A Practical Guide to Wireless Breast Localization Devices
CME credit for this article is available here.
Introduction
Despite the increasing incidence of breast cancer in the US, the 5-year survival rate for all stages of breast cancer approaches 91%, with more than 4 million breast cancer survivors living in the US. Breast-conserving surgery with or without adjunct endocrine therapy, chemotherapy, and/or radiation has become the standard of care for noninvasive and localized invasive breast cancers. For breast-conserving surgery to be successful, that is, have clear margins with an acceptable cosmetic result, it is essential that the cancer be localized accurately.
Until recently, wire-guided localization was the gold-standard technique for preoperative localization of nonpalpable breast cancers and entails anchoring a flexible wire to a lesion under image guidance, usually on the morning of surgery. With 1 end anchored to the lesion and the other end protruding from the patient’s breast, the patient would be transferred from radiology to the operating room (OR) for surgery. This technique is prone to unique complications and challenges, including wire dislodgement or migration, interference with the dissection route, and the need for coupling radiology and surgery services. Wireless localization devices can avoid these limitations.
Initial alternative nonwire localization techniques involved tagging the lesion with radioactive material that would then be detected in the OR with gamma cameras. However, these methods come with their own unique set of challenges related to the use and management of radioactive materials.1, 2 Recently, nonradioactive localization methods have been introduced and use various techniques such as RADAR (Scout, Merit Medical), magnetic seed localization (Magseed, Endomag), and radiofrequency identification (RFID) tagging (LOCalizer, Hologic; EnVisio, Elucent Medical).
This article aims to review the wireless localization devices commonly used at our institution, including the techniques, advantages, and limitations. Of note, other wireless localization devices are on the market but will not be discussed here. These include Pintuition (Sirius Medical), MOLLI (MOLLI Surgical Inc.), and TAKUMI (Matrix Cell Research Institute Inc.).
Radioactive Seed Localization
The first of the nonwire localization techniques discussed is radioactive seed localization, which most commonly uses iodine-125 (I-125)-labeled radioactive seeds. The 5-mm titanium radioseeds contain a maximum amount of 0.3 mCi of I-125 and are detected in the breast via a handheld gamma counter.2 The radiologist places them in the breast up to 5–7 days before surgery under ultrasound or mammographic guidance. The seeds are preloaded in an end-deploy needle and advanced to the target lesion percutaneously under image guidance. The deployment mechanism and lengths of the needle vary based on vendor. Multiple radioseeds can be placed in the same breast either for bracketing a single lesion or localizing multiple lesions. On the day of surgery, the surgeon precisely locates the seed using a gamma probe.
The surgeon can concurrently detect technetium-99mm for lymph node mapping, a unique advantage of the radioseed localization. Compared with wire-guided localization, there is a lower incidence of positive margins and decreased need for repeat surgery.3
The major disadvantage of this technique is the regulatory challenges associated with the use of radioactive material, including the need for oversight by a radiology safety officer and proper facility licensing.1, 2
Impulse-Reflecting System
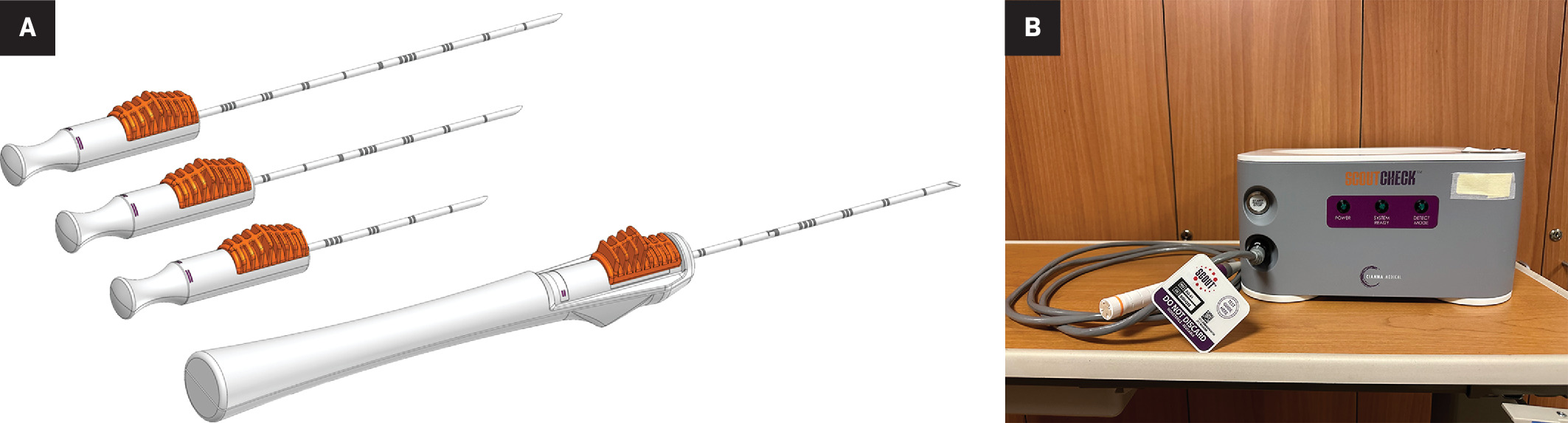
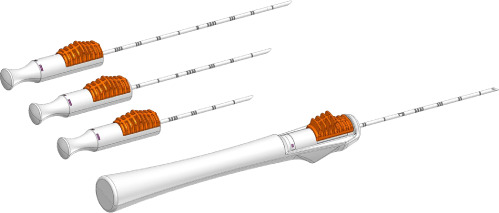
The Scout (Merit Medical) wireless localization device uses radar to locate the precise location of a breast tumor via an implanted 1.2-cm reflector and a handheld micro-impulse generating probe ( Figure 1 ). Using ultrasound or mammographic guidance, the reflector device is placed prior to surgery to mark the soft tissue intended for surgical removal. The reflector comes preloaded in a single-use deployment device consisting of a handpiece attached to a 16-gauge introducer needle of various lengths (5 cm, 7.5 cm, and 10 cm). After the target is identified on imaging, the needle can be percutaneously advanced. Once the needle tip is confirmed to be at the desired target, the Scout reflector can be deployed. The introducer needle is then removed, and the radiologist uses a console to confirm the placement of the reflector and its signal. Postplacement imaging with a 2-view mammogram is recommended ( Figure 2 ). No patient aftercare is required, and the reflector can be left in situ indefinitely. Multiple devices can be placed in the same breast.4
The Scout deployment devices are shown in (A). Radiology Scout console and probe (B) used to detect the wireless radar device once placed in the breast.
