Review of Clinically Significant Cancer in Lesions Labeled PI-RADS 3 on MRI Using PI- RADS Version 2.1
By Satei AM, Kaur M, MacLean J, Hakim B


Abstract
Objective and Hypothesis: Prostate Imaging Reporting and Data System (PI-RADS) category 3 represents an intermediate risk of clinically significant prostate cancer. These lesions are detected on magnetic resonance (MRI) using a combination of T2, DWI/ADC, and DCE sequences. Besides being labeled as equivocal, there is no definitively stated percent risk of clinically significant cancer for these lesions. This article reviewed literature surrounding PI-RADS version 2.1 category 3 prostate lesions on MRI.
Methods: The PubMed database was searched on October 4, 2022. Articles included in this review included only the newest version of PI-RADS, version 2.1, which was released in 2019. Primary endpoints included the incidence of prostate cancer and clinically significant prostate cancer.
Results: Eleven studies were included in this review, encompassing 1,481 PI-RADS 3 lesions exclusively evalu- ated using PI-RADS version 2.1. Clinically significant cancer was only found in 11.1% (n=164) of these lesions. The overall incidence of prostate cancer within these lesions was 21.4% (n=253), of the 1,185 PI-RADS 3 lesions included in this portion of the review.
Conclusion: The risk of clinically significant prostate cancer in PI-RADS 3 lesions evaluated using PI-RADS version 2.1 is low. However, individual patient factors, including age, previous biopsy status, prostate health index, and prostate specific antigen density should be considered when determining appropriateness of biopsy. Further research, including longitudinal studies involving future risk of clinically significant cancer in PI-RADS 3 lesions, would be beneficial for evaluation of the newest update of this increasingly popular reporting system.
Key words: PI-RADS, magnetic resonance imaging, prostate, prostate cancer, clinically significant cancer, structured reporting.
Introduction
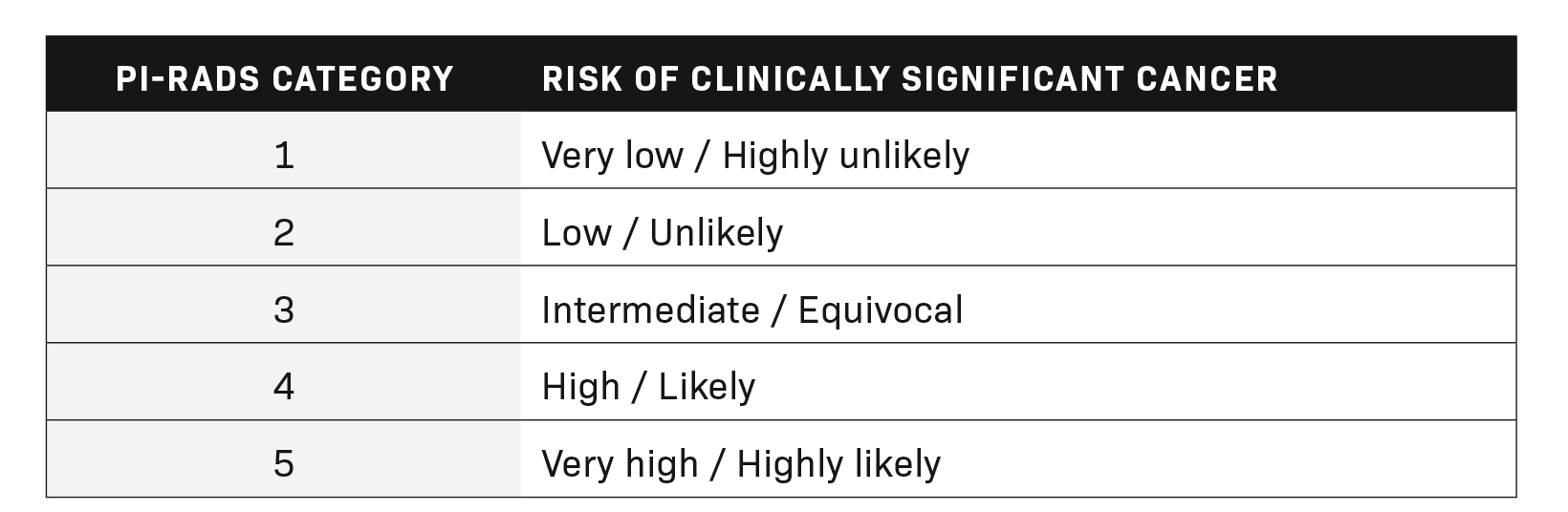
The Prostate Imaging Reporting and Data System (PI-RADS) represents a standard for the reporting of prostate lesions on magnetic resonance imaging (MRI). Initially published by the American College of Radiology (ACR) in late 2011, a second edition (v2.0) was released in 2017, which was further updated in 2019 and titled version 2.1 (v2.1). PI-RADS has since gained popularity among radiologists and hospital systems as a standardized reporting system for interpreting and dictating prostate MRI cases. These categories, ranging from PI-RADS 1 to PI-RADS 5, help give clinicians a better understanding of the risk a prostatic lesion carries for clinically significant cancer. Table 1 summarizes the most recent update of the risk of clinically significant prostate cancer, adapted from the ACR’s PI-RADS v2.1.1
An interesting conundrum arises from PI-RADS 3 lesions. Due to the ambiguity of the intermediate risk classification, manage- ment of these lesions can vary widely. The ACR specifically states, “for findings with PI-RADS Assessment Category 3, biopsy may or may not be appropriate”.1 Typically, in cases of PI-RADS 3 lesions, clinicians will correlate imaging findings with other patient factors, including age, prostate-specific antigen (PSA) level, digital rectal exam results, and individual patient history.
While each PI-RADS category carries a general descriptive risk of clinically significant prostate cancer, it does not provide a quantifiable risk of prostate cancer. Additionally, there are no definitive guidelines for management provided by the ACR based on the PI- RADS categorization. In reality, PI-RADS 1 and PI-RADS 2 lesions are managed conservatively as if they are benign, while PI-RADS 4 and PI-RADS 5 lesions are treated with biopsy and excision as if they are malignant.
PI-RADS v2.1 updates how each category is interpreted. One significant change includes the potential to upgrade transition zone lesions initially scored as PI-RADS 2 by T2 imaging (T2I) into category 3 lesions if diffusion weighted imaging (DWI) corresponds to a score ≥ 4.2 Additional descriptive criteria for PI-RADS 3 lesions, including specifying that the lesion needs to be “discrete and dif- ferent from the background” as well as the use of the term “marked” to describe apparent diffusion coefficient (ADC) and DWI lesion intensities, have been added.2
The new changes in v2.1 were enacted to help overcome the limitations of v2.0. However, v2.1 continues to use the rather vague intermediate/equivocal characterization of PI-RADS 3 lesions, without providing a definitive risk for clinically significant cancer. Review articles exploring the risk of clinically significant cancer in v2.0 noted such cancer in approximately 16-21% of biopsied PI- RADS 3 lesions, with variation depending on the patient’s prior biopsy and cancer history.3
With the previously mentioned changes to PI-RADS version 2.1, further research is required to clarify intermediate risk of pros- tate cancer in PI-RADS category 3
lesions. This article seeks to determine the risk of clinically significant cancer in PI-RADS 3 lesions through a review of literature. We hypothesize that the changes incorporated into PI-RADS version 2.1 will not significantly alter the incidence of clinically sig- nificant cancer diagnosed from lesions labeled as PI-RADS 3.
Methods and Materials
We conducted a literature search utilizing PubMed to find articles in the English language that include biopsy results of PI-RADS 3 lesions, using the term “PI-RADS 3”. The time frame included was 2017-2019. This time frame is appropriate as it includes only v2.1 of PI-RADS onward. Exclusion criteria included inappropriate study design; ie, case report, review articles, etc; an incorrect version of PI-RADS (only studies involving PI-RADS v2.1 were included), and published in non-English-language journals.
