Low Versus Ultra-High Field MRI: How to Select Your MRI Fleet
By Nikpanah M, Willoughby WR, Campbell-Washburn AE, Denney Jr TS, Malayeri AA, ver Hoef L, Porter KK
Abstract
Objective and hypothesis: Magnetic resonance imaging (MRi) is one of the most powerful non-invasive methods for clinical applications as well as biomedical research. Since the mid-1980s, there has been momentum for MRI scanners with higher field strengths to enhance contrast-to-noise ratio, signal-to-noise ratio, and spatial resolution. This resulted in the evolution of 0.3-0.6 Tesla (T) to conventional 1-1.5T and high-field 3T scanners, and eventually, ultra-high field scanners of 7T and beyond. The wide variety of available MR field strengths suits the demand for a multitude of research and clinical applications.
Methods and Materials: A structured literature search was performed for the terms “low-field magnetic resonance imaging” and “ultra-high field magnetic resonance imaging” by mapping their Medical Subject Heading (MeSH) tree structure utilizing the Public Library of Medicine’s (PubMed) Automatic Term Mapping (ATM). Then, the PubMed database and the Web of Science (Clarivate Analytics) Core Collection from 1980 to April 2022 were searched using all categories. Some specific searches for relevant topics such as the new MAGNETOM FreeMax MRI system were performed using Google, as well as some further exploration of topics around the future of MRI.
Results: Review articles, original research manuscripts, and editorial materials were reviewed. The selected manuscripts cited in this article represent a comprehensive review of the publications focused on low and ultra-high field MRI and the clinical applications of these systems. Our results showed that each of these MRI systems has unique clinical and research utilities that fit the needs of various healthcare settings or research facilities. Herein, we comprehensively discuss the technical features of these cutting-edge systems, their clinical uses, as well as advantages and disadvantages.
Conclusions: Developing a successful MRI program in a healthcare setting is a complex and comprehensive process that involves financial considerations for equipment procurement and maintenance as well as operational considerations.
Keywords: MRI, Low-Field MRI, Ultra-High Field MRI
Introduction
Magnetic resonance imaging (MRI) is one of the most effective noninvasive diagnostic tools in current medical practice, and it has played a vital role in cutting-edge research, yielding information regarding both structure and function.1 The early low-field MRI images were at times not interpretable, owing mainly to poor contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR). Since
the mid-1980s, there has been a push for MRI scanners with higher field strength (B0) to solve this limitation by improving CNR, SNR, and spatial resolution.1,2 These efforts led to the evolution from low-field 0.3-0.6 Tesla (T) scanners with textured images to conventional 1-1.5T and high-field 3T scanners with widespread clinical utilization due to higher image quality and faster acquisi- tion time.1,3 Application of ultra-high field (UHF, ≥ 7T) MRI in recent years has provided enhanced spatial resolution and improved SNR with decreased voxel size, allowing visualization of smaller structures in detail.1 However, UHF MRI has its disadvantages, and recently there has been renewed interest in contemporary low-field MRI systems equipped with modern hardware, advanced image acquisition methods, and advanced image reconstruction methods.
Currently, a variety of MRI field strengths are available to meet the need for a multitude of research and clinical utilities. Each of these MRI systems has unique features and capabilities, as well as advantages and disadvantages. In this manuscript, we review the technical aspects of state-of-the-art low, high, and UHF MRI systems and their clinical applications. Additionally, we share recommendations for radiology departments and practices regarding the selection of appropriate technologies that meet the clinical needs of their patient population.
Methods and Materials
A structured literature search was performed for the terms “low-field magnetic resonance imaging” and “ultra-high field magnetic resonance imaging” by mapping their Medical Subject Heading (MeSH) tree structure utilizing the Public Library of Medicine’s (PubMed) Automatic Term Mapping (ATM). Then, the PubMed database and the Web of Science (Clarivate Analytics) Core Collection from 1980 to April 2022 were searched using all categories.
Some specific searches for relevant topics such as the new MAGNETOM FreeMax MRI system were performed using Google, as well as some further exploration of topics around the future of MRI. Furthermore, by manual review of the references of these publications, additional references were identified for consideration. After removing the duplicates, the selected manuscripts cited in this article represent a comprehensive review of the publications focused on low-field and ultra-high field MRI, the advantages and disadvantages of these systems, as well as their clinical applications.
Results
Studies in languages other than English were excluded. Review articles, original research manuscripts, and editorial materials were reviewed. The selected manuscripts cited here represent a comprehensive review of the publications focused on low and ultra-high field MRI and the clinical applications of these systems.
Low-Field MRI
The first MRI scanners in the 1980s were low-field, typically with a field strength of 0.25–1.0T.4 These low-field models had poor spatial resolution, low temporal resolution, and limited image parameters and sequences.5 When these low-field MRI systems were used in clinical settings, it was assumed, and ultimately verified, that higher static field strengths would improve MRI performance.4,6
The MRI signal is proportional to the square of the magnetic field strength, and assuming a constant receive bandwidth, the noise is approximately proportional to the static magnetic field strength (B0). Therefore, the simplest technique to acquire a better SNR in an MRI system is to increase B0; however, B0 does not solely determine the image SNR.7,8 Developments in MRI technology, including SNR-efficient data acquisitions, parallel imaging, compressed sensing, and machine learning-based image reconstruction methods, have made the imaging methods of low-field MRI systems, which previously were rather restricted, more robust. These sequences are now comparable to those of standard clinical practice on 1.5T MRI systems.9,10 For example, the National Heart, Lung, and Blood Institute (NHLBI, National Institutes of Health), in collaboration with Siemens Healthineers, ramped down a whole-body 1.5T system to a 0.55T system equipped with high-performance software and hardware.11 This innovative technology showed significant promise for routine imaging and for novel applications of MRI.
Subsequently, on July 1, 2021, Siemens Healthcare announced that the U.S. Food and Drug Administration (FDA) cleared a 0.55T system (Magnetom FreeMax system by Siemens Medical Solutions Inc., Erlangen, Germany) for clinical applications.12 This high-performance system combines a 0.55T field strength with sophisticated image processing and deep learning technologies. ViewRay also offers a 0.35T whole-body MRI-linac system with high-performance hardware.13