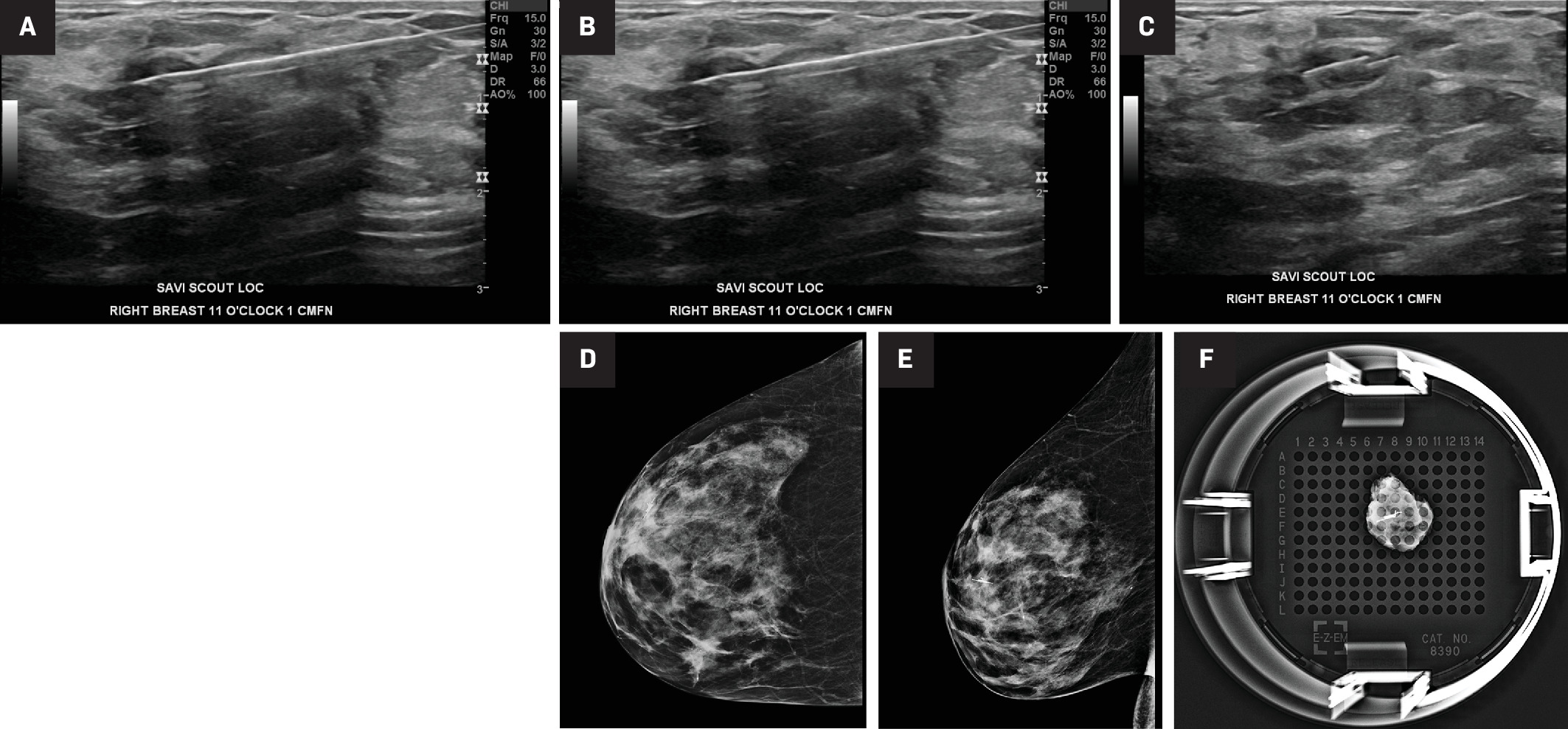
Ultrasound image (A) of the target lesion, a biopsy proven intraductal papilloma at 11:00 in the right breast, 1 cm from the nipple, marked by a ribbon marking clip. Scout 16-guage introducer needle (B), which was inserted percutaneously and advanced to the target lesion under ultrasound guidance. Inserted Scout localization device (C) after deployment and retraction of the needle. Cranial caudal (CC) and mediolateral oblique (MLO) mammographic views (D and E) of the right breast confirm the placement of the Scout localization device. The specimen radiograph (F) of the lumpectomy tissue showing the Scout device and biopsy clip within the specimen.
On the day of surgery, the surgeon uses a micro-impulse-generating probe to scan the breast and find the precise location of the reflector. The reflector contains 2 antennas that signal back to the probe. This signal is then processed by the console, which provides real-time directionality and proximity information via audible and visual cues to guide the surgeon during dissection. The probe can detect the location of the reflector at a maximum depth of 6 cm. The radiologist can use the handpiece and console, if available, to confirm the placement of the reflector after implantation.
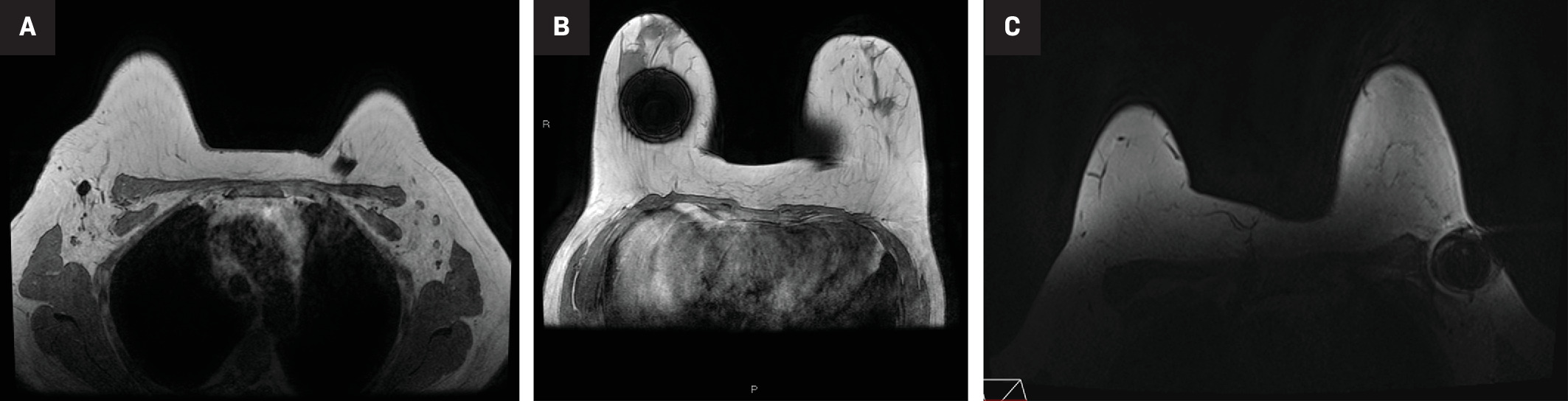
Compared with wire-guided localization, the Scout radar localization system results in statistically significant lower re-excision rates and positive margins.5 The Scout reflector also has an advantage over other nonwireless localizers due to its smaller MR artifact.6 The Scout reflector produces minimal signal void, whereas the Magseed and Loc RFID tag produce 2-6 cm and 2 cm of signal void, respectively ( Figure 3 ). When patients are newly diagnosed with breast cancer, they may need to undergo additional imaging with MR to assess the extent of the disease.7 Additionally, if a patient is treated with neoadjuvant chemotherapy, MR may be considered to evaluate treatment response. The minimal MR artifact of the reflector makes it a feasible option for localization of lymph nodes in the neoadjuvant setting8 ( Figures 4 , 5 ).