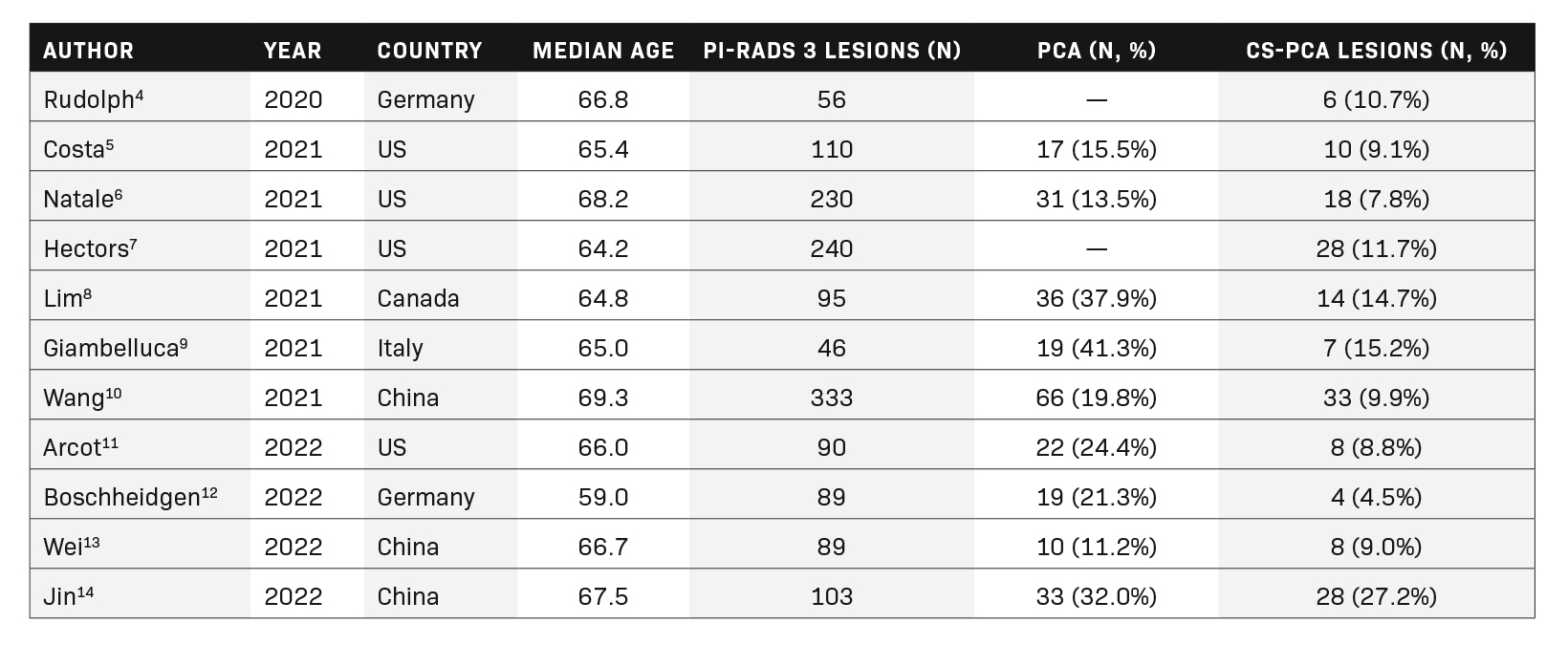
A total of 11 full-length articles were included in the final review. They were analyzed for the number of PI-RADS 3 lesions and the frequency of prostate cancer (PCa) and clinically significant prostate cancer (cs-PCa). The findings are summarized in Table 2.
Results
In our literature search, PI-RADS 3 lesions did not constitute a significant number of MRI results, with the majority of lesions be- ing labeled PI-RADS categories 1 or 2. The number and countries of origin of included studies were the United States (4), China (3), Germany (2), Canada (1), and Italy (1). The range of median age for the participants in the included studies was 59.0-69.3.
In total, 1481 PI-RADS 3 lesions were included in the current review, of which 164 (11.1%) contained clinically significant cancer. The range of clinically significant prostate cancer in the current review article was relatively wide, with a frequency of 4.5-27.2% of PI-RADS 3 lesions; however, most of the studies suggested a frequency of less than 10%.
Of the 1185 lesions described in articles that provided information about the frequency of prostate cancer, 21.4% were cancerous (n=253). These included both clinically insignificant and clinically significant prostate cancers.
A clear majority of PI-RADS 3 lesions were either benign or clinically insignificant cancer (n=1,317, 88.9%). Overall, the risk of clinically significant cancer for PI-RADS 3 lesions in our review was low.
Discussion
Defining PI-RADS 3
Prostate lesions are assigned a PI-RADS category based on their appearance on T2I, DWI/ADC, and dynamic contrast-enhanced (DCE) sequences. Like all PI-RADS category imaging findings, PI-RADS 3 lesions vary in appearance based upon their location within the prostate gland.
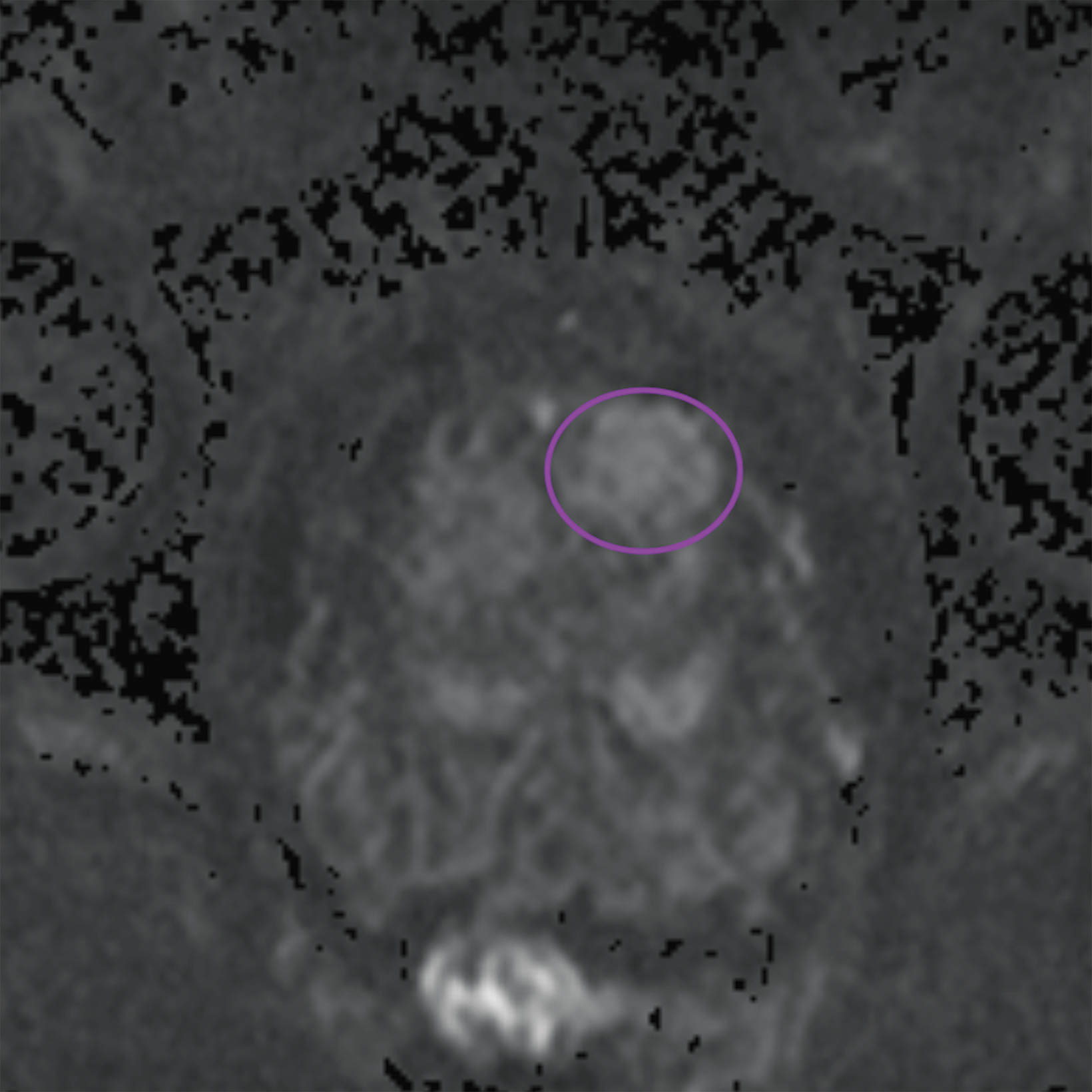