Researchers have developed several dedicated low-field MRI systems and demonstrated significant advantages for portable and point-of-care imaging.14–16 Hyperfine Research’s Swoop System (Guilford, CT) has created a first-of-its-kind portable low-field (0.064T) MRI scanner, which received 510(k) clearance from the FDA in February 2020 and addresses another critical niche for low-field MRI: patient-side imaging.17 Other application-specific MRI systems include Synaptive’s Evry 0.5T head-only MRI system, Promaxo’s 0.066T prostate MRI system, and Esaote’s MSK MRI systems.18–20
Given these considerations, what are currently considered novel low-field MRI scanners have grown in popularity, particularly in areas where higher static field MRI systems are difficult to install or when concerns exist regarding the cost, especially for maintenance.21
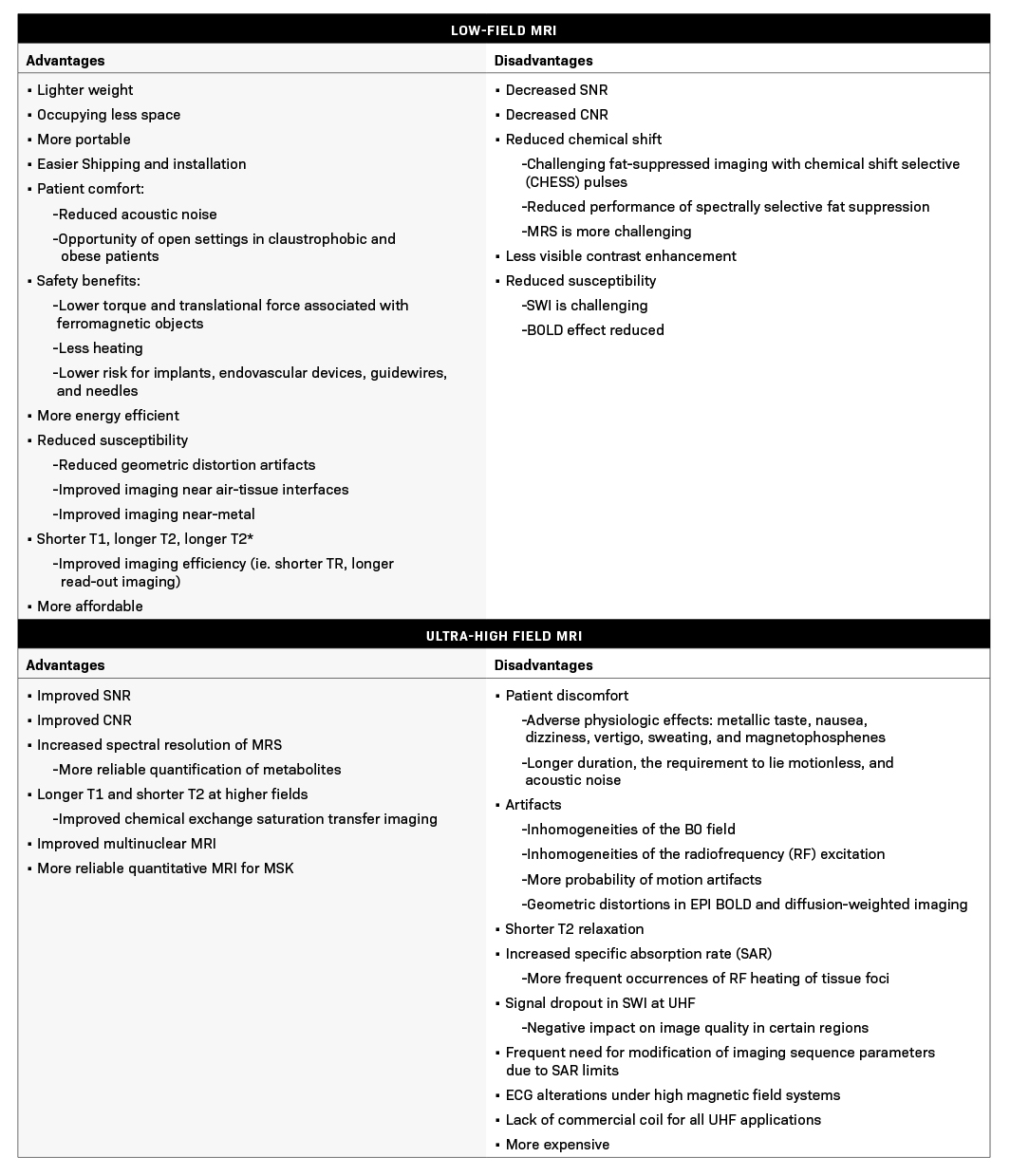
Advantages and Disadvantages of Low-Field MRI
Diagnostic imaging is a significant cost driver in patient care globally.5 Therefore, lower costs and simplified installation, operation, and maintenance are required for increased worldwide access.22 Hospitals also face significant logistical challenges when it comes to site selection.22 Generally, low-field MRI systems are more lightweight, occupy less space compared to high-field systems, require less shielding, and are more portable.5,23 This greatly simplifies and lowers the cost and difficulties associated with shipping, installation, and finding an optimal site.5 There are also patient acceptance benefits: their larger bore diameter allows for a less anxiety-provoking experience for patients with claustrophobia and easier positioning for obese patients.24
Imaging in the presence of susceptibility-induced magnetic field inhomogeneities is far superior in low-field; the source of susceptibility can be the lung, bowel, cranial sinuses, or metallic implants. This may afford new clinical opportunities to apply MRI and may improve imaging for the increasing number of patients with implanted devices.10
Low-field MRI also provides additional safety benefits. The torque and translational force associated with ferromagnetic objects are lower.25 Heating is also less of a concern due to the lower specific absorption rate (SAR). The lower magnetic field has the effect of lowering the implant risk profile.26 These considerations apply to MRI-guided interventions, as well as the associated endovascular devices, guidewires, and needles.25 This could expand MRI imaging to include cardiovascular interventional procedures, with promising preliminary findings.27 Some low-field MRI systems still use electromagnets or permanent magnets.28 For these, simple water cooling might be sufficient, eliminating the need for complicated and costly cryogenic cooling systems used in high-field MRI systems.23 These advantages highlight how low-field MRI systems may be more energy-efficient and align with environmental sustainability, a topic that has recently been a major focus in the radiology community.21,29
On the negative side, low SNR is the most noteworthy limitation of low-field MRI, as previously described. Low SNR can result in reduced image quality, lower resolution, and increased scan time. Although image acquisition, reconstruction, and processing strategies can compensate for the reduced SNR, these are not consistently available. Additionally, in low-field MRI systems (up to 0.3T), the application of fat-suppressed imaging with chemical shift selective (CHESS) radio frequency (RF) pulses is challenging.30,31 This is due to the decreased chemical shift of water and fat spectra at lower field strengths, which makes the application of fat suppression pulses more difficult. Exacerbating this, the water spectral width expands in an inversely proportional ratio to T2*, and the water signal can be inadvertently readily suppressed even with minor inhomogeneities in the magnetic field.31
Magnetic susceptibility is proportional to field strength, and while this is advantageous for reduced artifacts in many sequences, it is a challenge for others.23 As a result, 3T is widely accepted as the preferred field strength for some clinically significant MRI studies, including time of flight (TOF) MRI angiography (MRA) and susceptibility-weighted imaging.26 However, more recently, TOF MRA and susceptibility-weighted imaging have been acquired with image quality comparable to the 3T studies using the high-performance 0.55T low-field systems.23 Also, while still evolving, the current opinion is that the acquisition time on the 0.55T low-field systems can also be kept constant compared to 1.5T systems by leveraging SNR-efficient acquisitions and utilizing advanced reconstruction techniques.
Diffusion-weighted imaging (DWI) provides qualitative and quantitative information regarding tissue cellularity. The b-value is directly related to water diffusion effects and reflects the strength and timing of the gradients used to generate diffusion-weighted images. High-performance gradient systems with high maximum gradient amplitudes and slow rates result in increased spatial resolution and faster acquisitions; however, at higher field strengths, susceptibility and T2* effects are greater. At lower field strengths, imaging parameters for DWI may be manipulated such that it is possible to acquire similar high-quality images (eg, time to echo and readout bandwidth).
Clinical Applications of Low-field MRI
Low-field MRI has applications across body systems such as musculoskeletal (MSK), lung, cardiovascular, and abdomen. As initially investigated at the NIH, comprehensive lung imaging using high-performance low-field MRI provides novel imaging data to complement existing standard assessments such as spirometry, exercise testing, and CT. For instance, MRI measures of regional ventilation-perfusion (VQ) mismatch would be preferable over nuclear imaging approaches, which have limited resolution and are frequently unavailable.32 Oxygen-enhanced MRI has shown particular promise in low-field, where the T1 relaxivity of oxygen is higher.33,34
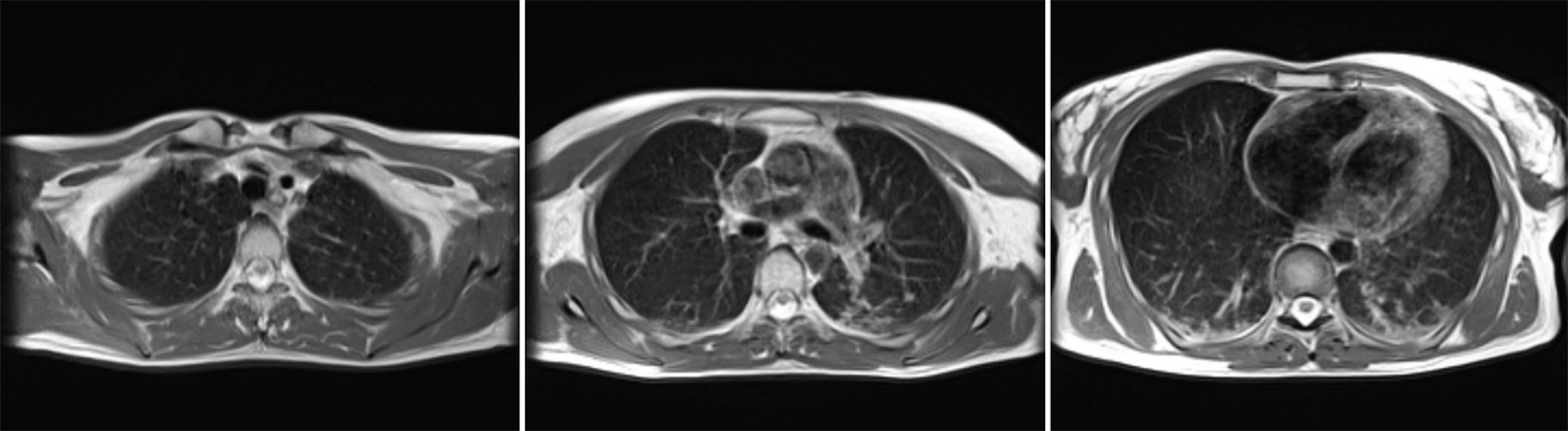
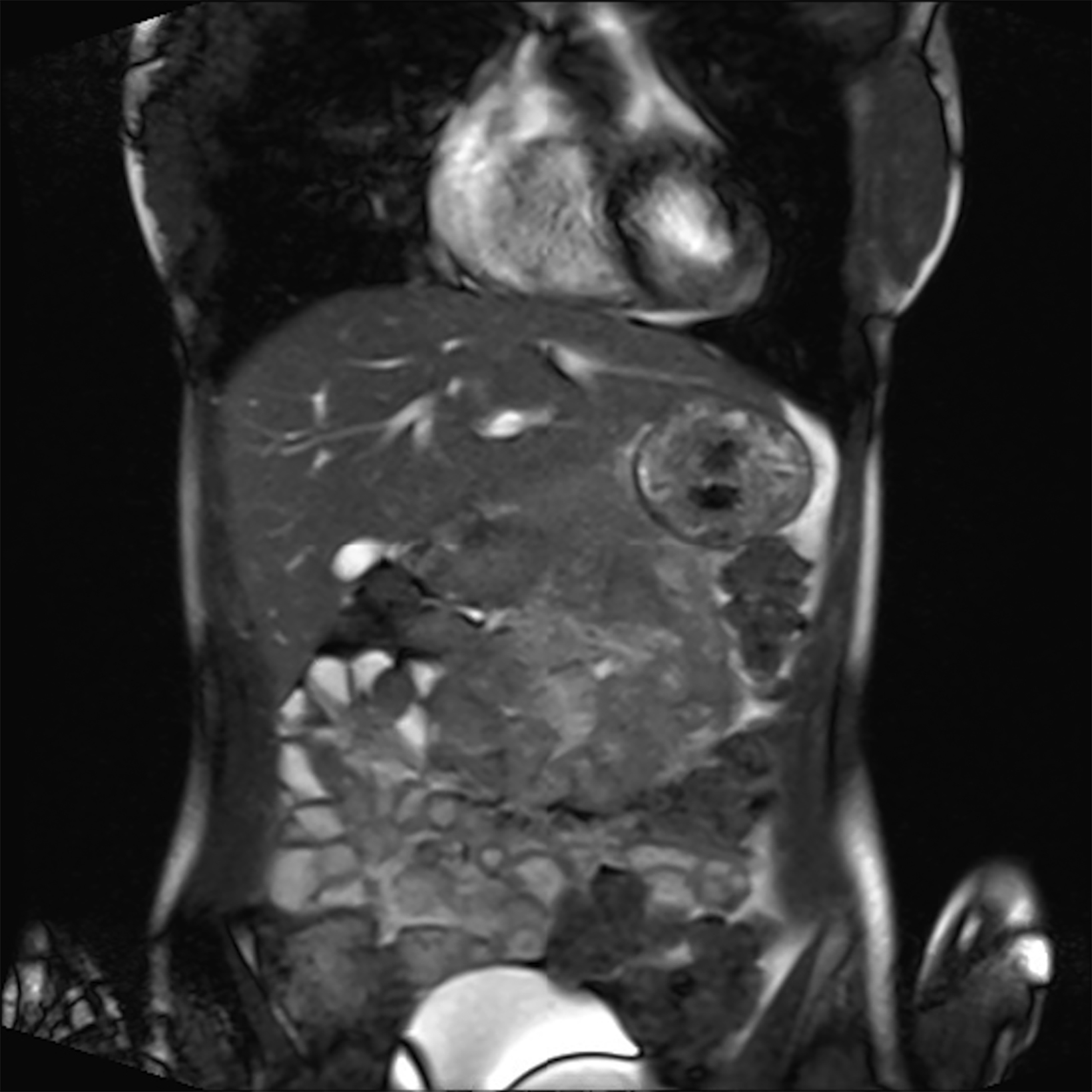
Compared to the current clinical workflow, in which patients are imaged multiple times with CT imaging, MRI tissue characterization provides higher specificity and lower radiation exposure on serial lung imaging.32 Recent studies have demonstrated high-quality structural lung imaging and similar pulmonary findings on high-performance MRI images and CT studies in several patients, including patients with COVID-19, suggesting the potential for repetitive lung assessments in these patients.35–38 Since MRI does not emit ionizing radiation, the ability to acquire MRI images with high quality is important in the pediatric population and may have clinical applications for younger patients with chronic pulmonary diseases, such as cystic fibrosis.32 Low-field MRI offers a specific advantage for lung imaging due to the reduced susceptibility of the air in the lung parenchyma (Figure 1).