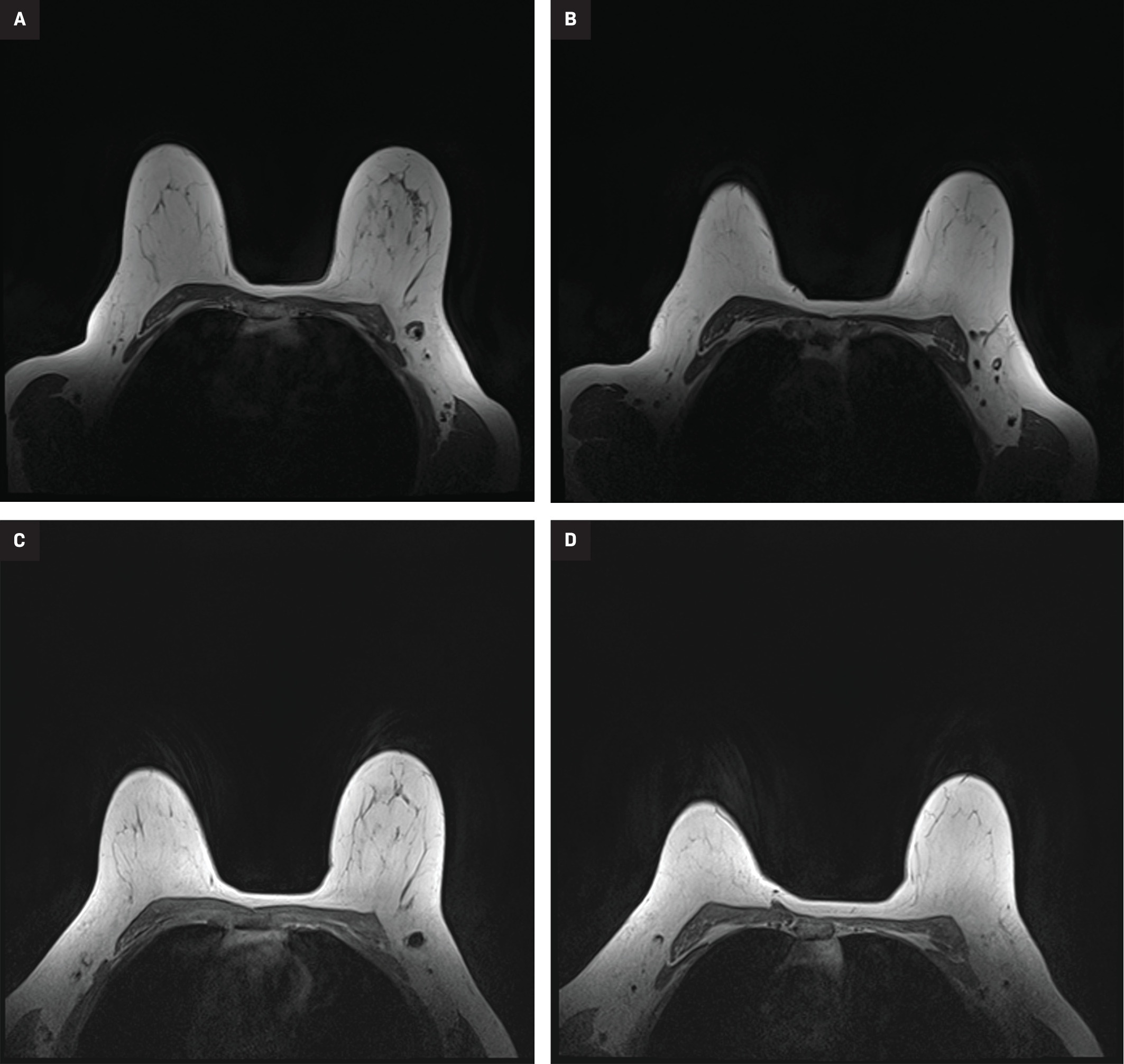
MR susceptibility artifact produced by the Scout, Magseed and LOC localization devices. Axial MR image (A) showing a small amount of susceptibility artifact emanating from the Scout localization device in the right axillary region. Note the susceptibility artifact in the left chest wall from the patient’s subcutaneous port. Axial MR (B) from a different patient showing a 6-cm signal void artifact stemming from a first-generation Magseed localization device in the right breast. Newer, second-generation Magseed devices produce less artifact, about 2-4 cm. Axial Vibrant MR image (C) from a third patient showing the susceptibility artifact associated with the LOC wireless localization device in the left axillary region.
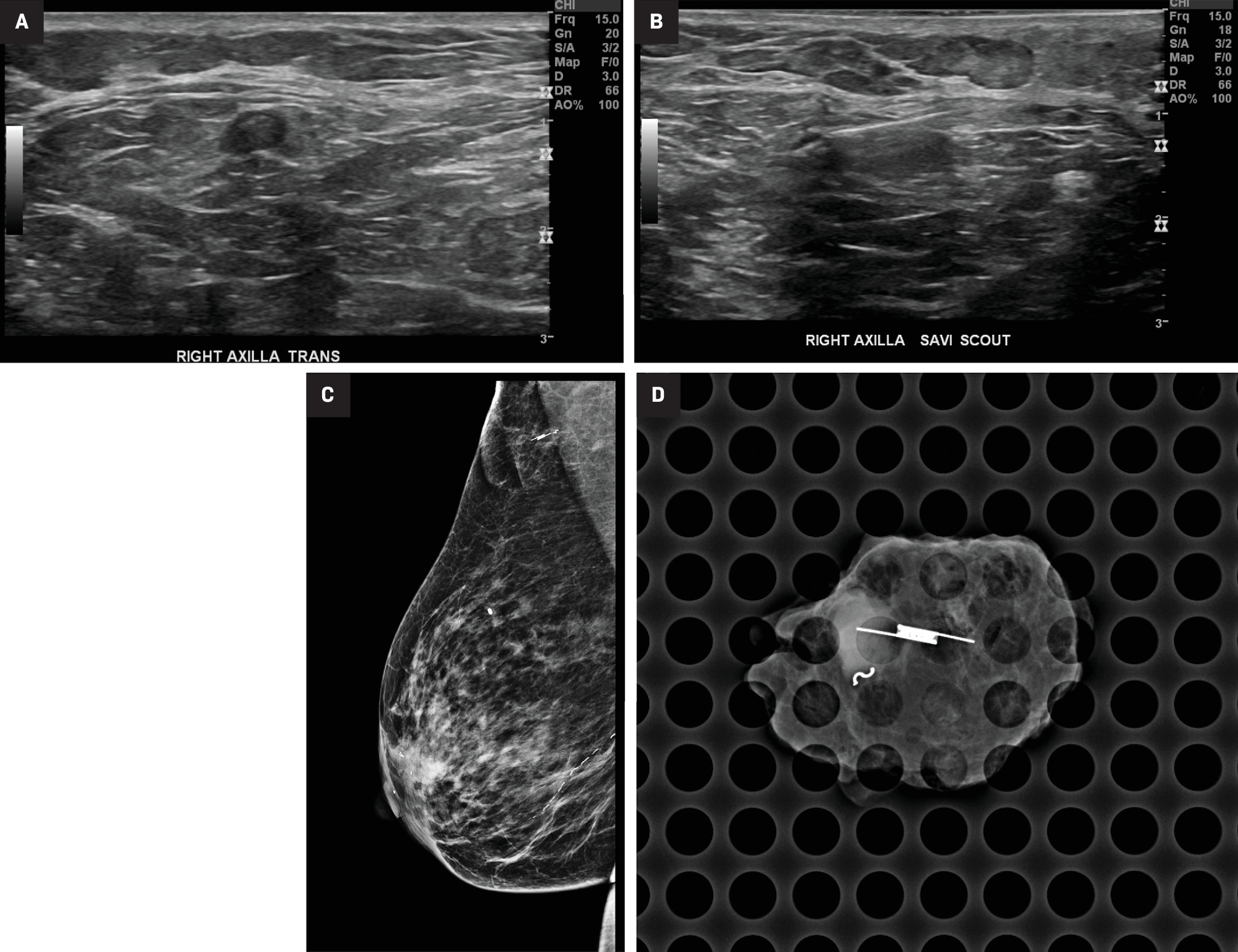
Localization of an axillary target. Target metastatic lymph node (A) in the right axilla, marked by an S-shaped marking clip. Scout localization device (B) inserted at the target. Post MLO mammographic view (C) of the right breast confirms placement of the localization device. Specimen radiograph (D) showing both the Scout and biopsy clip within the resected tissue.
