sonography, and Doppler sonography of the ductus venosus.
Nuchal translucency
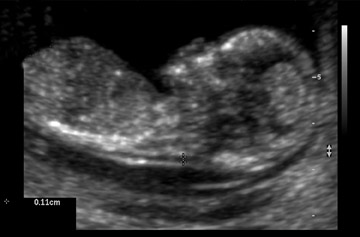
The normal space between the spine and the overlying skin at the back of the fetal neck during the first trimester is referred to as the NT (Figure 1). A large NT has been associated with an increased risk for aneuploidy, genetic disorders, anatomic abnormalities, and poor pregnancy outcomes. 7-9 When obtained between 10 weeks 3 days and 13 weeks 6 days' gestation, measurement of the NT has been shown to be a powerful sonographic marker for trisomy 21. 4 It is unlikely that one pathophysiologic mechanism leads to an enlarged NT. Possible etiologies include heart failure secondary to structural malformations, abnormalities in the extracellular matrix, and abnormal lymphatic development. 10
Nuchal translucency sonography is technically challenging and can be obtained both transabdominally and transvaginally. The fetus should be in a perfect midsagittal view. Careful attention should be paid to distinguish the nuchal skin from the amnion. The fetus should have a neutral neck, and the image should be magnified significantly. Calipers need to be placed at the inner borders of the NT space. Inter-and intraobserver variations in NT measurements have been noted. Therefore, both continued education and ongoing quality assurance are essential when NT measurements are used as a screening modality. 4 The ability to measure NT depends on the sonographer, the proper magnification of the fetus, the correct placement of the calipers, the ultrasound equipment, the fetal position, and the maternal body habitus. 11,12
In a portion of cases, obtaining an adequate NT may not be possible. When measuring the NT is difficult, the patient may return for a repeat examination in 1 week, provided that the gestational age is <14 weeks when the patient returns. Once the NT measurement is acquired, a special software program is used to convert the millimeter measurement into a multiple of the median (MoM) value. The MoM value takes into account gestational age variation in NT size, while allowing for the integration of maternal age and serum results.
In multiple pregnancy, NT appears to be a promising modality for screening for aneuploidy. As suggested previously, maternal serum screening in multiples has been limited because of the potential for discordance between fetuses and because of the impact of the various placentas on analytes. The NT distribution does not seem to differ significantly in singletons compared with twins. Thus, the Down syndrome detection rate in multiples should be similar to that of singletons. Further research is still needed, but this screening modality appears to be an improvement over maternal serum screening. 10 Some centers are already using NT measurements for screening for aneuploidy in patients with multiples, and NT measurements have been used for fetus selection in patients undergoing multifetal pregnancy reduction. 13
Cystic hygroma
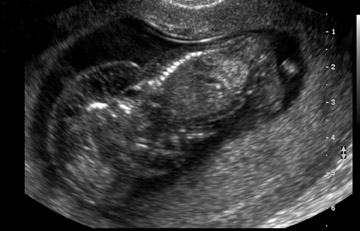
When a cystic hygroma is present, the NT is markedly enlarged and extends along the entire length of the fetus, with septations clearly visible within the space (Figure 2). It is important to remember that a cystic hygroma must be differentiated from a large NT. A cystic hygroma occurs in approximately 1 in 300 first-trimester ultrasounds. The finding of a cystic hygroma has been associated with a high risk for aneuploidy. The majority of cases are trisomy 21, but cystic hygroma has been associated with Turner syndrome, trisomy 18, trisomy 13, and triploidy. While many cases of cystic hygroma will have a normal karyotype, a large proportion of these cases will be complicated by a fetal malformation, such as a cardiac defect or a skeletal anomaly. Patients with a cystic hygroma on first-trimester ultrasound should be referred to genetic counseling, as only a small proportion of all cases of first-trimester cystic hygroma are associated with a normal live-born infant. 14
First-trimester maternal serum screening
First-trimester maternal serum screening utilizes maternal serum levels of pregnancy-associated plasma protein A (PAPP-A) and free beta hCG between 10 and 14 weeks' gestation. In pregnancies affected by Down syndrome, PAPP-A is lower compared with unaffected pregnancies at 10 to 14 weeks' gestation. Meanwhile, maternal serum levels of free beta hCG are higher in pregnancies affected by trisomy 21 than in unaffected pregnancies. These 2 markers, combined with maternal age, can detect up to 60% of cases of Down syndrome, with a 5% false-positive rate. 15