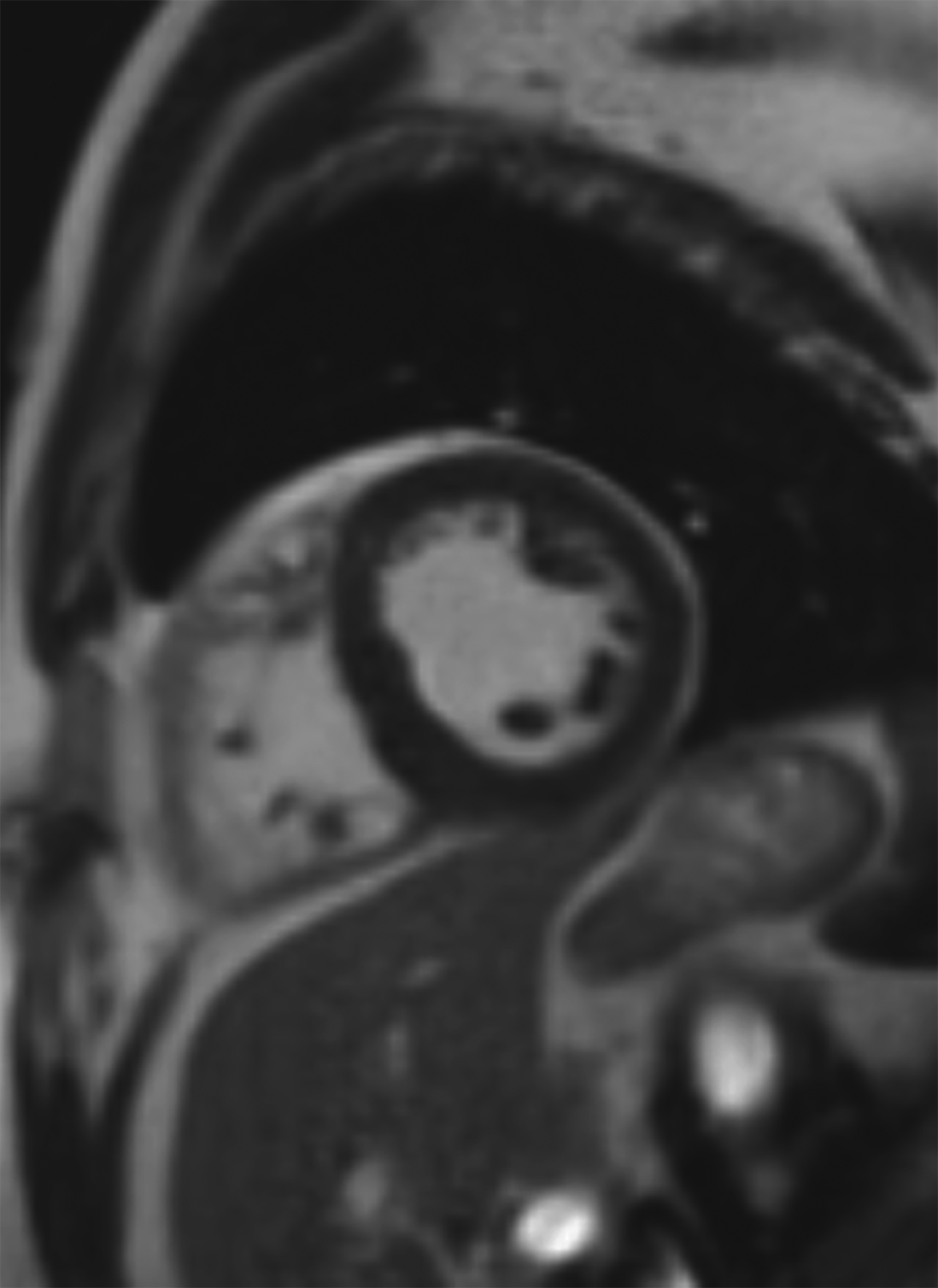
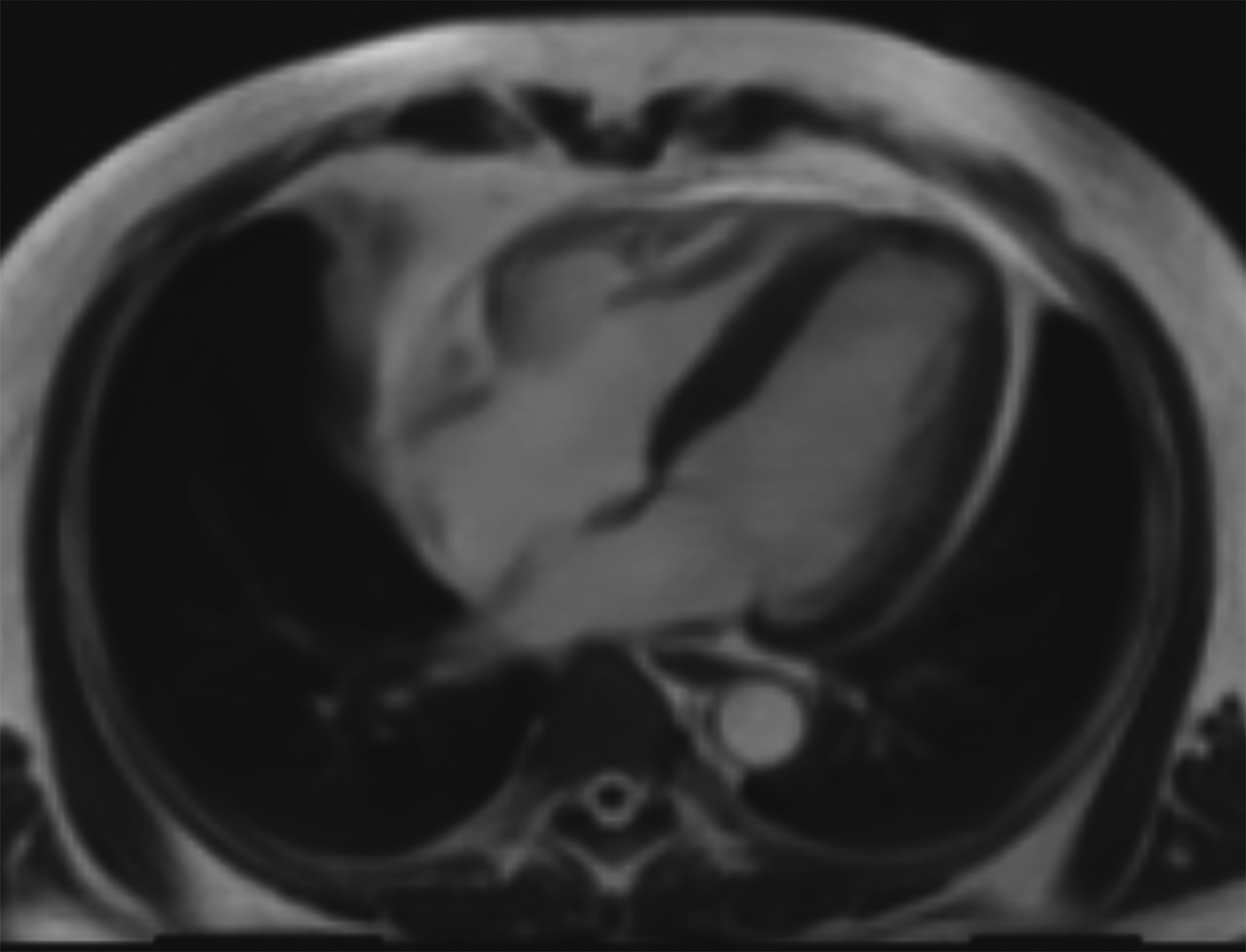
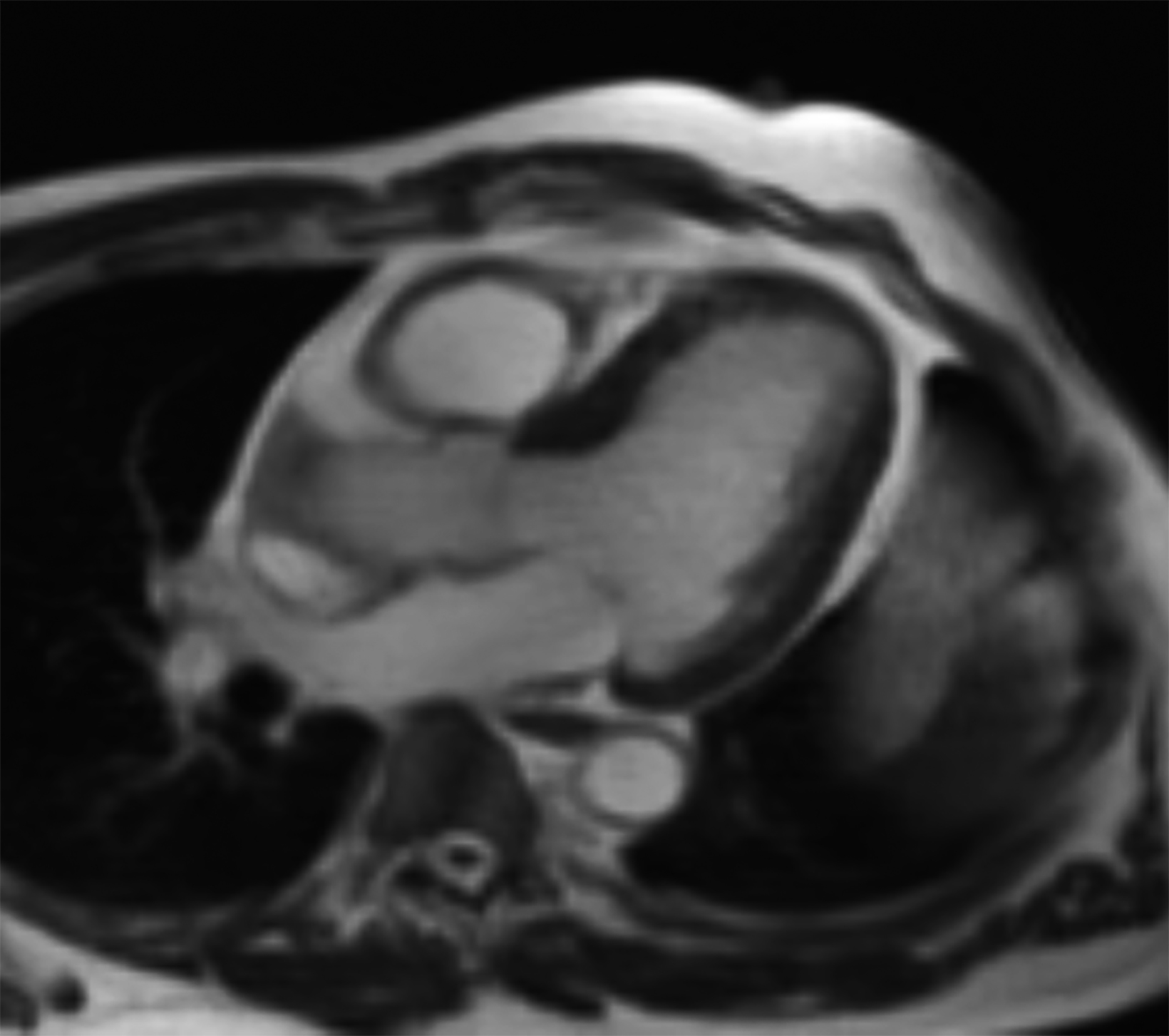
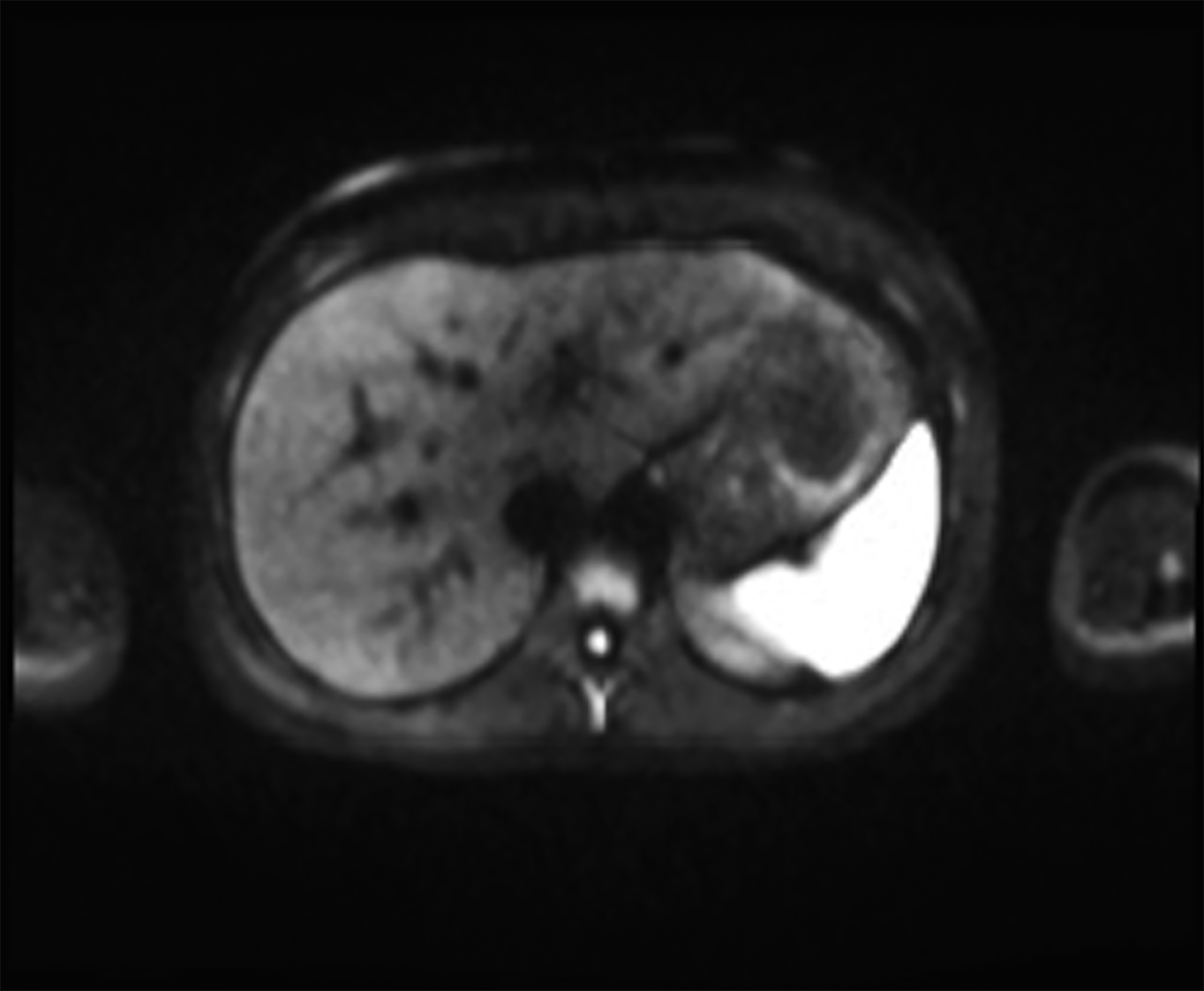
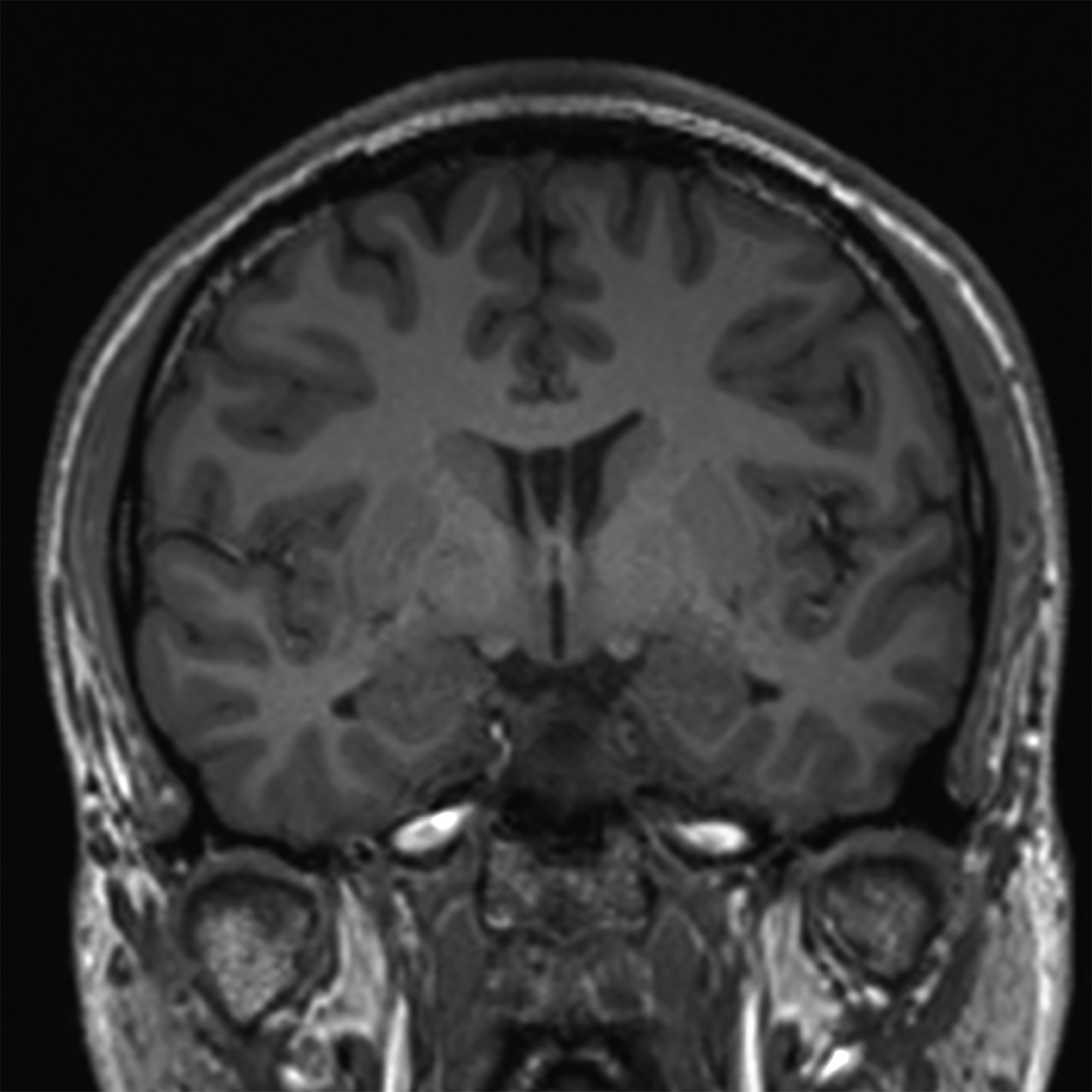
New low-field MRI has been applied to cardiovascular imaging and provides accurate and consistent clinical measurements for cine and late gadolinium enhancement imaging (Figure 2).39,40 Additionally, low-field MRI offers advantages