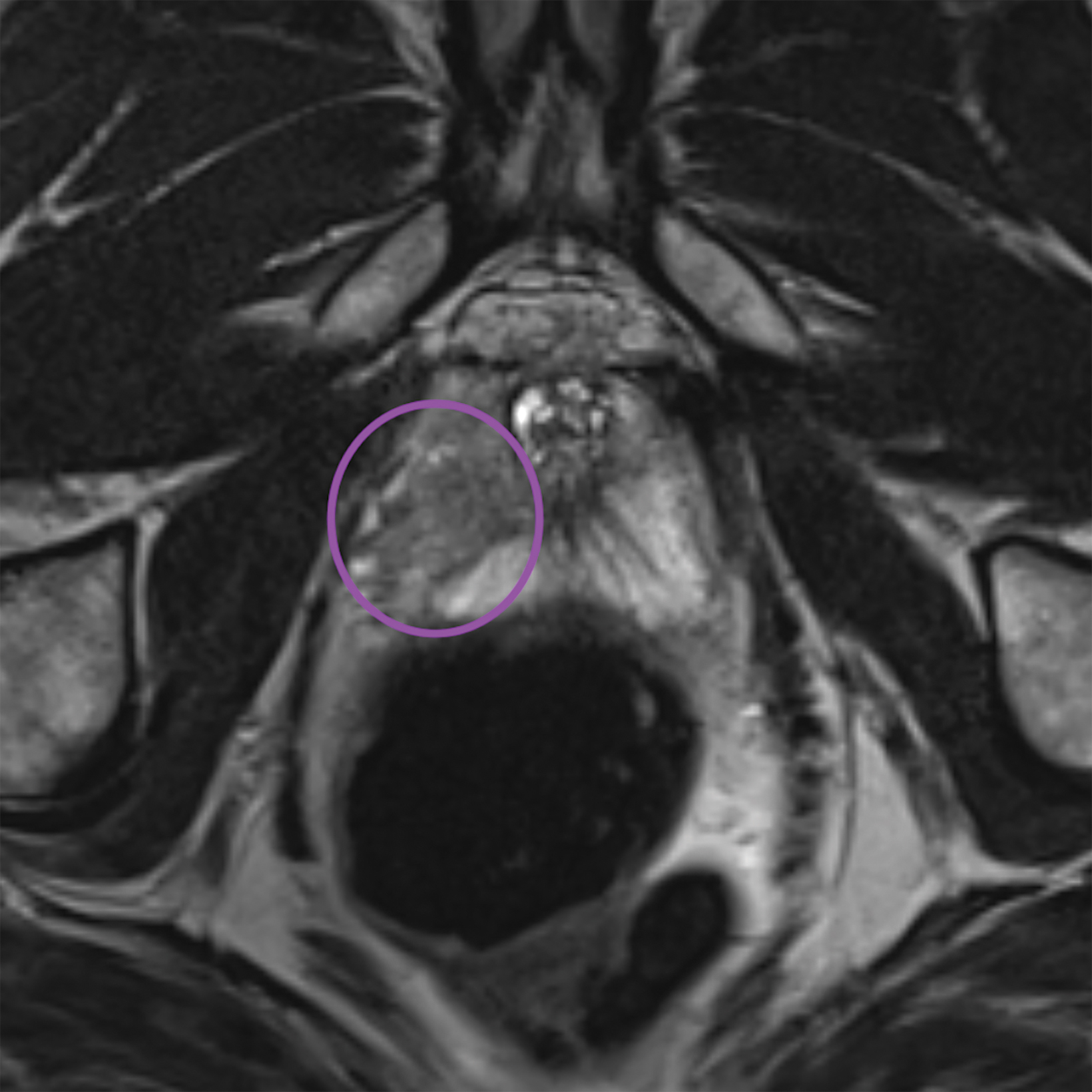
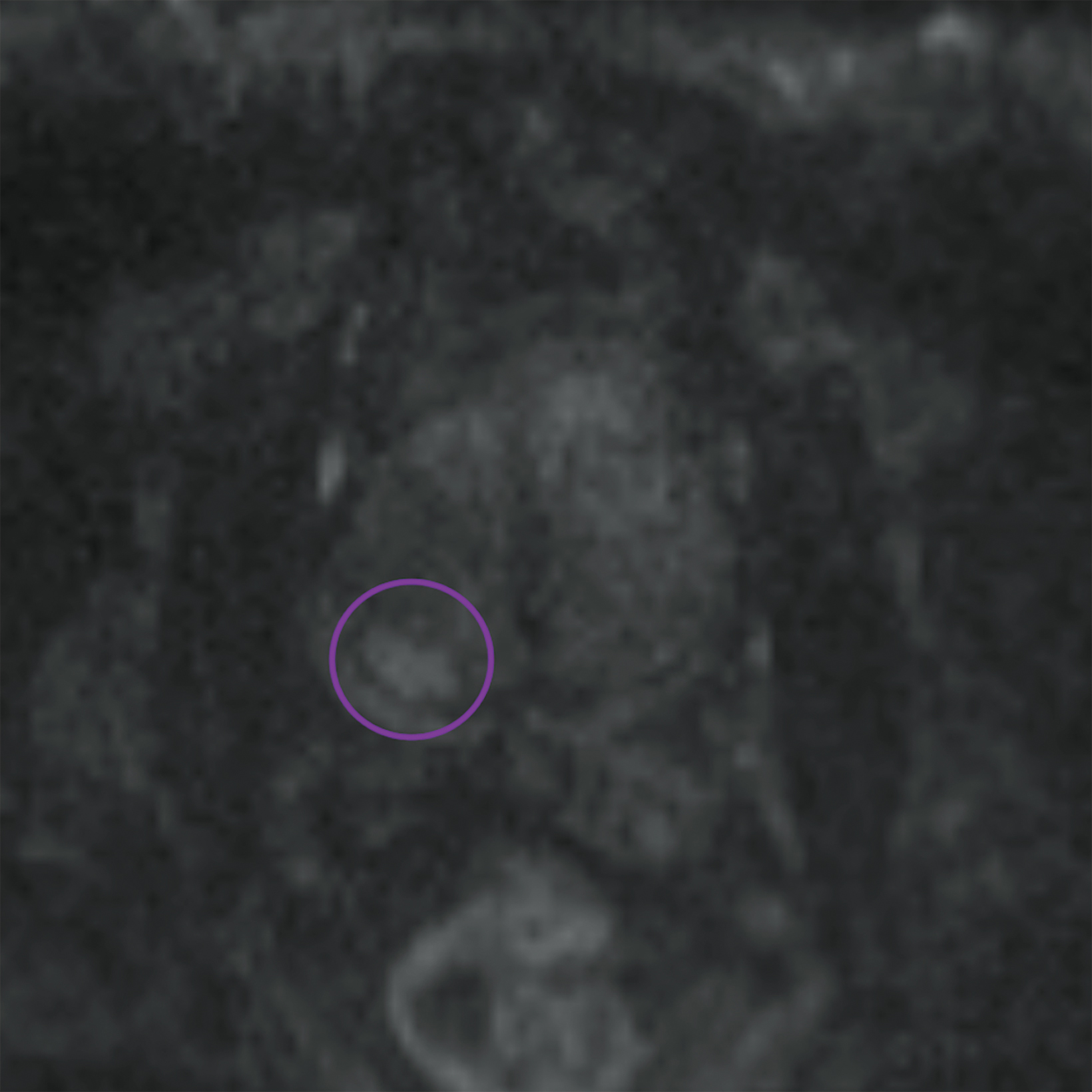
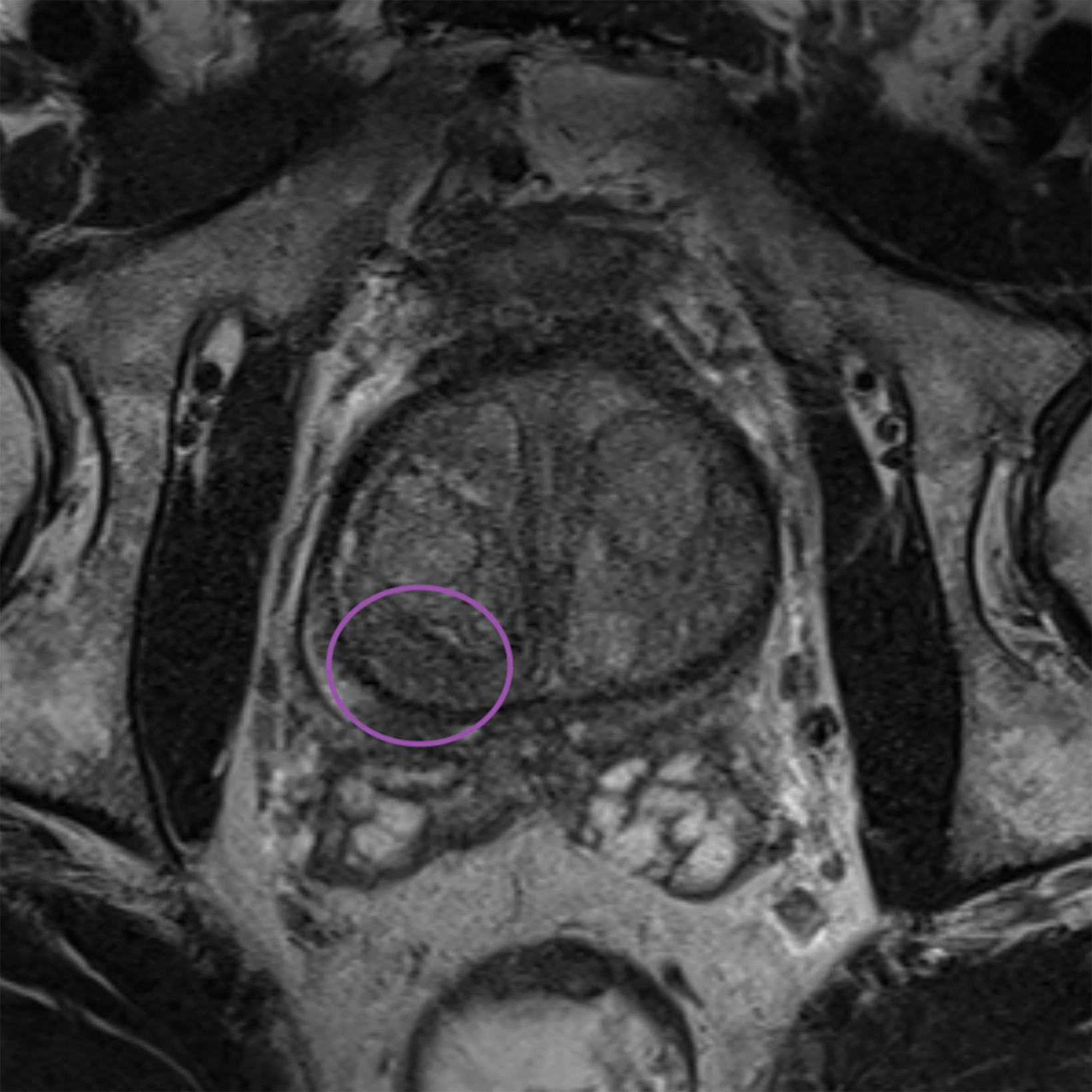
Lesions within the peripheral zone utilize DWI/ADC as the dominant sequence for categorization. To be classified as a PI-RADS 3 lesion, a lesion must display “[f]ocal (discrete and different from the background) hypointense on ADC and/or focal hyperintense on high b-value DWI; may be markedly hypointense on ADC or markedly hyperintense on high b-value DWI, but not both.”1 PI-RADS v2.1 clarified that “marked” restriction refers to “a more pronounced signal change than any other focus in the same zone.”1Figure 1 illustrates an example of a peripheral zone PI-RADS 3 lesion.
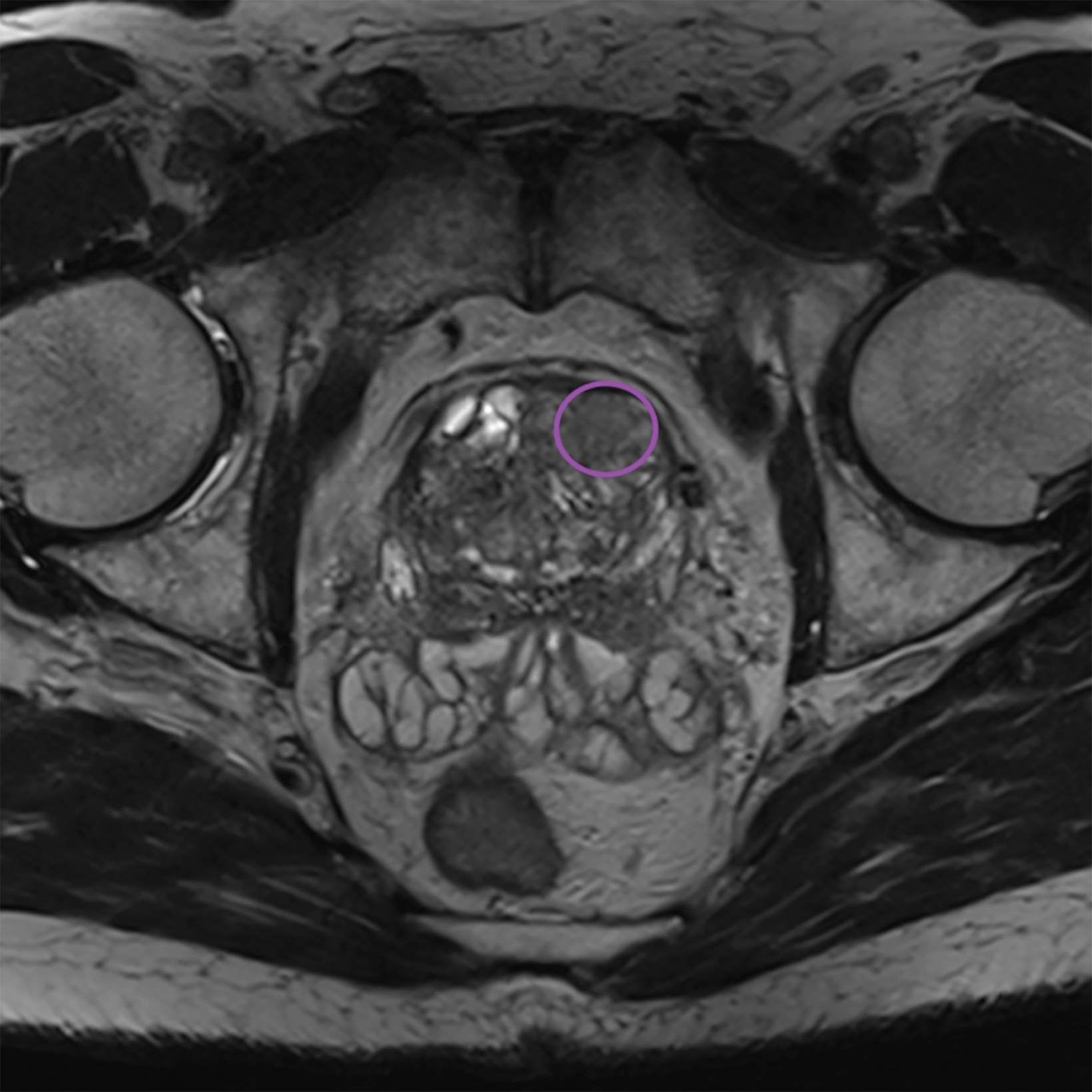
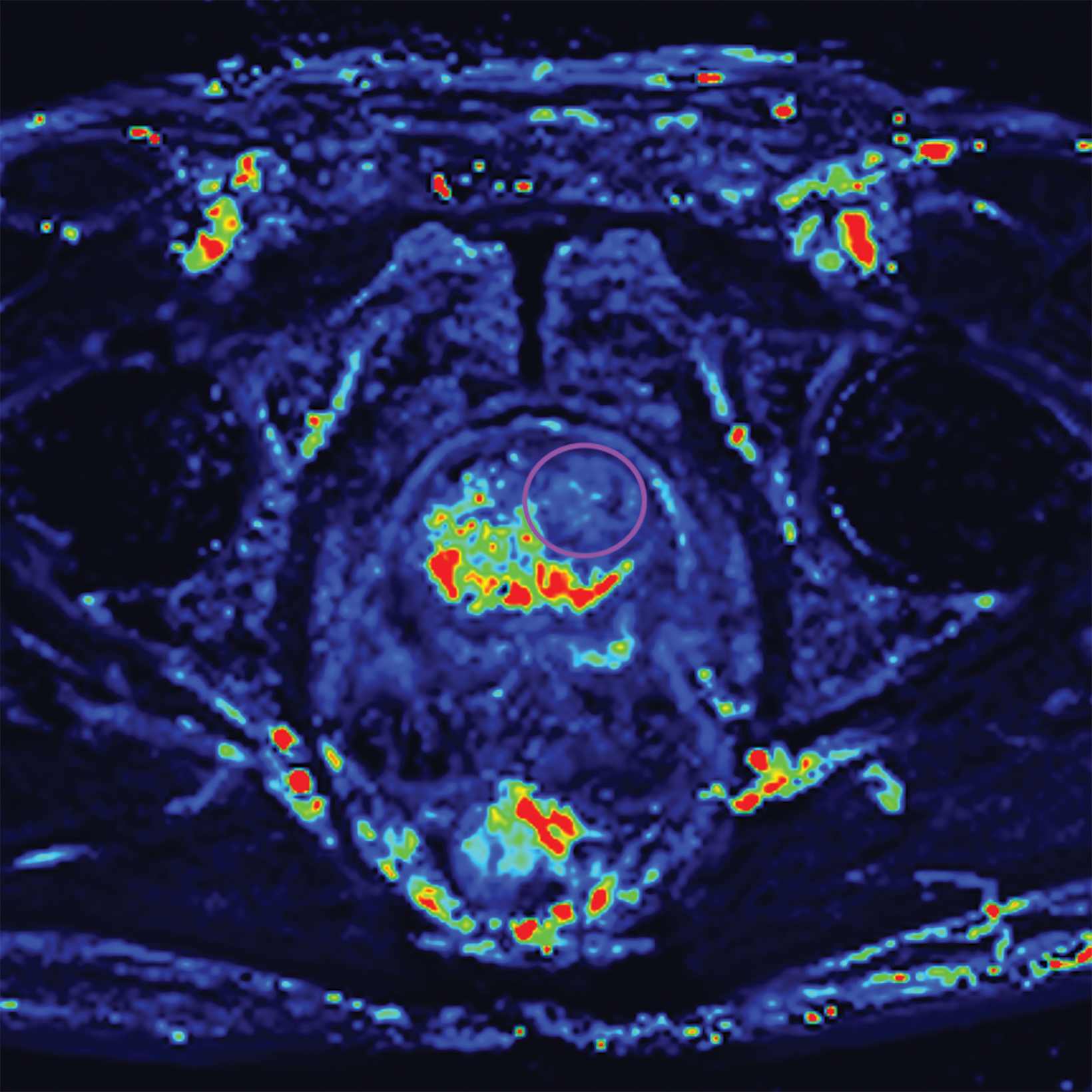
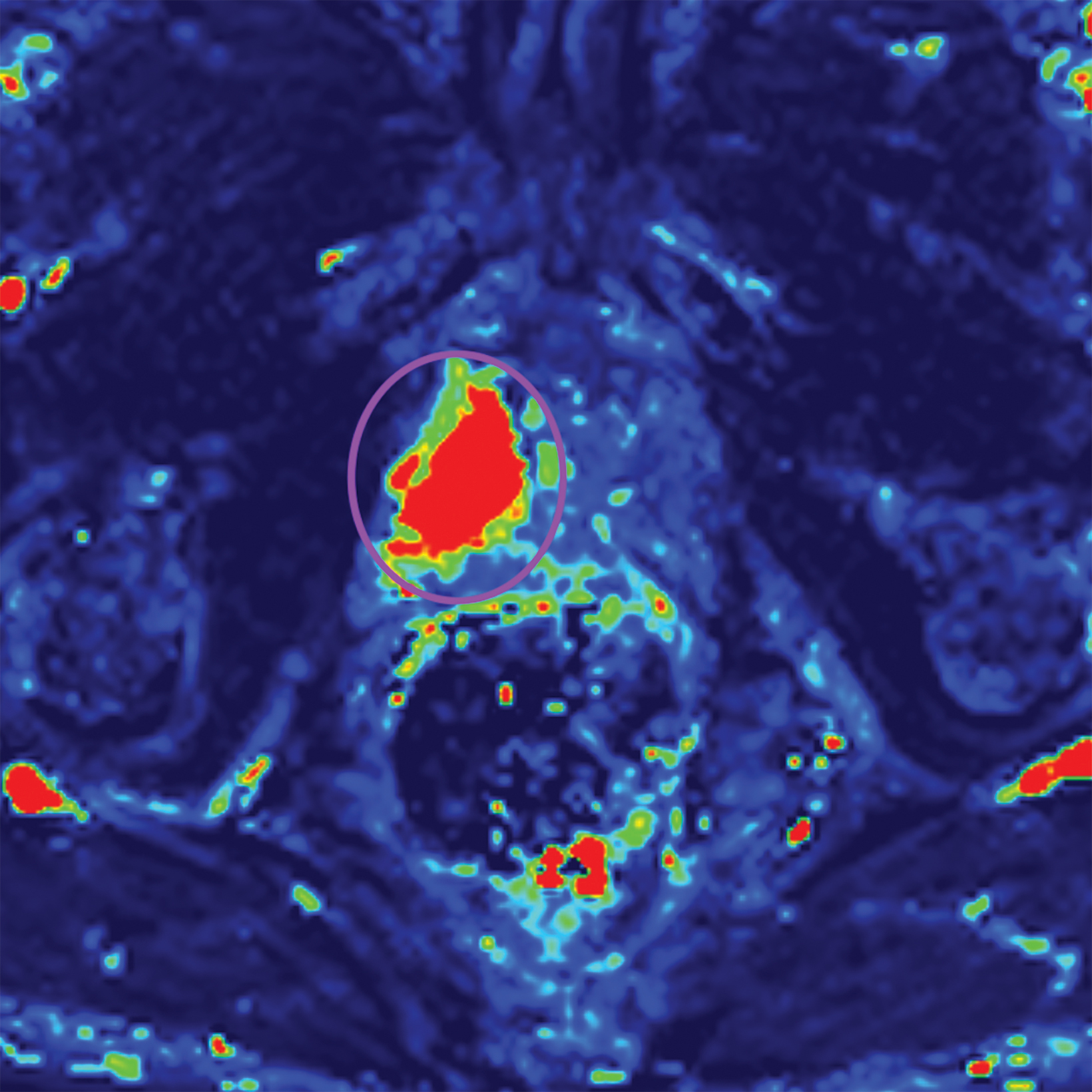
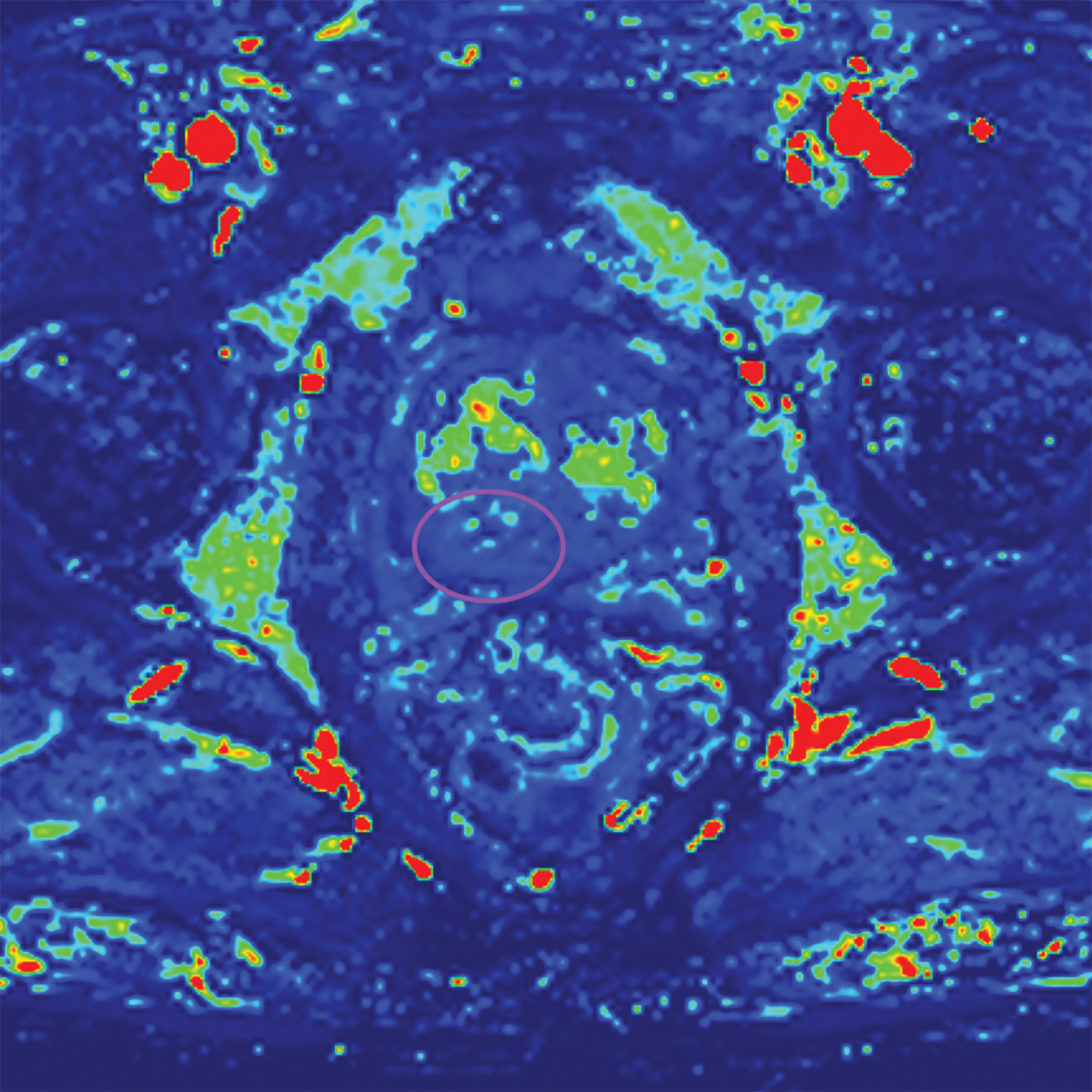
Additionally, to be categorized as PI-RADS 3, a lesion must be negative for DCE; ie, “no early or contemporaneous enhancement; or diffuse multifocal enhancement NOT corresponding to a focal finding on T2W and/or DWI or focal enhancement corresponding to a lesion demonstrating features of BPH on T2WI (including features of extruded BPH in the PZ).”1 If the lesion is positive for DCE; ie, “focal, and; earlier than or contemporaneously with enhancement of adjacent normal prostatic tissues, and corresponds to suspicious finding on T2W and/or DWI”, that lesion is upgraded to PI-RADS 4.1 Figure 2 illustrates an example of a peripheral zone lesion upgraded from PI-RADS 3 to PI-RADS 4 based on positivity on DCE; this patient underwent prostatectomy, which showed prostatic adenocarcinoma with a Gleason Score of 9 (4+5).
Lesions within the transitional zone utilize T2 as the dominant sequence. PI-RADS 3 is described as “[H]eterogeneous signal intensity or with obscured margins,” as well as other lesions “that do not qualify as 2, 4, or 5”.1
