Device to Treat Stroke Gets CE Marking
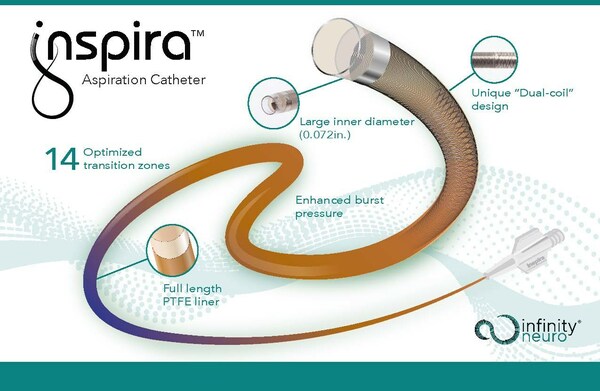 Infinity Neuro has received CE Mark approval for its Inspira aspiration catheter, designed to treat stroke. The Inspira aspiration catheters are physician inspired to deliver next-level navigation and aspiration performance; designed to access targeted vessels easily, quickly restore cerebral blood flow, and retrieve clots in patients experiencing acute ischemic stroke (AIS) due to a large vessel occlusion (LVO).
Infinity Neuro has received CE Mark approval for its Inspira aspiration catheter, designed to treat stroke. The Inspira aspiration catheters are physician inspired to deliver next-level navigation and aspiration performance; designed to access targeted vessels easily, quickly restore cerebral blood flow, and retrieve clots in patients experiencing acute ischemic stroke (AIS) due to a large vessel occlusion (LVO).
According to the company, Inspira is the first phase of a complementary integrated system which will offer greater ease-of-use and performance with first-to-market product innovations. Additionally, this milestone represents the company's commitment and ability to attain class III medical device approvals through the new, rigorous European MDR CE certification process which in today's market, medical device companies find challenging.
This marks the first neurovascular device approved under the new European MDR CE certification process as an original submission. The European commercial launch of Inspira aspiration catheters are underway through a network of EU distributors.