for MRI-guided cardiovascular catheterization and interventional research.40,41 Heating of implanted materials may also be reduced in low-field MRI as compared to high-field given the lower SAR; although, questions regarding the impact of implant length, shape, orientation, insulation, and position with respect to the transmit coil at low-field remain incompletely explored to date. 21
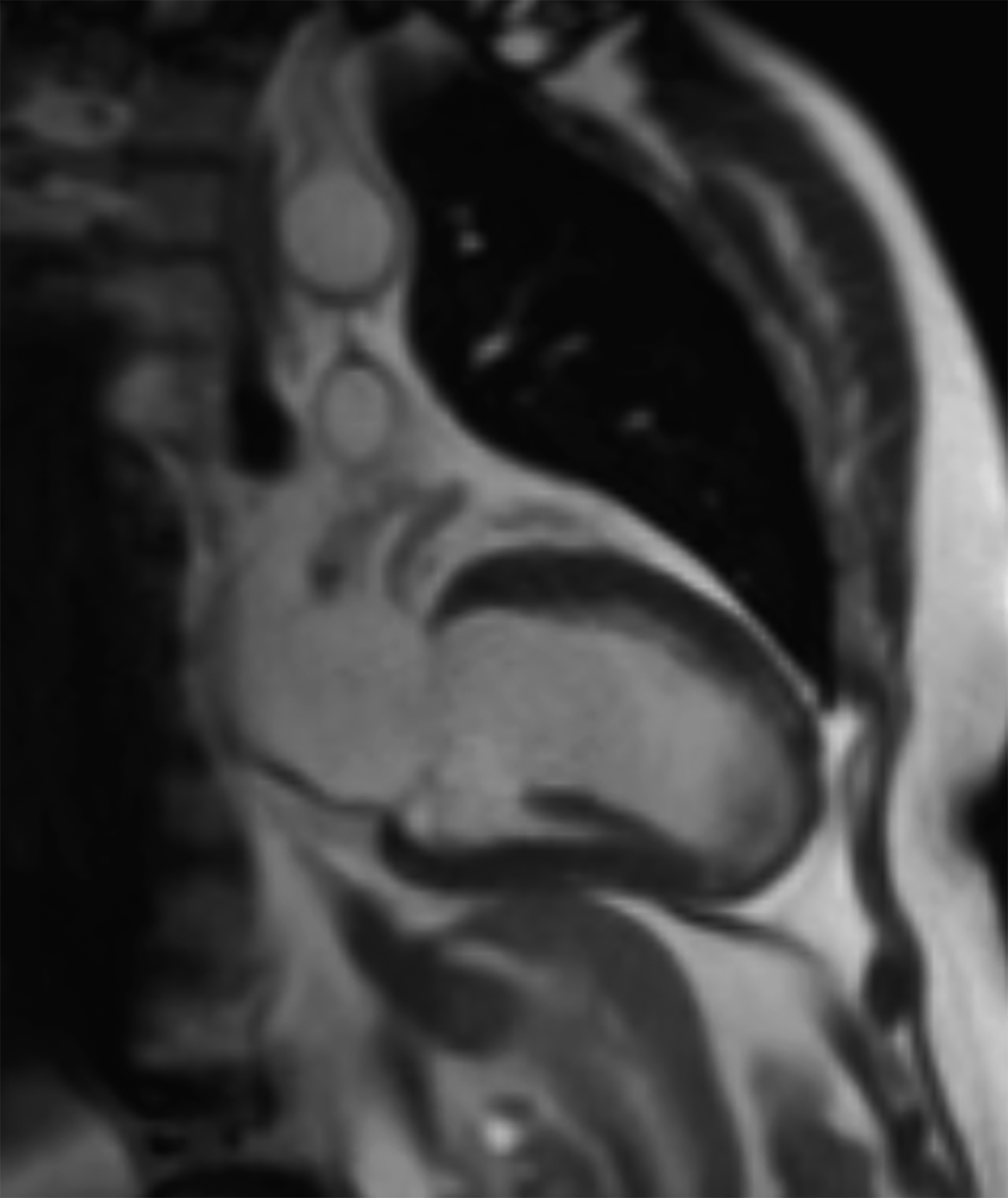