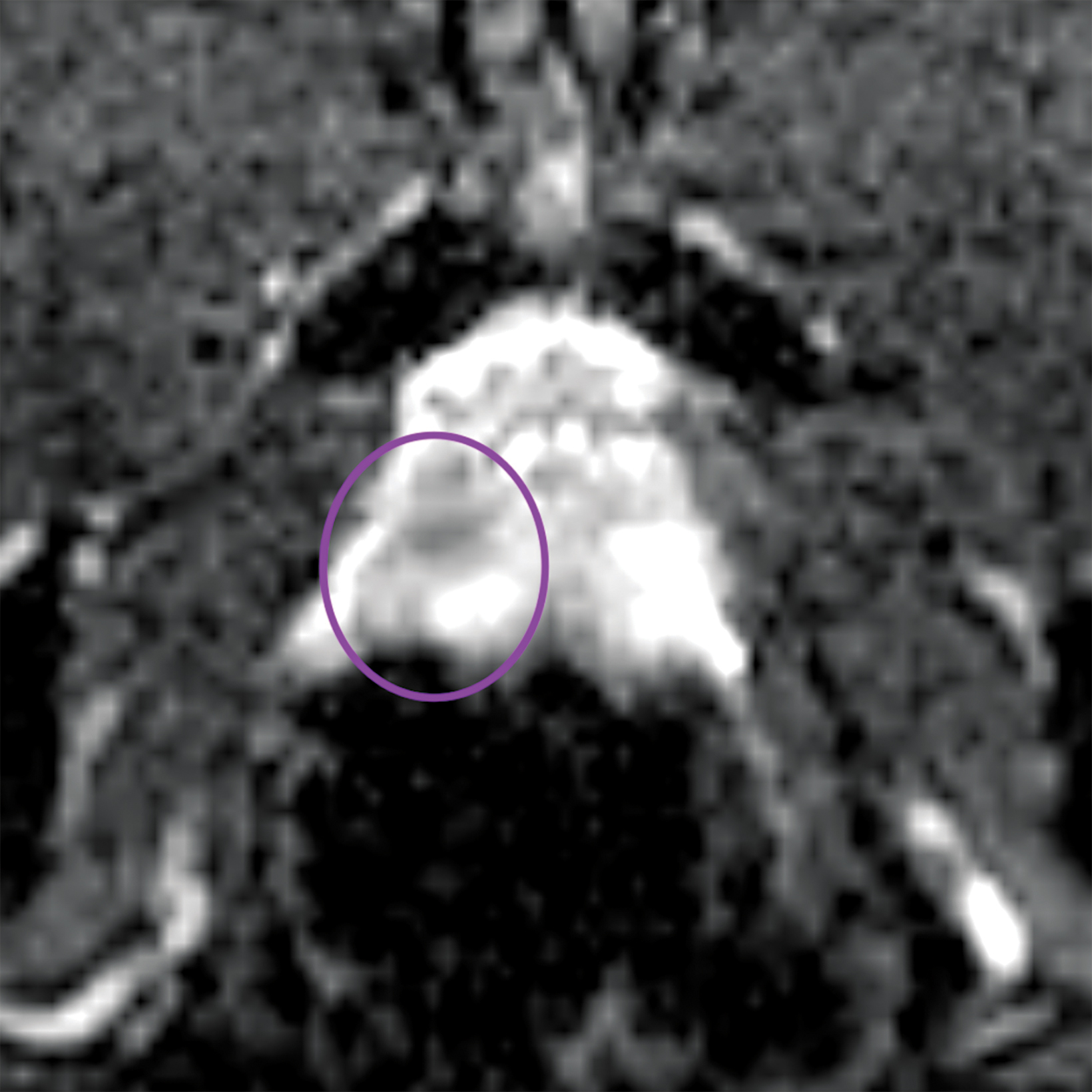
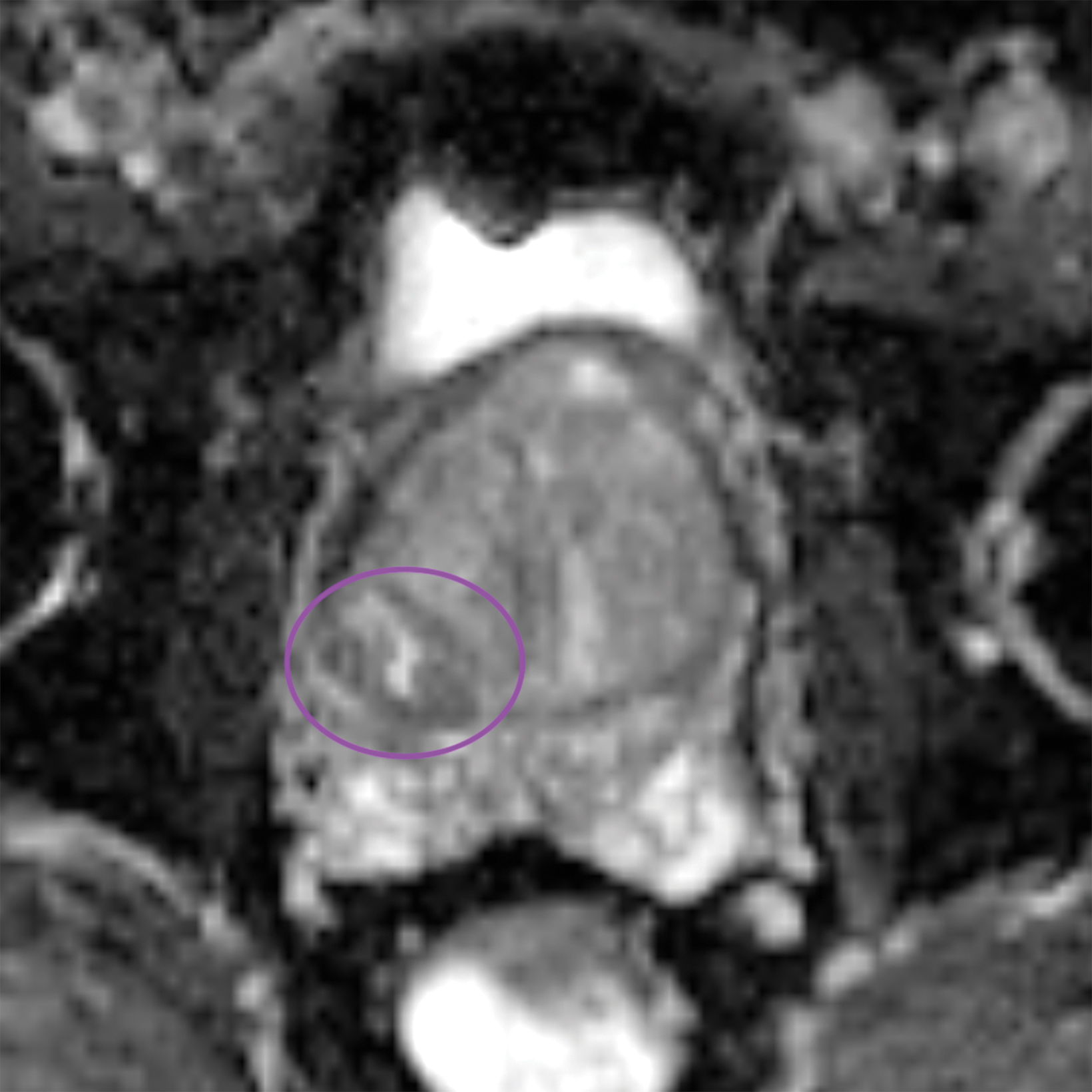
Similar to the peripheral zone, PI-RADS 3 lesions in the transitional zone can be recategorized based on additional imaging char- acteristics. Lesions which initially receive a score of PI-RADS 2 on T2I can be upgraded to PI-RADS 3 “if they have a DWI score greater than or equal to 4;” ie, if they have marked diffusion.1 Lesions initially categorized as PI-RADS 3 can be upgraded to PI-RADS 4 “if they have a DWI score of 5;” ie, marked diffusion and greater than or equal to 1.5 cm in size).1 Figure 3 illustrates an example of a transitional zone PI-RADS 3 lesion.
Despite the new forms of lesion categorization in v2.1, the limited number of studies comparing PI-RADS v2.0 to v2.1 have been mixed. Some studies have suggested v2.1 improves detection of transitional zone lesions, while others have shown no significant difference in the diagnostic performance between the two versions.4,15,16 Issues remaining with PI-RADS v2.1 include the lack of a category for lesions that do not fit into the currently existing five categories and lack of standardization for the way background changes are evaluated.17
Defining Clinically Significant Prostate Cancer
There is no universal definition of clinically significant prostate cancer. However, within the PI-RADS system, clinically significant cancer is defined through pathologic examination of a lesion. PI-RADS defines a clinically significant lesion as one which contains any of the following1:
- A Gleason score ≥ 7. This includes 3+4 lesions with prominent but not predominant Gleason 4 components.
- Tumor volume totaling ≥ 0.5cc.
- The presence of extraprostatic extension (EPE).