MR images of a patient status post biopsy of a left breast mass and axillary lymph node revealing metastatic breast carcinoma. Initial MR images (A, B) showing multiple left axillary lymph nodes, including the enlarged biopsy-proven metastatic lymph node marked by a biopsy marking clip as seen in (A). Repeat imaging 3 months later (C, D) after undergoing neoadjuvant chemotherapy and interval Scout localization of the biopsy marking clip in the left axilla, showing that the left axillary lymph nodes have decreased in size and the biopsy-proven metastatic node has normalized.
Merit Medical recently received FDA approval for the Scout Bx delivery system, which allows the Scout reflector to be placed at the time of stereotactic or MRI-guided biopsy. This newer device is MR-compatible with an end-deploy mechanism and can be used with most biopsy devices.
A disadvantage of the Scout localization device is disruption of the signal when placed within a hematoma or calcified fibroadenoma.9 Also, the device cannot be detected at depths greater than 6 cm, limiting its use in deep lesions. Owing to its shape, the Scout device can be more difficult to deploy than the alternative wireless localization devices. Additionally, if either antenna is damaged during deployment, issues detecting the signal may occur. Contraindications to placing a Scout localizer include a nickel allergy and the presence of a pacemaker (Table 1).
Breast Localization Devices
| Localization Seed | Deployment Device | Seed Size | Deployment Device Size | MR Artifact | Mammogram Appearance | Contraindications | |
|---|---|---|---|---|---|---|---|
| Scout (Merit Medical) Radar Reflector |  |
 |
12 mm | 16-Gauge; 5 cm, 7.5 cm, 10 cm | Minimal signal void on MR | 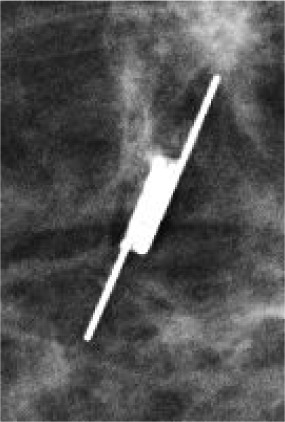 |
Nickel allergy, presence of pacemakers, evidence of infection |
| Magseed (Endomag) Magnetic Seed | 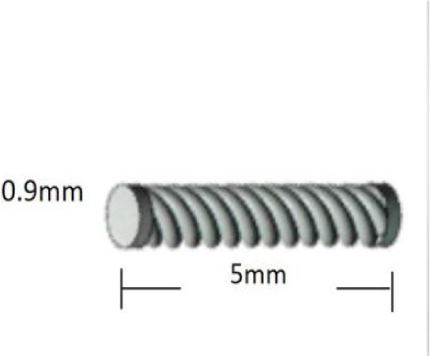 |
 |
5 mm | 18-Gauge; 7 cm or 12 cm | 4-6 cm signal void on MR | 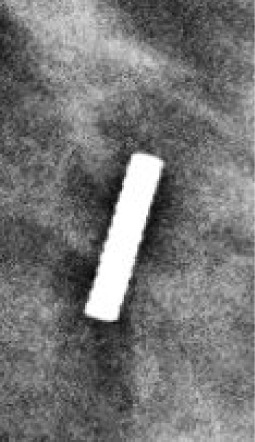 |
Nickel allergy, presence of pacemaker, clinical evidence of infected tissue. |
| LOCalizer (Hologic) Radiofrequency ID Tag |  |
 |
11 mm | 12-Gauge; 5 cm, 7 cm, 10 cm | ~2 cm of signal void on MR | 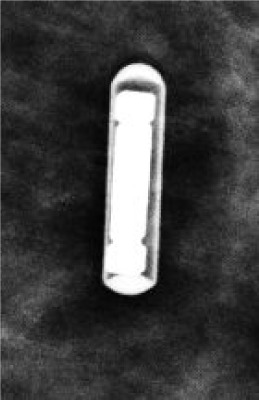 |
Clinical evidence of infected tissue |
| Smartclip (Elucent) Radiofrequency ID Tag |  |
 |
8 mm | 15-Gauge; 5 cm, 7.5 cm, 10 cm | ~2 cm of signal void on MR | 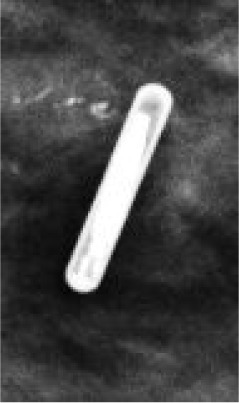 |
Active cardiac device, clinical evidence of infected tissue |
Radiofrequency Identification Systems
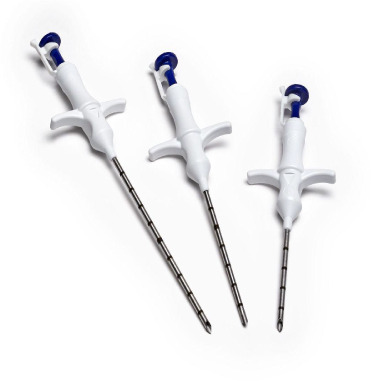