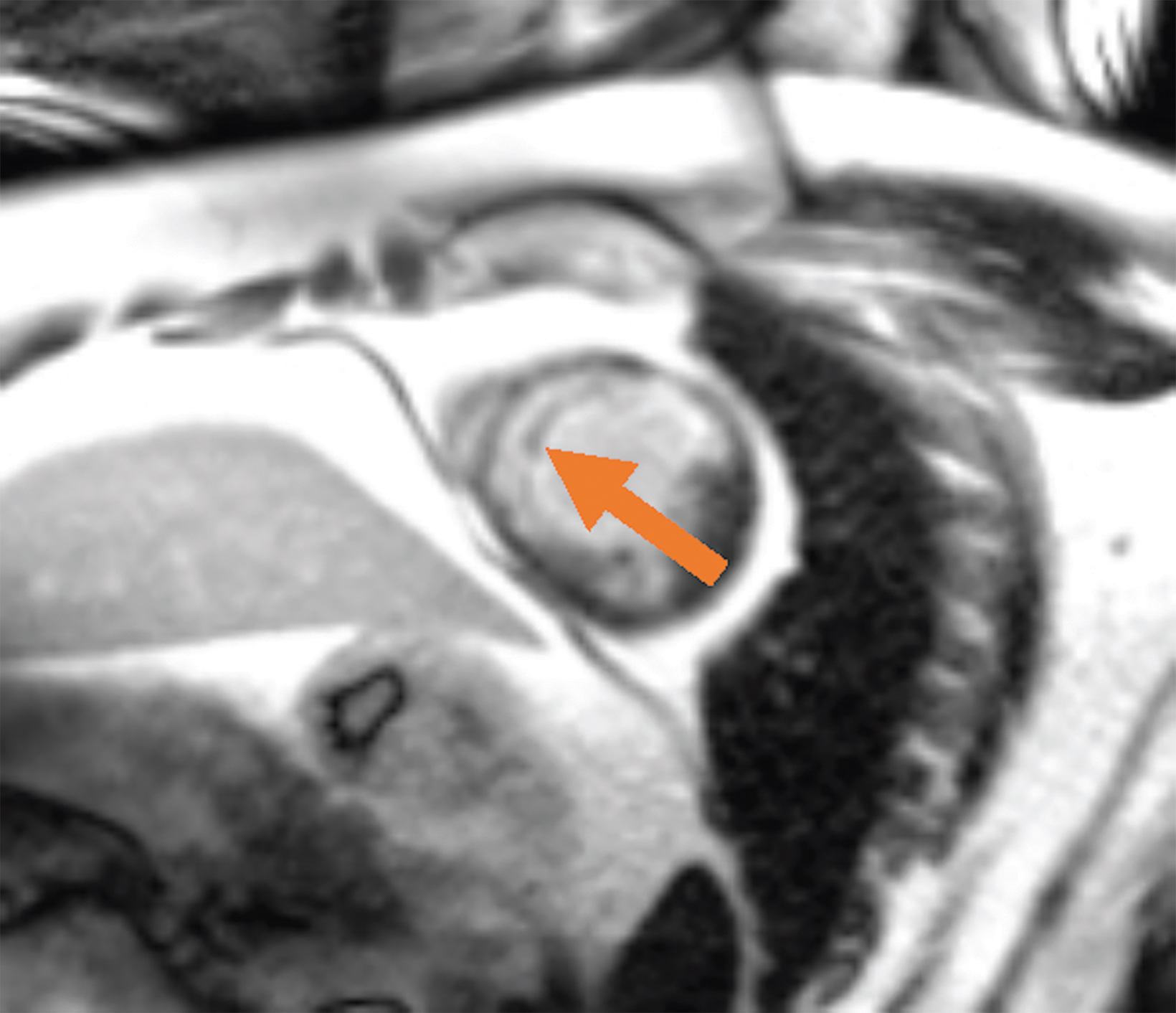
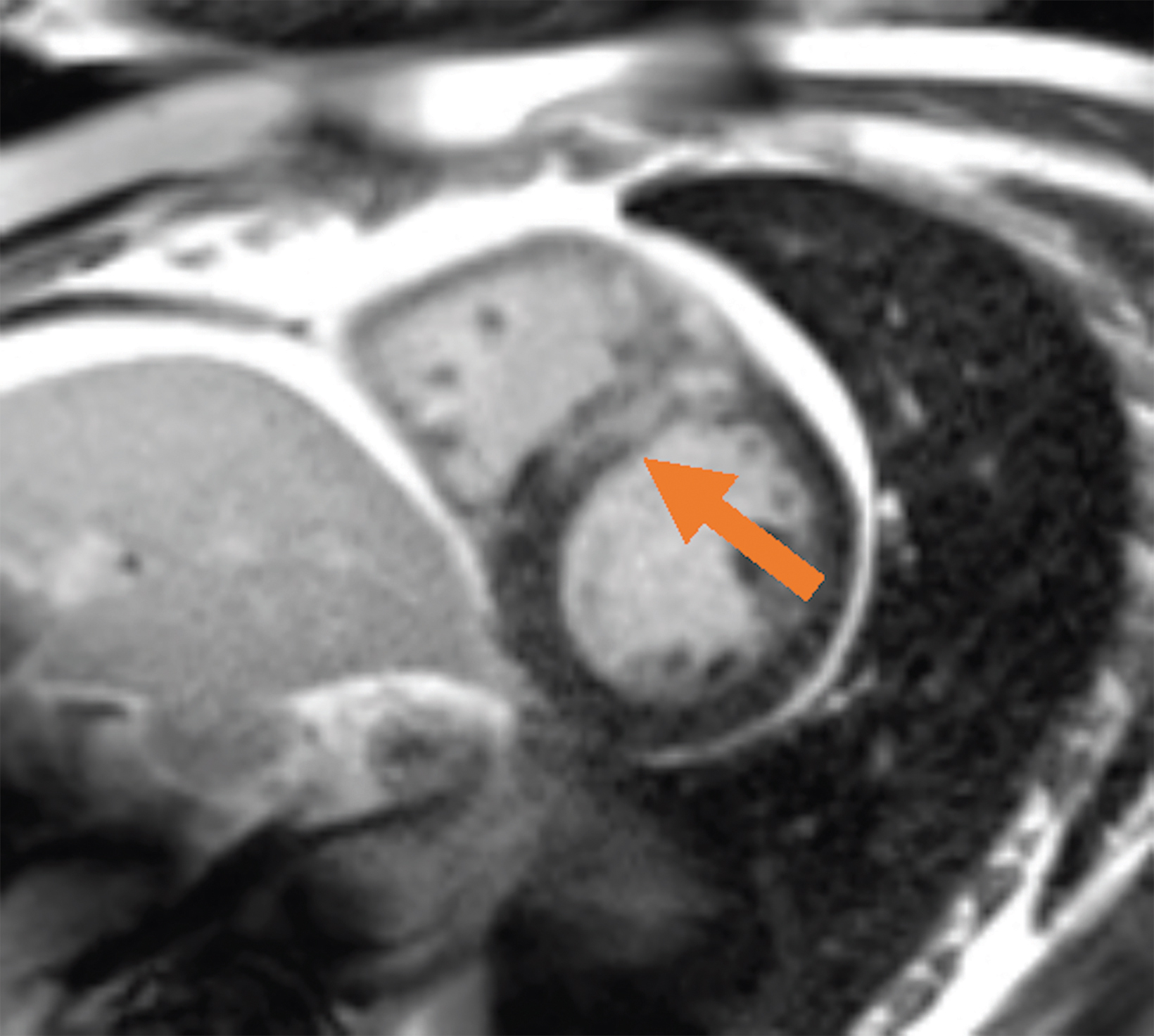
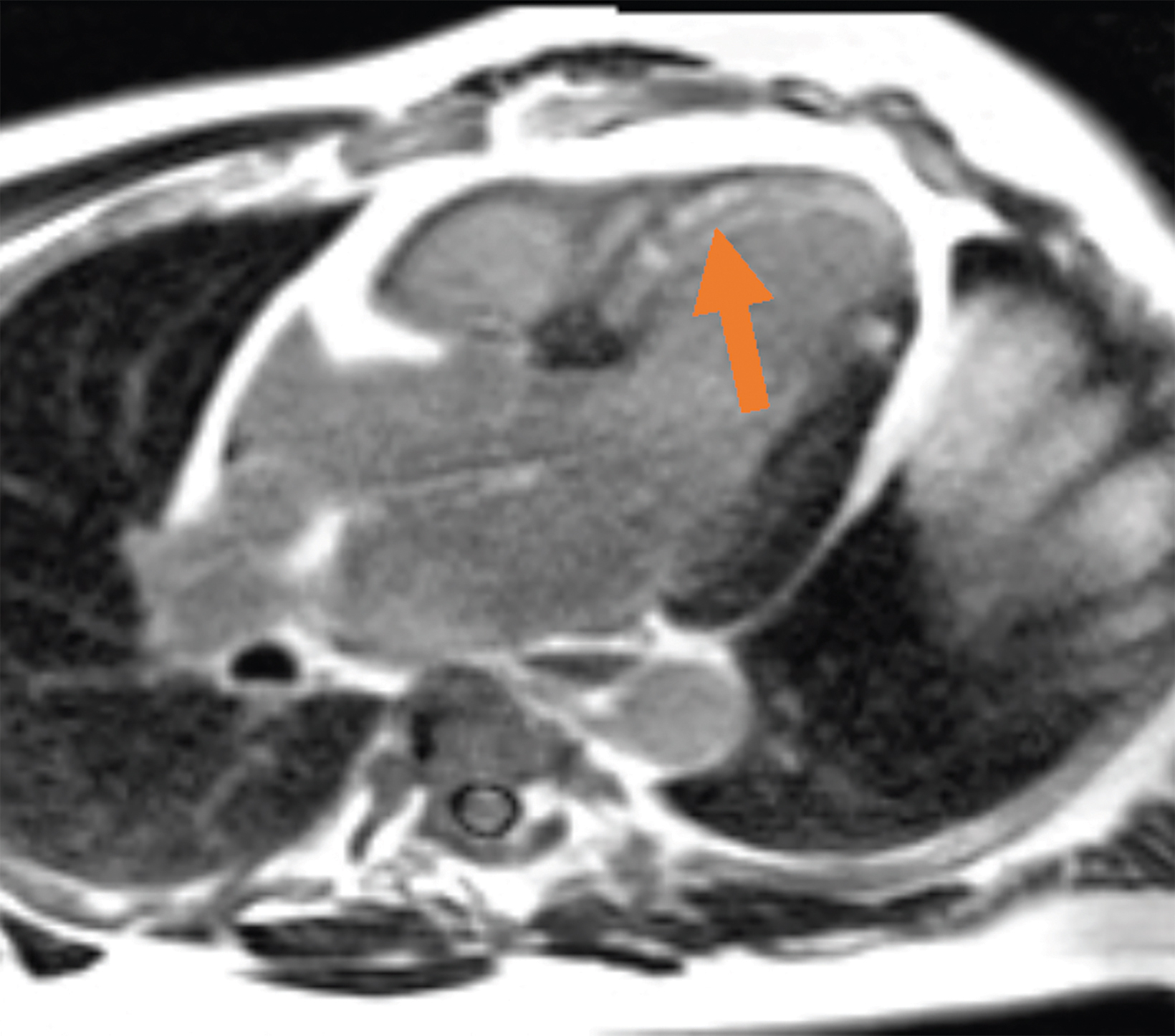
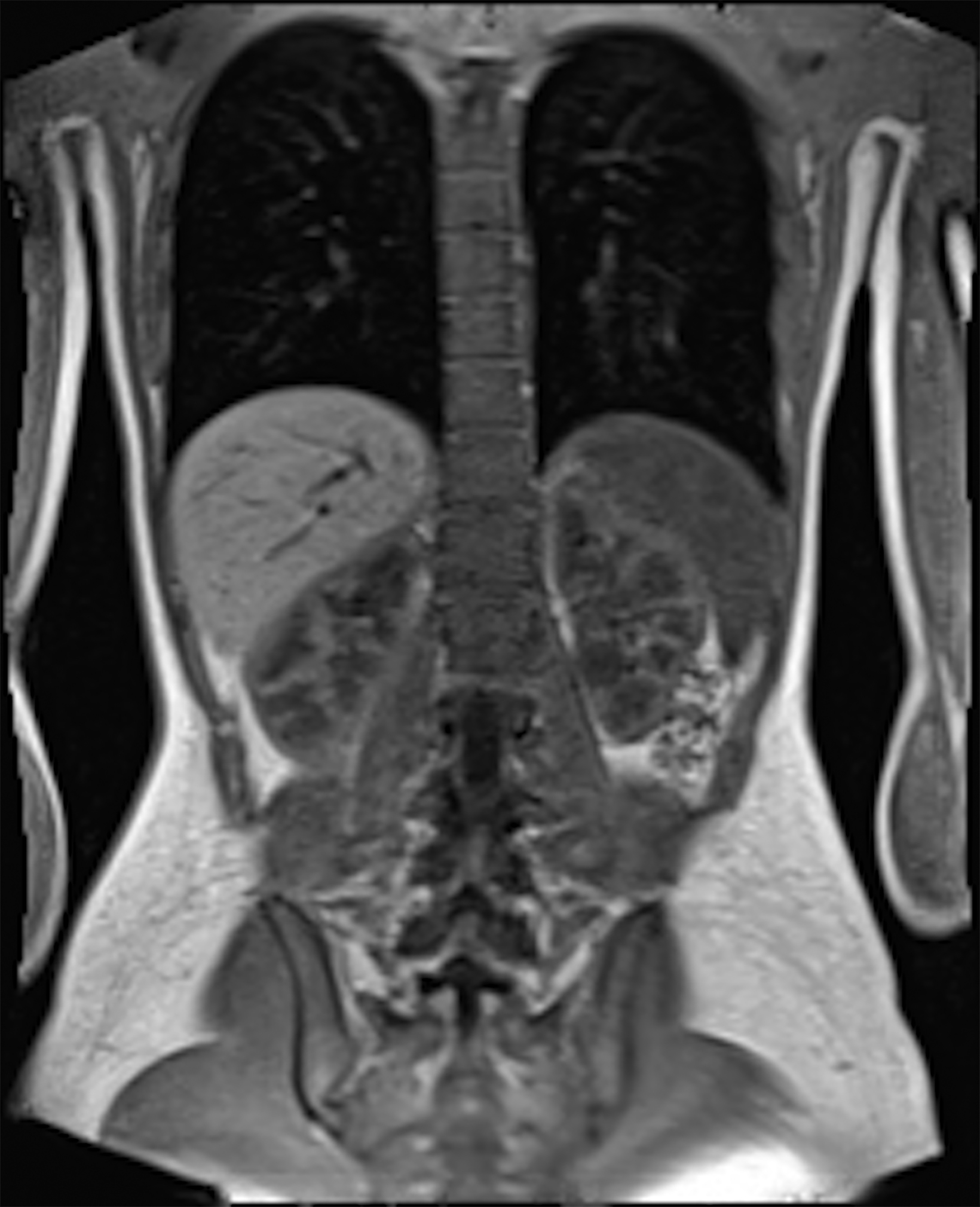
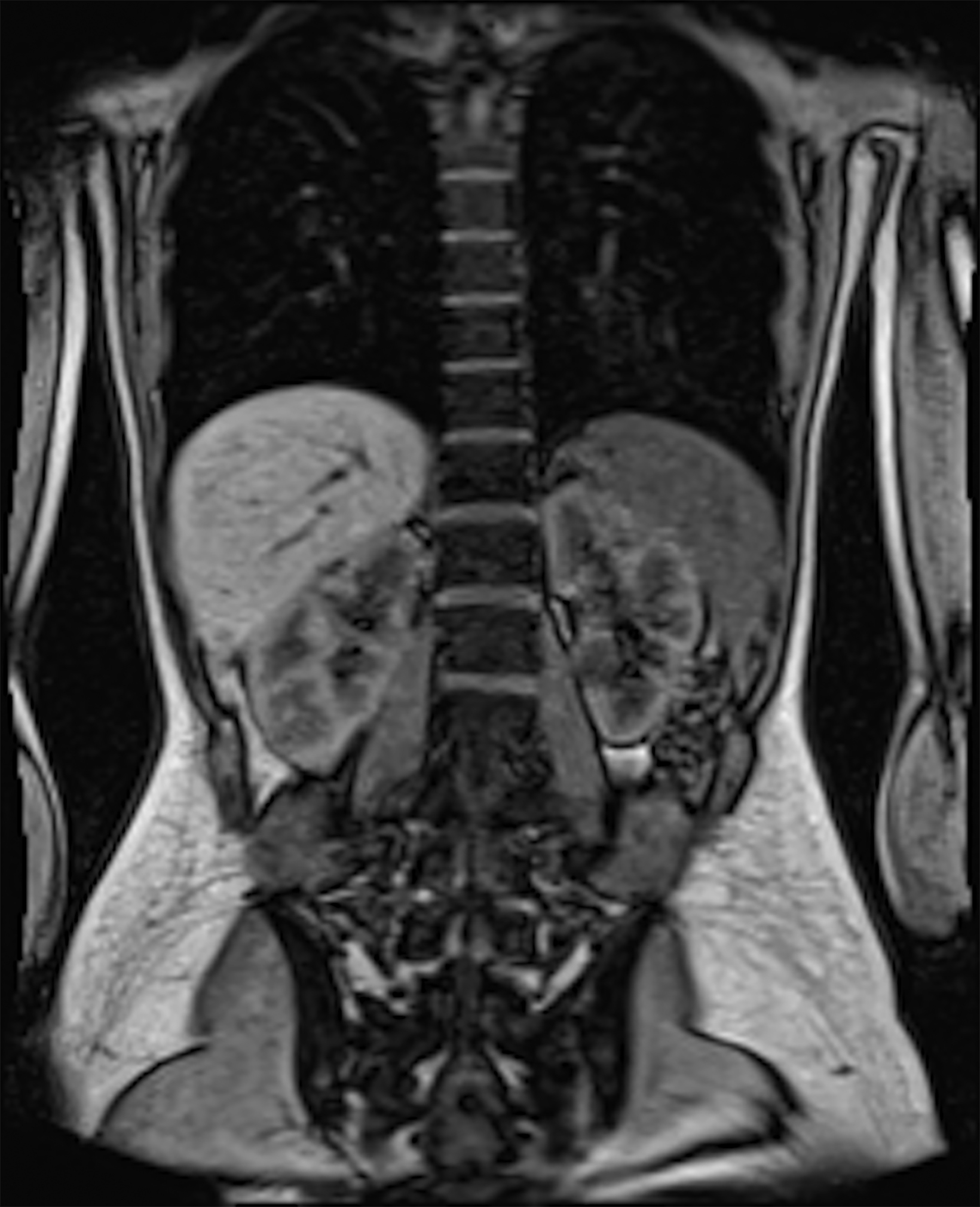
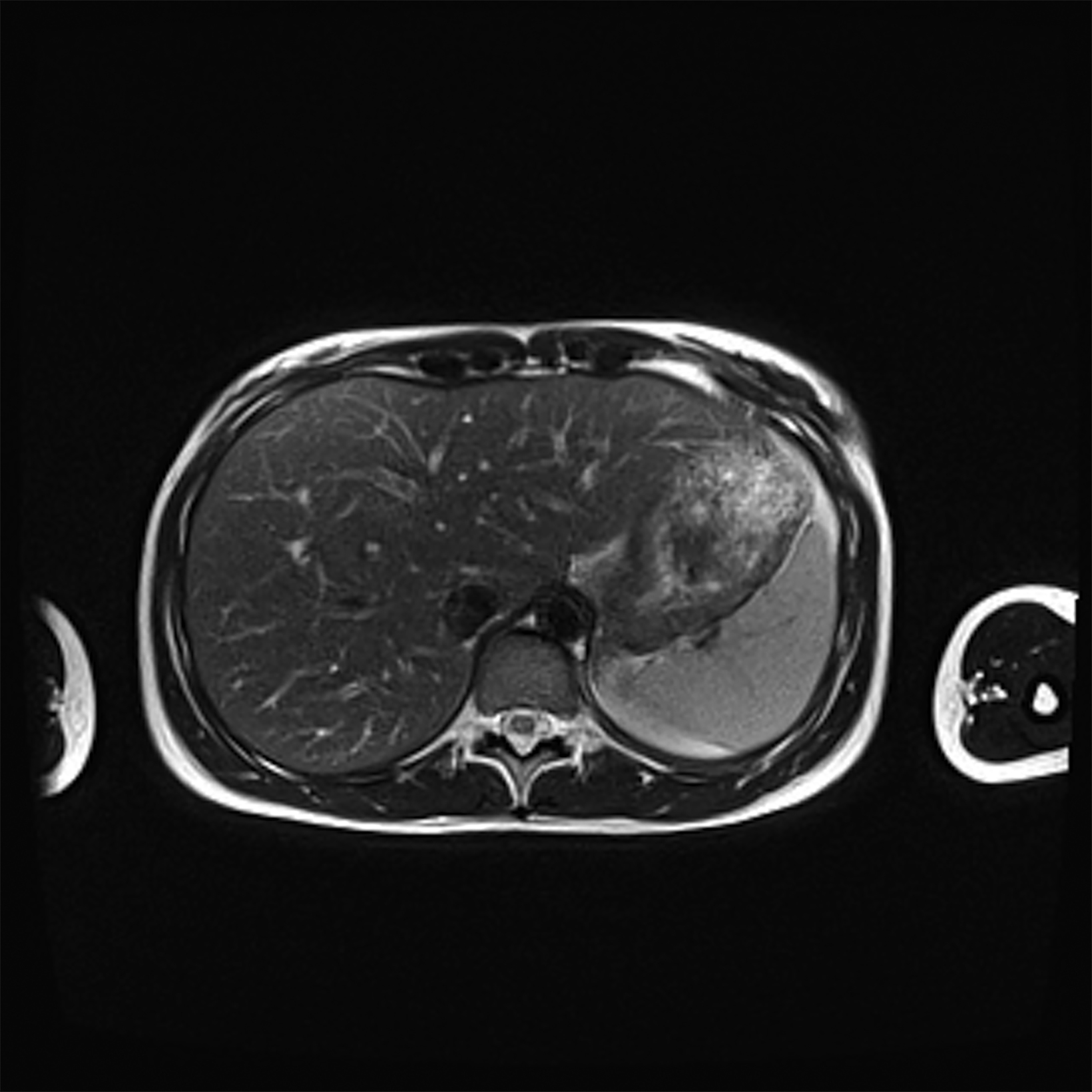
Low-field imaging may be a preferable MRI technique for joint imaging,42 particularly when considering the reduced artifacts around implants. A study by Reil, et al, showed that the 0.2T MRI method has low sensitivity but high specificity for articular cartilage lesions.43 Field strengths <100mT have demonstrated even more improvement in metallic artifacts.44 Low-field MRI has also been applied for diagnostic abdominal MRI and for quantification of iron overload (Figure 3).45,46
One important factor in utilizing imaging modalities in clinical settings is their accessibility. Hyperfine Research’s Swoop portable system makes MRI available and accessible.17 This novel system has beneficial utility in time-sensitive and point-of-care settings such as intensive care units, emergency rooms, and mobile stroke units.47 Portable MRI has the potential for neuroimaging of brain injuries, intracranial hemorrhage, hydrocephalus, and midline shift in high-risk clinical settings.48,49 Portable MRI systems may significantly expand the role of MRI in a variety of settings.