More recent literature may refer to the new Gleason scoring grades, where a score of 2 represents an old Gleason score of 3+4 (predominantly well-formed glands with a lesser component of poorly formed glands), while a score of 3 represents an old Gleason score of 4+3 (predominantly poorly formed glands with a lesser component of well-formed glands). This is to eliminate confusion as the latter represents more aggressive disease.18 Using the new Gleason scoring system, a grade of 2 or more represents clinically significant disease.
Management of PI-RADS 3 Lesions
PI-RADS 3 lesions are difficult to manage, owing to their overlapping findings with benign conditions like prostatitis, benign prostatic hyperplasia and fibrosis. Some tumors are smaller and infiltrative, which may further hinder the diagnosis.3 A balance must be attained to lower overdiagnosis and not to miss clinically significant prostate cancer.
British NICE guidelines regarding prostate cancer are based on a 5-point Likert prostate scale, a similar standardized scoring tool that includes clinical parameters.19 The 5-point Likert scale is considered equivalent to the 5-point PI-RADS scale. NICE guidelines recommend biopsy for lesions with a Likert score of 3 or more.20 Meanwhile, the European Association of Urology guidelines, which rely on PI-RADS scoring, recommend biopsy for all PI-RADS 3 lesions.21
The ACR does not provide any management recommendations. It invariably depends on the discretion of the clinical team on how to proceed. Patient factors such as a patient’s age, comorbidities, and treatment preferences, must be taken into account.
Contributory Factors to Clinically Significant Cancer
Only a handful of articles have been published under the latest version of PI-RADS assessing the relationship of clinical factors and clinically significant prostate cancer in PI-RADS 3 lesions.