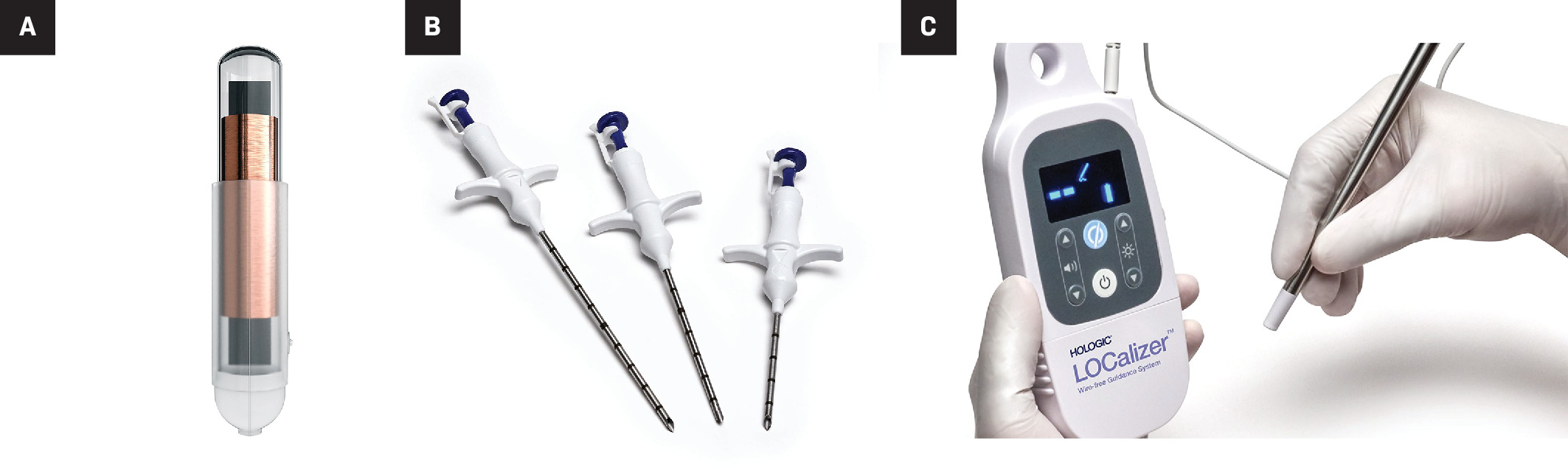
The LOCalizer (Hologic) wireless device uses unique RFID numbered tags to localize breast lesions. The tags are approximately 10.6 mm × 2 mm with a polypropylene cap to prevent migration postimplantation ( Figure 6 ).10 Each tag is preloaded into a 12-gauge, stainless steel needle (5 cm, 7 cm, or 10 cm). Under sonographic or mammographic guidance, a tag is inserted proximal to the focal tumor within 6 cm of the breast surface by depressing the tag applicator’s plunger and removing the safety lock ( Figure 7 ). If more than 1 tag is placed, they must be placed at least 2 cm apart to reduce RFID signal interference during tag localization. Each device has a distinct signal, and the console displays the unique ID number of each tag. To localize the tag on the day of surgery, the single-use surgical probe plugs into a handheld reader, which displays the distance to the tag in millimeters and emits audible cues.11 The small, portable, handheld reader device is a unique advantage of the LOCalizer device. The size of the tag, however, poses disadvantages to this system over the alternatives. The larger tag requires a larger deployment needle. To prevent skin deformations, a small preplacement skin incision is needed.12 When placed in superficial lesions, intraoperative migration is a risk.13
Single tag (10.6 mm × 2 mm) (A) that may be inserted into a breast to localize a lesion, each with a unique radiofrequency identification (RFID) number for localization. Twelve-gauge, stainless steel needles (B) in 3 lengths (5 cm, 7 cm, 10 cm) to insert the tags into the breast. Integrated reader, loop probe, and pencil-shaped surgical probe (C). The reader is a digital screen that shows the distance from the probes to each tag in millimeters, along with its unique RFID number. The loop probe (the circular shape on top of the reader) is integrated into the reader to confirm tag placement. The pencil-shaped surgical probe (attached to the reader by a wire) localizes each tag during surgical excision.
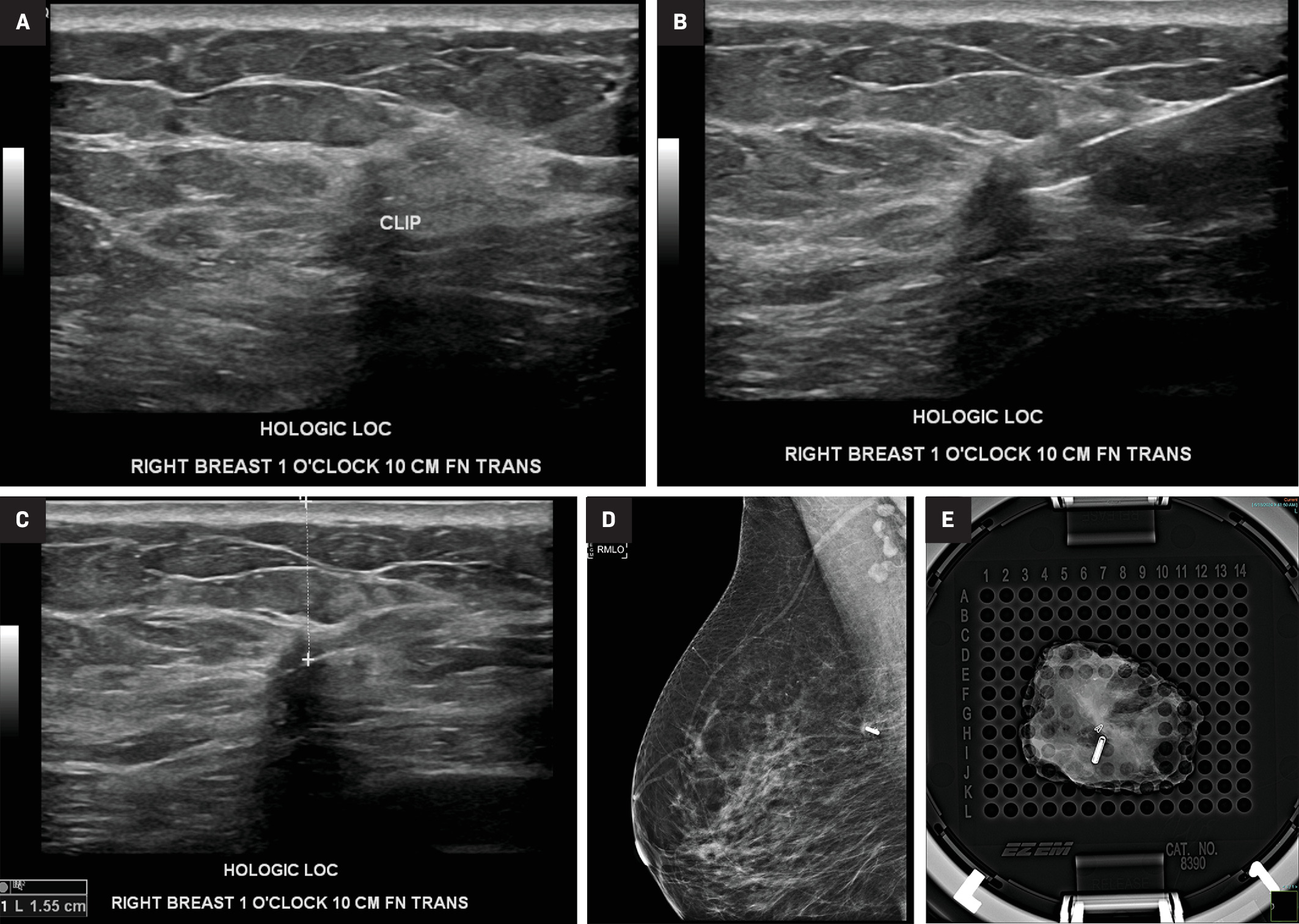
Ultrasound-guided localization of a biopsy-proven invasive ductal carcinoma, marked by a biopsy clip, with a Hologic LOC device. Target marked by a clip (A). Deployed LOC device with the needle still in site (B), which was subsequently removed (C). Post-MLO mammographic view (D) of the right breast confirms the position of the wireless LOC device in the upper breast. Perioperative radiograph of a specimen (E) from another patient shows both the LOCalizer and biopsy clip within the resected tissue.