Combined sonographic and serum screening
The most efficient method of first-trimester screening for aneuploidy involves combining maternal age, NT, and the serum markers PAPP-A and free beta hCG. Between 10 and 14 weeks' gestation, this screening method may detect up to 82% of cases of trisomy 21, with a 5% false-positive rate. 4 Timing is important, as studies have shown that the accuracy of this screening protocol deteriorates over time. With this method, trisomy 21 detection rates are 87% at 11 weeks and 82% at 13 weeks, both at a 5% false-positive rate. 16
Nasal bone
Nasal bone sonography is another first-trimester ultrasound technique that has been proposed for screening for aneuploidy. 17 Similar to NT, this technique may be difficult to master and elusive to obtain. Nasal bone sonography is performed between 10 and 14 weeks' gestation. A perfect midsagittal image is required, and the fetal spine should be down with slight neck flexion. When the fetal profile is facing upwards, it may be possible to distinguish 2 echogenic lines at the fetal nose profile. The superficial echogenic line is the skin, and the deeper line is the nose bone. Failure to visualize the fetal nasal bone may be an independent risk factor for fetal aneuploidy. The studies suggesting that the absence of the fetal nasal bone may be used as a marker for trisomy 21 are limited by the fact that they are derived from high-risk populations. More recent research has suggested that nasal bone evaluation may not be useful for general population screening. 18
Ductus venosus Doppler sonography
Doppler of the ductus venosus is another technique that has been proposed for the first-trimester screening of fetal aneuploidy. The ductus venosus usually demonstrates a triphasic flow pattern, with forward flow reaching peaks during ventricular systole and early ventricular diastole. Absent or reversed flow at the time of atrial contraction is considered abnormal and has been suggested as a marker for aneuploidy. 19-24
Doppler of the ductus venosus at this point is not used in the general population for screening for aneuploidy. It is technically challenging during the first tri-mester. 4 At 10 to 14 weeks' gestation, the ductus venosus vessel may be as small as 2 mm while the Doppler gate size may be
0.5 to 2 mm in size. As a result, obtaining accurate flow velocity waveforms from such a tiny vessel may be difficult without contamination of the waveforms from the neighboring vessels. In addition, it is uncertain whether the NT measurement and ductus venosus flow are independent findings. If they are not independent, it becomes statistically complex to use one test to alter risk assessment derived from another test. At this time, screening for aneuploidy using Doppler of the ductus venosus should be considered investigational. 13
Second-trimester screening
While the advances in first-trimester screening are exciting, there will always be a role for second-trimester screening. Many patients may not present in time for first-trimester screening. Likewise, many patients may not have access to providers skilled at NT sonography or skilled at CVS. A portion of patients may prefer amniocentesis to CVS. In addition to second-trimester maternal serum screening (which was discussed previously), other second-trimester screening methods include ultrasound detection of anatomic abnormalities as well as ultrasound detection of minor markers for aneuploidy.
Second-trimester ultrasound
At the time of second-trimester anatomic ultrasound, any major fetal structural anomaly (such as a cardiac defect, omphalocele, diaphragmatic hernia, duodenal or esophageal atresia, renal abnormalities, clubbed or rocker-bottom feet, holoprosencephaly, meningomyelocele, and/or proboscis) should prompt immediate consultation with a genetic specialist, as these findings have been associated with trisomy. Invasive prenatal diagnosis should be offered for any major congenital anomaly. The optimal time to perform an anatomic survey is at approximately 18 weeks' gestation, as evaluation of the fetal anatomy is maximized at this time. Likewise, amniocentesis results can be obtained in a timely manner, and termination of pregnancy is still an option if the patient desires.