The prostate-specific antigen (PSA) score is widely accepted as a marker for screening and management, and the relationship between PSA density (PSAD), calculated by dividing total PSA by prostate volume, and PI-RADS lesions has previously been investigated. One study assessed the role of elevated PSAD in transitional zone PI-RADS lesions, with elevated PSA ranging from 4-20 ng/ ml. The evidence showed a higher prediction of clinically significant prostate cancer for PSAD levels greater than 0.15 ng/ml/ml.6 Another study subclassified PI-RADS 3 lesions into 3a (lower-risk lesions with volume < 0.5 ml) and 3b (higher-risk lesions with volume ≥0.5 ml). They found a 100% sensitivity and positive predictive value in detecting clinically significant prostate cancer in patients in the 3b category with a PSAD greater than 0.15 ng/ml/ml.22
PSA-based tools such as the prostate health index (PHI) are also becoming more widely used. The PHI takes into account the [-2] proPSA and free PSA levels. A study evaluating the role of PHI in a group of 143 men evaluated using PI-RADS v2.0 found that PHI was useful for avoiding unnecessary biopsies.23 In patients with PHI value of 49 and more, approximately 55% of biopsies could be avoided without missing clinically significant cancers.
Other factors, including older age and biopsy naive status, have been associated with clinically significant prostate cancer. In contrast, men with at least one negative biopsy were found to have a lower risk.24 A retrospective study of 141 patients evaluated using PI-RADS v2.0 showed a mean interval of approximately 12.4 months to be optimal for follow-up MRI rather than immediate biopsy in PI-RADS 3 lesions.25
Limitations and Opportunities for Further Research