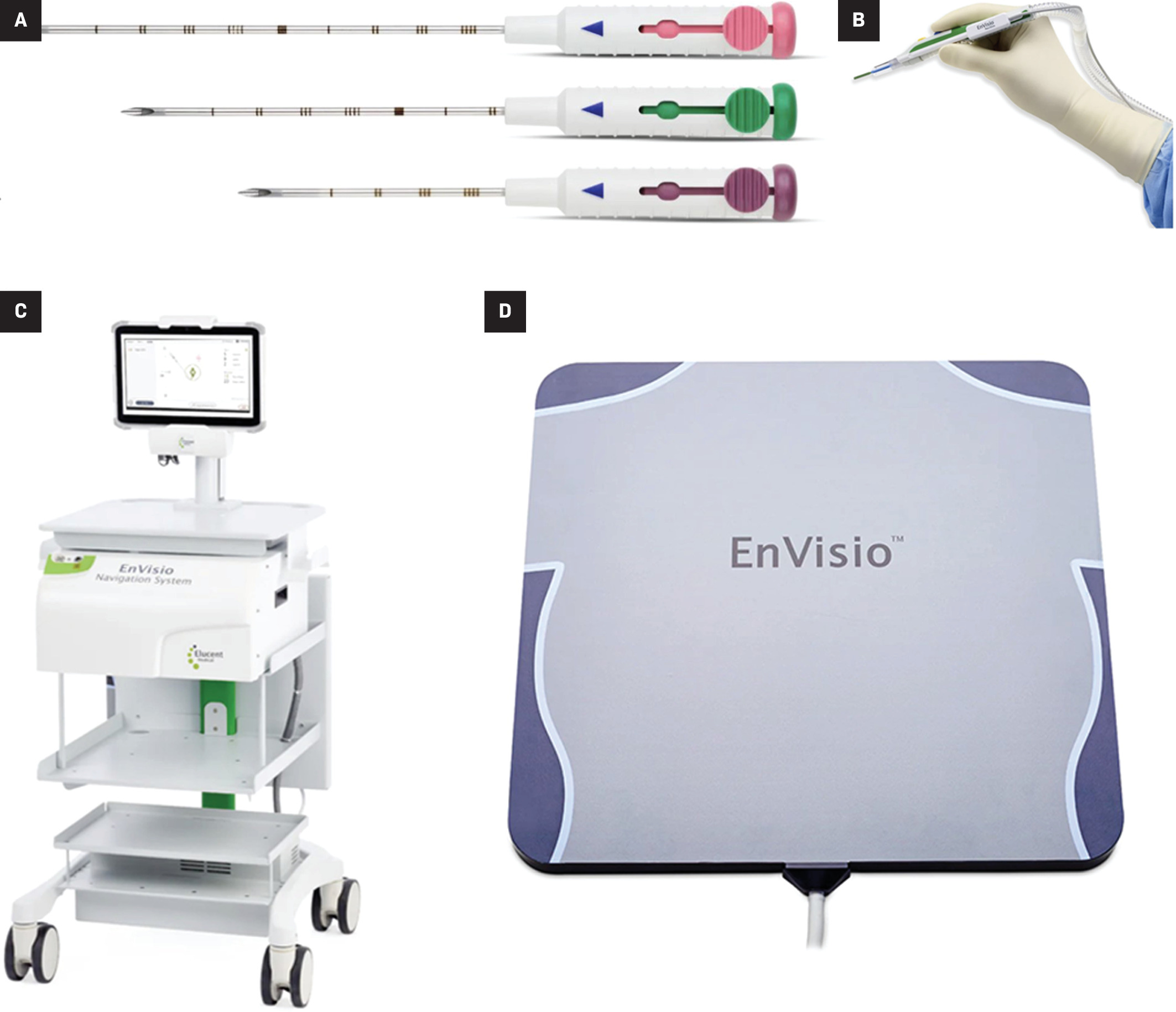
The EnVisio navigation system (Elucent Medical) is another common RFID tag system for wireless localization. This system allows deployment of up to 3 different colored SmartClips, each measuring 1.4 mm × 8 mm ( Figure 8 ). Under mammographic or ultrasound guidance, a SmartClip can be placed at any time prior to surgery using a preloaded 15-gauge deployment device, available in lengths of 5 cm, 7.5 cm, and 10 cm. The deployment device has an unlock-and-slide deployment to insert the preloaded SmartClip, which can be placed in the breast indefinitely ( Figure 9 ). On the day of surgery, the patient lies on an EnVisio Patient Pad for the surgeon to localize the signal of each SmartClip using a single-use NavSlim transducer attached to an electrocautery device. Each SmartClip emits a unique number that can be detected by a transducer on the surgeon’s electrocautery device, providing coordinates in 3 planes. The single-use, attachable transducer prevents the need for sterilization between use, decreases possible high costs of maintenance, and reduces surgical clutter.
Fifteen-gauge SmartClip unlock-and-slide deployment devices of differing lengths (5 cm, 7.5 cm, 10 cm) (A) to insert the SmartClips into the breast. Single-use NavSlim transducer (B) attached to an electrocautery device to localize each SmartClip. Heads Up Display (C) providing real-time navigation during localization of each SmartClip, providing coordinates in 3 planes: medial/lateral, superior/inferior, and anterior/posterior (x, y, z). EnVisio Patient Pad (D) generates electromagnetic waves that detect the implanted SmartClip.