In addition to major structural anomalies, second-trimester ultrasound can detect minor markers for aneuploidy. 25-27 These findings are not fetal malformations. Rather, they are specific ultrasound findings that have been associated with an increased risk for fetal aneuploidy in certain high-risk populations, such as those of advanced maternal age or those with abnormal serum screening results. The role of these markers in the low-risk population remains uncertain. These markers include nuchal thickening, mild ventriculomegaly, a short humerus or femur, echogenic bowel, enlarged cisterna magna, renal pyelectasis, echogenic intracardiac focus, hypoplastic nasal bones, a single umbilical artery, choroid plexus cysts, and overlapping fingers. Detection of these minor markers should prompt consultation with a genetic or maternal fetal medicine specialist who is experienced at counseling patients regarding the risks associated with these markers. Use of likelihood ratios may be useful for integrating these markers into a precise risk assessment. A detailed discussion of these markers is beyond the scope of this review.
Combined first- and second-trimester screening for aneuploidy
Another option for screening for aneuploidy that is gaining popularity is combining first- and second-trimester screening. Combined first-trimester NT and serum screening has shown similar results as second-trimester serum screening with the quad test with regard to screening for aneuploidy. 7 Combining first- and second-trimester screening maximizes the performance of both of these screening modalities. 28 At this point in time, patients receiving care at centers offering first-trimester screening and CVS may choose between 2 types of first- and second-trimester combined screening: Integrated screening and step-wise screening.
Integrated screening
Integrated screening is a 2-step approach in which results are withheld until both the first- and second-trimester screening tests have been obtained, at which time a single risk assessment is provided. Patients who opt for integrated screening have NT performed between 10 and 14 weeks' gestation. A measurement of PAPP-A is also obtained during this time period. Between 15 and 16 weeks' gestation, the quad test is performed, and all results are integrated together. The trisomy 21 detection rate with this method of screening is 94%, with a 5% false-positive rate. 29 This test is very attractive to many patients, as it maximizes the Down syndrome detection rate while minimizing the false-positive rate. 4 If NT is unavailable, "serum-integrated" testing that includes obtaining PAPP-A in the first trimester followed by the quad test in the second trimester may be performed.
Step-wise screening
In step-wise screening, patients undergo first-trimester testing and are given the results at that time. The second-trimester screening then utilizes the first-trimester results as the new a priori risk. The advantage of this screening method is that patients may opt for CVS in the first trimester if their screening results indicate that their pregnancy is at increased risk for aneuploidy. Otherwise, they can wait until the second-trimester screening is completed and receive results that afford a better Down syndrome detection rate.
Advances in the diagnosis of fetal congenital abnormalities
Fast magnetic resonance imaging
While ultrasound remains the imaging modality of choice for the fetus because of its widespread availability and reasonable cost, it has several limitations. These include small field-of-view, limited soft-tissue acoustic contrast, poor image quality in pregnancies complicated by oligohydramnios, beam attenuation by adipose tissue, and limited visualization of the posterior fossa after 33 weeks' gestation because of bone cal-cification. 30,31 Magnetic resonance imaging (MRI) is now being used in conjunction with ultrasound to provide additional information for prenatal diagnosis. The advantages of MRI include the use of multiple planes for reconstruction and a large field-of-view, making the visualization of complicated anomalies easier.