Limitations of this study primarily center around available data. This review only examines lesions classified by the most recent version of PI-RADS, with few studies utilizing this criteria undertaken since its debut in 2019. Additionally, within the available data some articles included in our review did not discriminate between lesions and patients. For example, a single patient may have multiple lesions, confounding their risk for clinically significant cancer.
As more data is collected and more research is conducted utilizing the PI-RADS v2.1 approach, sample sizes will become more robust, allowing for increased confidence in conclusions. Further longitudinal studies to allow for evaluation of PI-RADS 3 lesions over time would be beneficial in determining future risk for clinically significant cancer. Studies incorporating clinical tools such as PSAD, PHI, and patient history could evaluate the benefit for clinical teams to include this information in their decision-making process.
Finally, additional research comparing v2.0 to v2.1 can help in the development of future PI-RADS versions by exposing the possible limitations of this continually developing reporting system.
Conclusion
The present literature review of 11 articles evaluating prostate cancer and clinically significant prostate cancer using PI-RADS version 2.1 reveals low levels of clinically significant cancer. However, contributory individual patient factors such as age, PSAD, PHI, and biopsy status should be considered before deciding whether to perform diagnostic biopsy. As PI-RADS continues to gain popularity as a standardized reporting system for prostate lesions, continued research will be instrumental in the further evolution of the PI-RADS system.
References
- ACR.org. PI-RADS prostate imaging – reporting and data system. 2019. Available at: <https://www.acr.org/-/media/ACR/Files/RADS/PI-RADS/PIRADS-V2-1. pdf> [Accessed 4 October 2022].