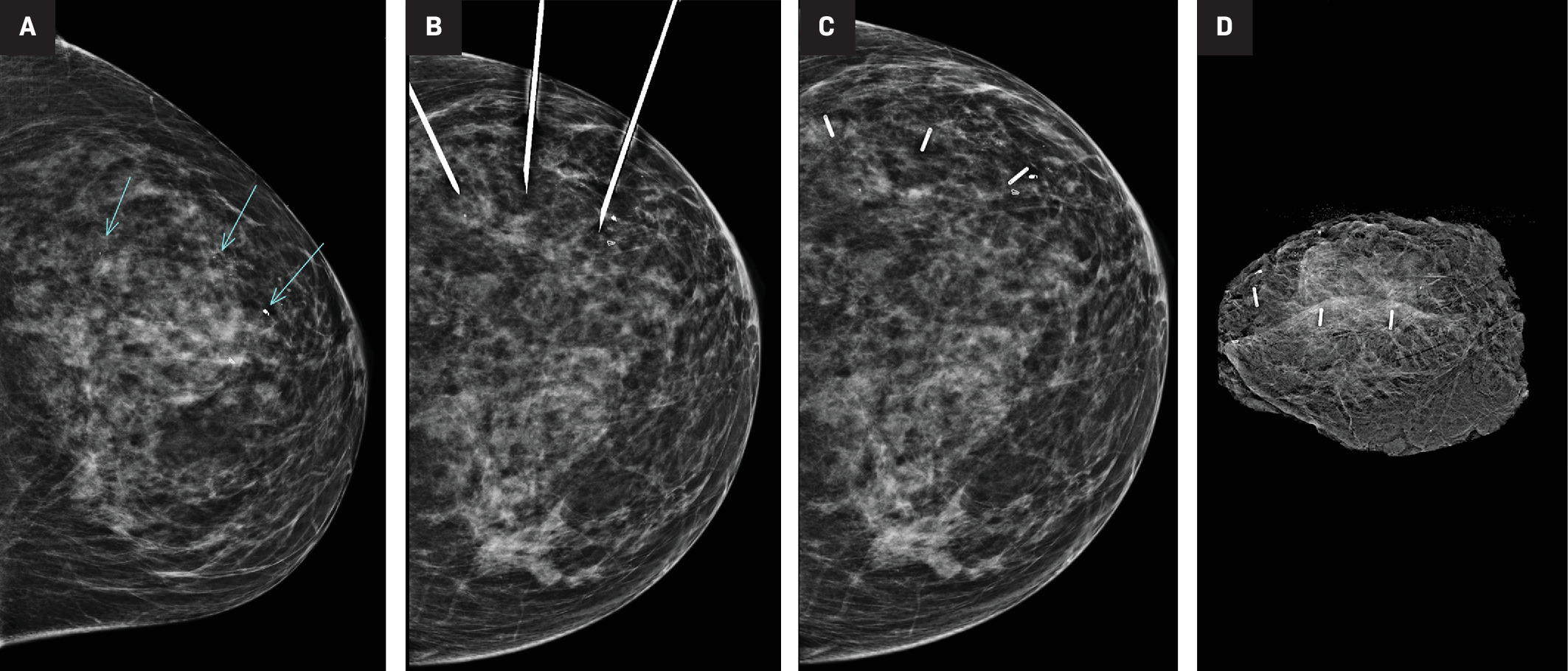
Placement of 3 SmartClips under mammographic guidance. CC mammographic view (A) of the left breast showing 3 separate targets of calcifications, the most anterior of which is marked by a biopsy-marking clip and pathologically proven to be ductal carcinoma in situ. Three separate deployment needles in place (B). Post-CC view (C) of the left breast confirms the position of all 3 SmartClips, denoted by their corresponding colors. Specimen radiograph of the lumpectomy tissue(D), showing all 3 clips and the targets calcifications within the specimen.
A potential disadvantage of the EnVisio Surgical Navigation System is the smooth exterior coating of each SmartClip, decreasing its ability to anchor to its surroundings. However, 1 study found that this posed no significant risk of postplacement migration.14 Another limitation is that the patient must remain in the supine position throughout the operation to stay within the boundaries of the EnVisio Patient Pad. Alternative positions may disrupt the localization signal. Furthermore, an active cardiac device may interfere with the signal from the electromagnetic pad, although the cardiac device may be deactivated with a magnet during the operation. In addition, the Patient Pad precludes the use of certain intraoperative equipment, such as padding for prolonged operative cases. Lastly, although SmartClips are MRI safe, they create a 2-cm signal void artifact.
Magnetic Localization System
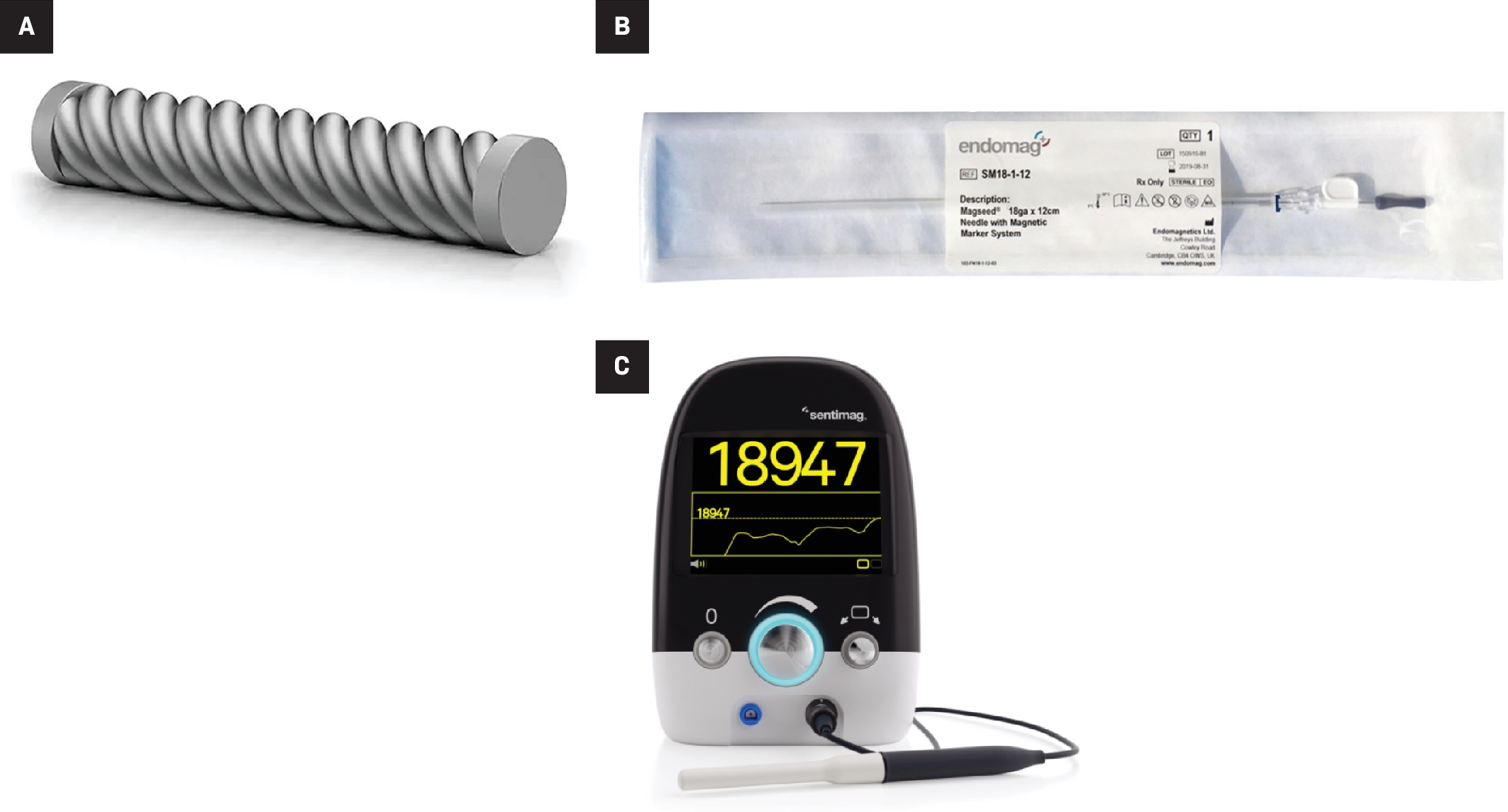
Magseed (Endomag) is a 5 mm × 1 mm paramagnetic wireless localization device made of medical-grade stainless steel and iron oxide ( Figure 10 ). The seed is preloaded in an 18-gauge deployment device, available in lengths of 7 cm or 12 cm, and can be placed under ultrasound or stereotactic guidance. Similar to the other wireless devices, multiple Magseeds can be placed within the same breast. After the target is visualized on imaging, the Magseed needle is advanced percutaneously to the target, and then deployed via a 1-step push mechanism. Placement is confirmed using 2-view mammography ( Figure 11 ). On the day of surgery, the surgeon detects the Magseed via the SentiMag probe, which generates a temporary magnetic field. The Magseed is transiently magnetized and the SentiMag displays the distance of the Magseed to the probe in millimeters while providing audible cues.