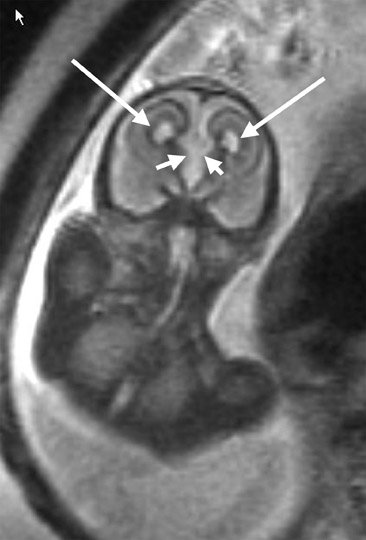
Use of MRI was first described in pregnancy in 1983. 32 Its initial application was for maternal and placental abnormalities. Fetal MRI was initially limited because of fetal motion artifact. In the 1990s, fetal MRI became practical because of the development of single-shot rapid acquisition sequence with refocused echoes. This high-quality T2-weighted sequence has a slice acquisition time of <1 second and essentially "freezes" fetal motion. 33,34 As a result, excellent fetal imaging can now be obtained without maternal or fetal sedation (Figure 3). 34
Provided that there is no maternal contraindication, most studies suggest that MRI is safe in pregnancy, as it allows the acquisition of excellent soft-tissue contrast without using ionizing radiation. 34 However, several animal studies have suggested the possibility of teratogenetic effects in early pregnancy. 35-41 While these studies may not be applicable to humans, they indicate that MRI should be used with caution in the first trimester. The risk of acoustic damage to the fetus is thought to be negligible. 42,43 Patients can be informed that according to the Safety Committee of the Society for Magnetic Resonance Imaging, MRI is reasonable when other nonionizing forms of radiation are inadequate, or when the study would provide information that would otherwise require exposure to ionizing radiation. While there is no evidence that MRI can harm the fetus, 44 it is important to note that the United States Food and Drug Administration asserts that the safety of fetal MRI "has not been established," and that most of the centers using this imaging technique in pregnancy limit its use to after the first trimester and require informed consent prior to the procedure. 34,45
The main indication for fetal MRI is further evaluation of inconclusive ultrasound findings. It is also useful for evaluation prior to fetal surgery. 46 Fetal MRI has proven to be extremely helpful for the examination of many suspected fetal anomalies. Definitive indications for fetal MRI have not been established, and recommendations are based on case reports, small case series, and expert opinion. 30 Ultrasound of the fetal brain can be limited because many anomalies have a non-specific appearance. Likewise, technical factors can limit the appearance of the brain, such as bone ossi-fication. 47 In addition, severe parenchymal abnormalities often cannot be visualized with ultrasound.
Fetal MRI has been particularly helpful with the diagnosis and management of suspected fetal central nervous system (CNS) abnormalities and is suggested if a CNS abnormality is suspected on prenatal ultrasound. 48 Fetal MRI can aid in the diagnosis of ventriculomegaly, agenesis of the corpus callosum, posterior fossa abnormalities, cortical gyral malformations, hemorrhage, holoprosencephaly, arachnoid cysts, neural tube defects, and vascular malformations. 34 In fact, MRI has changed the diagnosis and aided in the management of many cases of suspected CNS abnormalities on ultrasound. 47,49 MRI can help better clarify the diagnosis and help patients make decisions such as whether or not to continue with the pregnancy. Levine et al 47 compared 242 ultrasound studies and 242 MRI studies of the CNS in 214 fetuses with suspected CNS abnormalities or who were at high risk for CNS abnormalities. At confirmatory ultrasound, 69 fetuses had normal CNS imaging. Approximately 80% of fetuses in the study (171 of 242) had postnatal follow-up. MRI provided additional information in 50% (72 of 145) of cases and actually revealed a new major finding in 32% (46 of 145) of fetuses with abnormal ultrasound findings. In patients with previable fetuses, this information was utilized to help patients decide whether to continue or terminate the pregnancy. In patients with viable fetuses, this information was used to help determine the mode and the location of delivery (community hospital versus tertiary care center).
In monochorionic twin pregnancies complicated by intrauterine fetal demise (IUFD), fetal MRI can be used to diagnose multicystic encephalomalacia, a devastating neurologic disorder that may occur in up to 20% of monochorionic twins complicated by single IUFD. 30,50 Fetal MRI is usually performed 2 weeks following demise of a fetus. A normal MRI following single IUFD in a monochorionic twin pregnancy is thought to be reassuring. In addition, fetal MRI has been used in other complicated monochorionic twins at risk for a possible neurologic ischemic episode, such as cases complicated by the twin-twin transfusion syndrome and cases that have undergone invasive prenatal procedures, such as selective reduction using cord ablative techniques. 30,51 The imaging modality may also be helpful in the diagnosis of conjoined twins. 52
With regard to the fetal neck, MRI can help distinguish between lymphangioma and cervical teratoma. 30 As neck masses can compromise fetal breathing at birth, MRI can help with the assessment of the fetal airway so that the proper precautions are taken at delivery.