- Purysko AS, Rosenkrantz AB, Turkbey IB, Macura KJ. Radiographics update: PI-RADS version 2.1-a pictorial update. Radiographics. 2020 Nov-Dec;40(7):E- 33-E37. doi: 10.1148/rg.2020190207
- Schoots IG. MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions? Transl Androl Urol. 2018 Feb;7(1):70-82. doi: 10.21037/tau.2017.12.31
- Rudolph MM, Baur ADJ, Cash H, Haas M, Mahjoub S, Hartenstein A, Hamm CA, Beetz NL, Konietschke F, Hamm B, Asbach P, Penzkofer T. Diagnostic performance of PI-RADS version 2.1 compared to version 2.0 for detection of peripheral and transition zone prostate cancer. Sci Rep. 2020 Sep 29;10(1):15982. doi: 10.1038/s41598-020-72544-z
- Costa DN, Jia L, Subramanian N, Xi Y, Rofsky NM, Recchimuzzi DZ, de Leon AD, Arraj P, Pedrosa I. Prospective PI-RADS v2.1 atypical benign prostatic hyperplasia nodules with marked restricted diffusion: detection of clinically significant prostate cancer on multiparametric MRI. AJR Am J Roentgenol. 2021 Aug;217(2):395-403. doi: 10.2214/AJR.20.24370
- Natale C, Koller CR, Greenberg JW, Pincus J, Krane LS. Considering predictive factors in the diagnosis of clinically significant prostate cancer in patients with PI-RADS 3 lesions. Life (Basel). 2021 Dec 19;11(12):1432. doi: 10.3390/life11121432
- Hectors SJ, Chen C, Chen J, Wang J, Gordon S, Yu M, Al Hussein Al Awamlh B, Sabuncu MR, Margolis DJA, Hu JC. Magnetic resonance imaging radiomics-based machine learning prediction of clinically significant prostate cancer in equivocal PI-RADS 3 lesions. J Magn Reson Imaging. 2021 Nov;54(5):1466-1473. doi: 10.1002/jmri.27692
- Lim CS, Abreu-Gomez J, Carrion I, Schieda N. Prevalence of prostate cancer in PI-RADS version 2.1 transition zone atypical nodules upgraded by abnormal DWI: correlation with MRI-directed TRUS-guided targeted biopsy. AJR Am J Roentgenol. 2021 Mar;216(3):683-690. doi: 10.2214/AJR.20.23932
- Giambelluca D, Cannella R, Vernuccio F, Comelli A, Pavone A, Salvaggio L, Galia M, Midiri M, Lagalla R, Salvaggio G. PI-RADS 3 lesions: role of prostate MRI texture analysis in the identification of prostate cancer. Curr Probl Diagn Radiol. 2021 Mar-Apr;50(2):175-185. doi: 10.1067/j.cpradiol.2019.10.009
- Wang ZB, Wei CG, Zhang YY, Pan P, Dai GC, Tu J, Shen JK. The role of PSA density among PI-RADS v2.1 categories to avoid an unnecessary transition zone biopsy in patients with PSA 4-20 ng/mL. Biomed Res Int. 2021 Oct 11;2021:3995789. doi: 10.1155/2021/3995789
- Arcot R, Sekar S, Kotamarti S, Krischak M, Michael ZD, Foo WC, Huang J, Polascik TJ, Gupta RT. Structured approach to resolving discordance between PI-RADS v2.1 score and targeted prostate biopsy results: an opportunity for quality improvement. Abdom Radiol (NY). 2022 Aug;47(8):2917-2927. doi: 10.1007/ s00261-022-03562-w
- Boschheidgen M, Schimmöller L, Doerfler S, Al-Monajjed R, Morawitz J, Ziayee F, Mally D, Quentin M, Arsov C, Albers P, Antoch G, Ullrich T. Single center analysis of an advisable control interval for follow-up of patients with PI-RADS category 3 in multiparametric MRI of the prostate. Sci Rep. 2022 Apr 25;12(1):6746. doi: 10.1038/s41598-022-10859-9
- Wei X, Xu J, Zhong S, Zou J, Cheng Z, Ding Z, Zhou X. Diagnostic value of combining PI-RADS v2.1 with PSAD in clinically significant prostate cancer. Abdom Radiol (NY). 2022 Oct;47(10):3574-3582. doi: 10.1007/s00261-022-03592-4
- Jin P, Yang L, Qiao X, Hu C, Hu C, Wang X, Bao J. Utility of clinical-radiomic model to identify clinically significant prostate cancer in biparametric MRI PI-RADS v2.1 category 3 lesions. Front Oncol. 2022 Feb 24;12:840786. doi: 10.3389/fonc.2022.840786
- Wang Z, Zhao W, Shen J, Jiang Z, Yang S, Tan S, Zhang Y. PI-RADS version 2.1 scoring system is superior in detecting transition zone prostate cancer: a diagnostic study. Abdom Radiol (NY). 2020 Dec;45(12):4142-4149. doi: 10.1007/s00261-020-02724-y
- Bhayana R, O’Shea A, Anderson MA, Bradley WR, Gottumukkala RV, Mojtahed A, Pierce TT, Harisinghani M. PI-RADS versions 2 and 2.1: interobserver agreement and diagnostic performance in peripheral and transition zone lesions among six radiologists. AJR Am J Roentgenol. 2021 Jul;217(1):141-151. doi: 10.2214/AJR.20.24199. Epub 2020 Sep 9. PMID: 32903060.
- Purysko AS, Baroni RH, Giganti F, Costa D, Renard-Penna R, Kim CK, Raman SS. PI-RADS version 2.1: a critical review, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021 Jan;216(1):20-32. doi: 10.2214/AJR.20.24495. Epub 2020 Nov 19. PMID: 32997518.
- Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016 Feb;40(2):244-52. doi: 10.1097/PAS.0000000000000530
- Latifoltojar A, Appayya MB, Barrett T, Punwani S. Similarities and differences between likert and PIRADS v2.1 scores of prostate multiparametric MRI: a pictorial review of histology-validated cases. Clin Radiol. 2019 Nov;74(11):895.e1-895.e15. doi: 10.1016/j.crad.2019.08.020
- NICE.org.uk. Prostate cancer: diagnosis and management. Dec 2021. Available at: <https://www.nice.org.uk/guidance/ng131/chapter/ Recommendations#assessment-and-diagnosis> [Accessed 4 October 2022].
- Uroweb.org. Guidelines on prostate cancer. 2022. Available at: <https://uroweb.org/guidelines> [Accessed 4 October 2022].
- Rico L, Blas L, Vitagliano G, Contreras P, Rios Pita H, Ameri C. PI-RADS 3 lesions: Does the association of the lesion volume with the prostate-specific antigen density matter in the diagnosis of clinically significant prostate cancer? Urol Oncol. 2021 Jul;39(7):431.e9-431.e13. doi: 10.1016/j.urolonc.2020.11.010
- Carbunaru S, Stinson J, Babajide R, Hollowell CMP, Yang X, Sekosan M, Ferrer K, Kajdacsy-Balla A, Abelleira J, Ruden M, King-Lee P, Dalton DP, Casalino DD, Kittles RA, Gann PH, Schaeffer EM, Murphy AB. Performance of prostate health index and PSA density in a diverse biopsy-naïve cohort with mpMRI for detecting significant prostate cancer. BJUI Compass. 2021 Jun 15;2(6):370-376. doi: 10.1002/bco2.91
- Fang AM, Shumaker LA, Martin KD, Jackson JC, Fan RE, Khajir G, Patel HD, Soodana-Prakash N, Vourganti S, Filson CP, Sonn GA, Sprenkle PC, Gupta GN, Punnen S, Rais-Bahrami S. Multi-institutional analysis of clinical and imaging risk factors for detecting clinically significant prostate cancer in men with PI-RADS 3 lesions. Cancer. 2022 Sep 15;128(18):3287-3296. doi: 10.1002/cncr.34355
- Steinkohl F, Gruber L, Bektic J, Nagele U, Aigner F, Herrmann TRW, Rieger M, Junker D. Retrospective analysis of the development of PIRADS 3 lesions over time: when is a follow-up MRI reasonable? World J Urol. 2018 Mar;36(3):367-373. doi: 10.1007/s00345-017-2135-0