Magseed wireless localization device (A), the deployment needle (B), and the SentiMag probe and console (third generation) used to detect it (C).
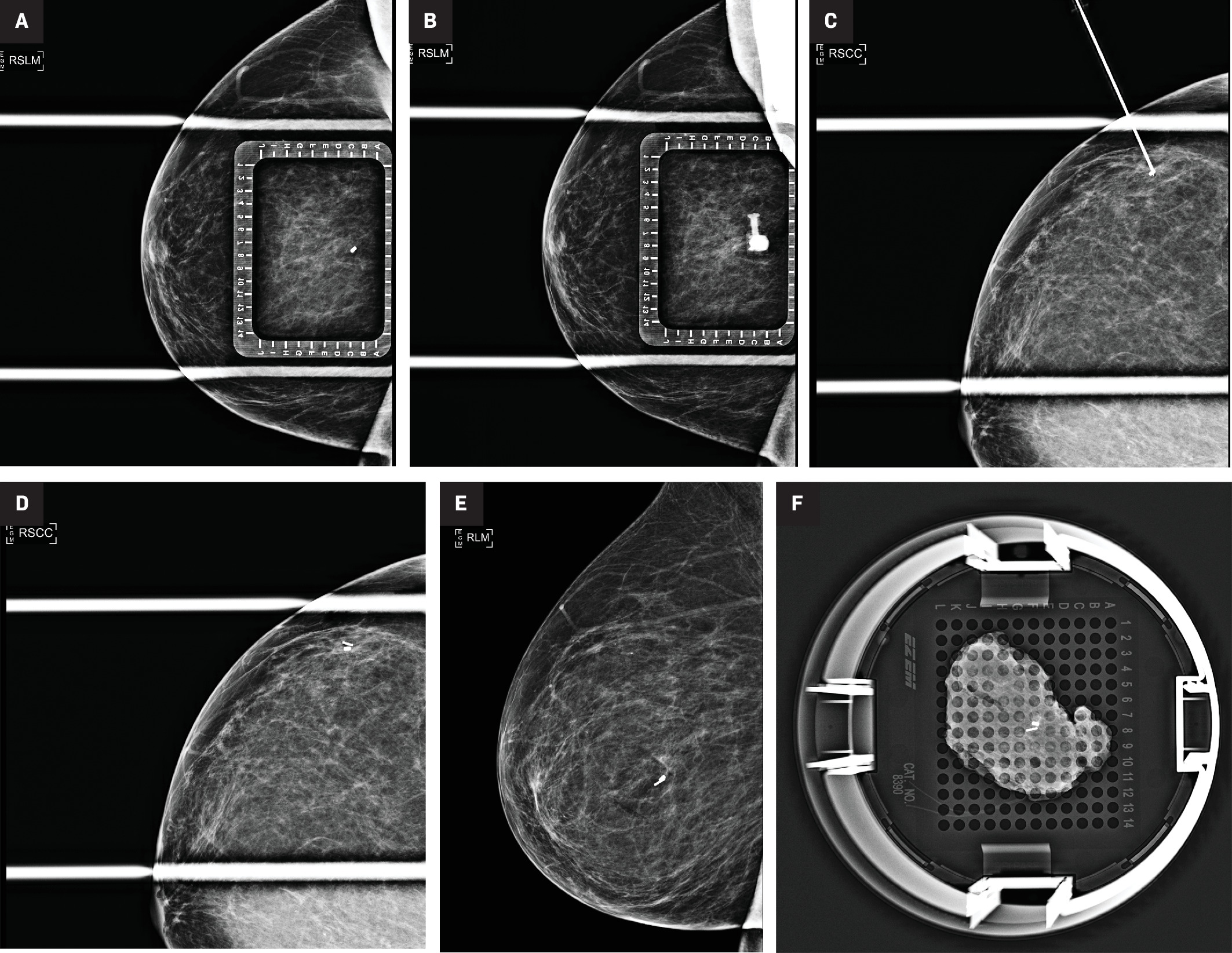
Step by step placement of the Magseed localization device under mammographic guidance. Spot compression lateromedial (LM) view (A) of the right breast with an overlying alphanumeric grid showing the target, a cork clip marking the site of biopsy-proven atypical ductal hyperplasia, at C-7. Repeat imaging (B) after introduction of the Magseed introducer needle showing the needle hub en face at EC-7 on the grid. CC view (C) of the right breast confirms the needle tip is at the target. Deployed Magseed postplacement (D). Postplacement MLO view (E) was also performed. Postlumpectomy specimen radiograph (F) showing both the biopsy clip and the Magseed within the resected tissue.
Magseed has a 99.9% successful localization rate.15 Compared with wire-guided localization, it is more successful in terms of index lesion removal and fewer failed localizations. It has no significant difference in terms of identification rates, re-excision rate, specimen size and weight, and lesion-to-specimen size ratio.16
The major advantage of this system is its deployment device, which is the thinnest on the market and has a 1-step mechanism, which is likely more comfortable for patients. The SentiMag probe can also be used to localize sentinel lymph nodes through Magtrace. Magtrace is a nonradioactive superparamagnetic iron lymphatic tracer that can be used in place of traditional radioisotope tracers for localizing sentinel lymph nodes. Magseeds can be placed within a lymph node prior to neoadjuvant chemotherapy for targeted axillary dissection.17 However, its 2-6-cm signal void artifact on MR limits its utility in this setting.
Given this significant signal void, individual Magseeds within the same breast would need to be placed at least 2 cm apart for accurate detection on MR. A major drawback of the Magseed system is that intraoperative equipment such as electrocautery or paramagnetic surgical equipment can interfere with the electromagnetic signal and must be removed from the operating field. Nonconductive surgical tools must be used, and if there is interference with conductive surgical instruments, the SentiMag probe requires recalibration.18
Conclusion
Wireless localization devices are replacing wire-guided localization and radioactive localization methods to guide breast-conserving surgical treatment of breast cancer. While not all commercially available devices have been included, a variety of options have been presented, including their comparable technical specifications, advantages, and limitations. Breast imagers and their patients will benefit from familiarity with these devices.
References
Citation
JJ DS, J K, S S, N V, S K. A Practical Guide to Wireless Breast Localization Devices. Appl Radiol. 2024;(4):5 - 15.
doi:10.37549/AR-D-24-0001
August 1, 2024