Fetal MRI has also been helpful for the diagnosis of fetal thoracic abnormalities such as congenital diaphragmatic hernia (CDH), congenital cystic adenomatoid malformation of the lung, and broncho-pulmonary sequestration. Fetal MRI helps with the evaluation of the liver position in fetuses with CDH. 53 It may also be helpful with the assessment of fetal lung volumes in these patients. In addition, complex genitourinary anomalies such as cloacal exstrophy may be better imaged on MRI due to the larger field-of-view. 30
From the maternal aspect of care, if abnormal placentation is suspected, MRI may help assess the degree of invasion and help distinguish between placenta accreta, increta, and percreta. 54-57 MRI can be used to help distinguish the placenta from the myometrium. Levine et al 54 evaluated 19 patients at risk for placenta accreta with ultrasound and MRI. Five cases of accreta were diagnosed with vaginal ultrasound and Doppler studies. In 1 patient with a posterior placenta and history of myomectomy, MRI aided in the diagnosis of placenta accreta, as the ultrasound imaging had not been diagnostic. Other investigators have used MRI to assess placental invasion. 56,57 Accurate prenatal diagnosis of abnormal placentation is important because it reduces fetal and maternal morbidity by enabling specialized preoperative and postoperative care. 56
Fetal MRI is not currently recommended as a primary imaging method for any fetal anomaly or condition. The information obtained from MRI can be used in conjunction with the information obtained from ultrasound. Fetal sonographic examination is important for selecting appropriate fetuses whose management could benefit from MRI. Fetal MRI is suggested for cases in which ultrasound cannot make a definitive diagnosis and when a large field-of-view is required. In addition, it may provide additional specific information necessary for fetal intervention, including fetal surgery. It is important to remember that that the field of fetal MRI continues to rapidly evolve as technology advances and as new applications are determined.
Three-dimensional ultrasound
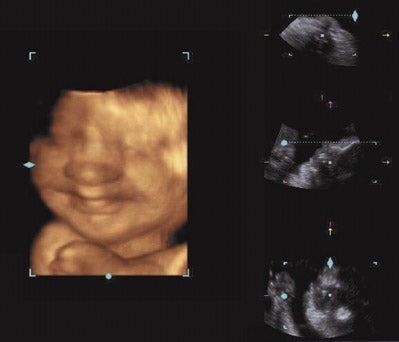
Similar to MRI, three-dimensional (3D) ultrasound allows for multiplanar imaging and allows the examiner to move back and forth between different planes because of the capability of viewing the fetus in 3 rather than 2 spatial planes (Figure 4). Images can be reconstructed, and the examiner can move the fetus into desired positions that are often not possible with conventional ultrasound. In addition, 3D scanning enhances imaging capabilities by permitting surface rendering of a structure. Acquisition of data-points through the entire volume of interest is required to produce 3D ultrasound pictures. Acquisition quality depends on acquisition speed. Slow acquisition speed results in more scanned slices and is used for nonmoving organs. Fast speeds are preferable for moving structures. The "four-dimension-al" (4D) real-time imaging technique requires ultrafast acquisition. Four-dimensional ultrasound displays a continuously updated and newly acquired volume in any rendering modality. This creates the impression of a moving structure. 58
Previously, it was thought that 3D ultrasound provided only aesthetic images without contributing to prenatal diagnosis. There are no accepted indications for 3D ultrasound in an obstetric patient at this point. Few outcome studies have confirmed whether or not this technology changes practices or clinical outcomes. 59 Nonetheless, this technology is rapidly advancing and has been shown to be helpful in a research setting. It seems to be useful as an adjunct to 2D ultrasound in certain clinical situations such as fetal echocardiography and in the diagnosis and further evaluation of certain fetal anomalies such as cleft lip and palate and skeletal anomalies. 60-65
Other advances
Preimplantation genetic diagnosis: In the field of reproduction endocrinology, preimplantation genetic diagnosis (PGD) is one of the most exciting new advances. It is a form of prenatal diagnosis for cytogenetic and Mendelian disorders. 66 This technique allows for genetic testing prior to embryo transfer in patients undergoing assisted reproductive technology (ART). One or two cells are biopsied from embryos at the 6- to 8-cell stage and are analyzed for chromosomal abnormalities or for single-gene disorders. Alternatively, polar bodies can be biopsied from oocytes for genetic testing as well. Preimplantation genetic diagnosis has been used for couples at risk for having pregnancies affected by single-gene or X-linked disorders. It has also been used for couples with an age-related risk for aneuploidy and for couples who carry balanced chromosomal rearrangements. 67 In addition, it is thought that patients who have experienced many failed in-vitro fertilization (IVF) cycles or who have experienced recurrent early pregnancy loss may benefit from PGD. Only embryos free of the genetic abnormalities under evaluation are replaced at the time of embryo transfer. The main drawback to this procedure is that patients must undergo IVF in order to have PGD even if they do not have any fertility issues. In-vitro fertilization has inherent risks such as ovarian hyperstimulation and multiple gestations. Besides being invasive, the technique is expensive. Furthermore, PGD is still investigational, as there is insufficient information to determine its impact on pregnancy and pediatric outcomes. At this point, PGD is reserved for patients at high risk for genetic abnormalities. 2
Fetal cells and DNA in maternal circulation: Noninvasive prenatal genetic diagnosis is an important area of research, as it would be preferable to invasive methods such as amniocentesis and CVS, which carry a small but definitive risk for fetal loss. Isolating fetal cells or free fetal DNA from maternal plasma is currently an exciting and promising area of research. However, these techniques are extremely challenging. Many different protocols have been developed using one or more techniques, such as fluorescence-activated and magnetic-activated cell sorting, to recover fetal cells after separation from whole blood. 68 Practical applications for the clinician have not yet been developed. 69 One of the main limiting factors seems to be the rarity of such cells in the maternal circulation. Enrichment techniques are needed to help increase the yield. 2 Estimates of the number of fetal cells in the maternal circulation vary depending on the gestational age and the technique used to obtain the cells. 2
Current potential applications for analysis of fetal cells and of cell-free fetal DNA in maternal circulation include noninvasive detection of certain paternally inherited genetic traits and noninvasive detection of fetal Rhesus (Rh) D genotype in Rh(D)-sensitized patients. Noninvasive fetal genotyping would be useful for the management of Rh(D)-sensitized patients whose partners are heterozygous for the Rh(D) gene because no further diagnostic or therapeutic procedures would be necessary if the fetus was confirmed Rh(D)-negative. 69 In X-linked genetic disorders in which half of the fetuses will be female and unaffected, fetal DNA determination of the fetal sex could reduce the number of invasive procedures required for the diagnosis of X-linked genetic disorders by 50%. 69 Additional clinical applications of fetal cells and fetal DNA in maternal circulation may include screening for aneuploidy, pre-eclampsia, or preterm labor. 70,71 These applications currently rely on the detection of Y-chromosomal sequences and are limited to male fetuses. 72 The above noninvasive diagnostic methods are not yet in clinical use but offer extremely exciting options for the future. 2,73
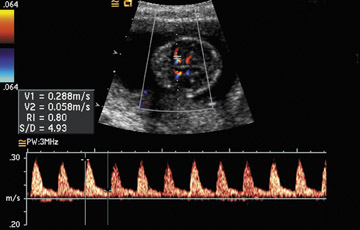
Noninvasive diagnosis of fetal anemia by Doppler ultrasound: Previously, the diagnosis of fetal anemia required percutaneous umbilical blood sampling (PUBS), an invasive procedure with an inherent risk for fetal loss. Doppler measurements of the middle cerebral artery peak systolic velocity can now be used to noninvasively diagnose anemia in fetuses without hydrops who are at risk for anemia secondary to conditions such as Rh(D) sensitization and parvovirus B19 infection (Figure 5). 74,75 This noninvasive method of detecting fetal anemia de-creases the number of invasive PUBS procedures needed in these high-risk patients. The value of the middle cerebral artery peak systolic velocity is expressed as multiples of the median. Middle cerebral artery peak systolic velocity >1.50 multiples of the median is considered suggestive of anemia and is considered an indication for PUBS (Table 1).
Conclusion
While there have been exciting advances in the field of prenatal diagnosis within the past few years, the future holds the promise of great breakthroughs. Advances in the areas of genomics, proteomics, and stem-cell research are anticipated. It is expected that imaging mo- dalities will continue to improve, and it is hoped that techniques used in the fields of noninvasive prenatal diagnosis and in preimplantation genetic diagnosis will continue to advance. Accurate prenatal diagnosis of fetal abnormalities improves patient care by optimizing patient counseling and allowing for informed patient and physician decision making.