Ultra-high Field MRI
While the majority of MRI examinations are currently performed at static magnetic fields of 1.5 and 3T, systems with higher fields up to 10.5T have been used for research purposes, particularly in neuroscience, since the late 1990s.50–52 In 1998, the first human MRI study using UHF (MRI scanners with field strength of ≥ 7T) was performed at Ohio State University on an 8T scanner.53 The results were remarkable, leading to the installation of several UHF research scanners across the world. Moreover, after the FDA declaration in 2014 classified MRI up to 8T as a negligible risk, in 2017, the first 7T system with FDA 510(k) clearance hit the market (Magnetom Terra system by Siemens Medical Solutions Inc., Erlangen, Germany) for clinical use, confined to examination of the head, and upper and lower extremities (neuro and MSK).54,55
As of 2018, sixty-two 7T human scanners were installed worldwide, and eight more 7T scanners were on order. Five 9.4T human scanners had been installed in Minnesota, Chicago, Tübingen, Jülich, and Maastricht. Moreover, several human scanners >10T had been installed or were in development, which included whole-body 10.5T (Minnesota), head-only 11.7T (Bethesda), whole-body 11.7 T (Paris), and 14T (Heidelberg).56–58
Advantages and Disadvantages of UHF MRI scanners
Improved SNR is the most commonly cited reason for justifying the expenditure and effort of obtaining higher magnetic field scanners. Many MRI applications can benefit from the increase in SNR associated with higher magnetic fields through better spatial resolution or, in the case of dynamic processes, higher temporal resolution.2 Higher SNR at UHF MRI also allows for higher resolution and/or high b-value acquisition on DWI.59 SNR is not always the most important measure to assess the potential of MRI to identify lesions of interest; CNR is a more precise parameter for this purpose.2
A wide range of imaging features are affected by field strength, and many MRI applications benefit from the resultant increase in both SNR and CNR. Enhanced contrast in susceptibility-weighted imaging (SWI) at higher fields is due to increased phase shifts at higher Larmor frequencies, and stronger blood oxygen level-dependent (BOLD) contrast on functional MRI (fMRI) is due to greater susceptibility (T2*) effects at higher field strengths.60 Improved CNR also leads to improved magnetic resonance spectroscopy (MRS) SNR and enhanced separation of MRS peaks/spectral resolution.61 Moreover, T1 is longer and T2 is shorter at higher fields, resulting in improved chemical exchange saturation transfer (CEST) imaging.62, 63
Structural MRI is based on the 1H MR signal from water molecules (H2O). Proton MRS (1H MRS) plays a complementary role to conventional MRI by delivering a plethora of biochemical and metabolic data.64 Multi-nuclear MRI captures MR signal from nuclei other than hydrogen such as sodium-23 (23Na), phosphorus-31 (31P), fluorine-19 (19F), and carbon-13 (13C). Because these nuclei are involved in many biological processes, they provide metabolic and functional information beyond that provided by hydrogen alone. Because these nuclei occur at a relatively low concentration, the increased signal available from UHF MRI systems has improved the ability to image using these physiologically relevant nuclei.65
Some of the challenges of UFH include inhomogeneities of the B0 field, inhomogeneities of the radiofrequency (RF) excitation, shorter T2 relaxation, and increased SAR.66 Technical developments such as improved coil designs, higher-order B0 shimming, radiofrequency pulse shaping (B1 shimming), and parallel transmission techniques can address some of these challenges (2), but others like shorter T2 and increased SAR are physical limitations that cannot be solved through engineering.
Moreover, even though UHF provides images with higher resolution and enhanced differentiation of tissue types, motion artifacts are more likely to appear on high-resolution MRI.67 A variety of prospective and retroactive motion correction approaches have been applied to address this concern.68,69 The increased occurrence of signal dropout in SWI at UHF, which is advantageous for certain clinical applications like the detection of microbleeds and other small lesions, negatively impacts image quality in certain regions of interest near air-tissue interfaces.2 Geometric distortions in EPI BOLD and diffusion-weighted imaging are also increased at UHF, which can offset gains in spatial or temporal resolution without special post-processing steps.70,71
Physiologically, at UHF there are more frequent occurrences of RF heating of tissue foci. Therefore, to stay compliant with regulatory standards, imaging sequence parameters must frequently be modified, such as repetition time lengthening or lowering the number of captured slices, which makes clinical utilization of UHF extremely challenging.2 The physiologic effects of higher magnetic fields are time-dependent and can be classified as transient or permanent. Transient effects vanish either promptly or in a reasonably short amount of time post-exposure and affect the daily function of body systems, whereas permanent consequences cause prolonged health concerns.
Some of the transient physiologic effects of UHF MRI systems include metallic taste, nausea, dizziness, vertigo, sweating, and magnetophosphenes.2,72 With the development of UHF systems, concerns exist that uncomfortable transient symptoms might impact patients’ desire to undergo imaging studies. Therefore, several studies comprehensively investigated these outcomes on 7T and 9T systems and reported that even though these effects were higher in high magnetic fields compared to lower fields, they had no major impact on the technique’s acceptability.72,73 However, the most observed causes of discomfort in patients were study duration, the requirement to lie motionless, and acoustic noise, which were irrelevant to magnetic field strength.73
Another important effect is electrocardiogram (ECG) alterations under high magnetic field systems, marked by high T-waves.74 Such ECG variations can make it challenging to interpret UHF imaging sequences that rely on cardiac triggering or gating. There has also been debate in the literature regarding whether UHF systems may impact blood pressure as a result of the extra effort to circulate blood through the high magnetic field. Initial modeling studies suggested this would pose a possible obstacle to 10T or higher MRI examinations; however, subsequent studies on humans and animals assessing blood pressure variations when exposed to high magnetic fields indicated no meaningful consequences at fields as high as 9.4T or 10.5T.75–77
Multiple investigational studies have also focused on temporary cognitive consequences, with some studies concluding no correlation while others reporting a positive correlation.78–81 Even though occupational exposure to magnetic fields is often far lower than the high field at the isocenter of the magnet, employees who are involved in maintaining or cleaning the interior of the machine may be subjected to high fields. Studies are ongoing regarding these effects and approaches to minimize potential negative outcomes.
Long-term or permanent effects of high magnetic fields are mainly assessed by evaluating DNA damage. According to the International Commission on Non-Ionizing Radiation Protection (ICNIRP) and most recent scientific reports and reviews, the possible effects of MRI on DNA are far lower than ionizing radiation outcomes.82,83 Few investigations have studied the effect of MRI on DNA, with controversial findings.84,85 In-vivo and in-vitro investigations on strength fields as high as 7T and large study populations found no substantial DNA alterations.84,86,87 MRI has been widely used to evaluate many patients, with an outstanding record for safety, even at 7T. Studies with larger patient populations are warranted to evaluate the safe utilization of even higher magnetic field strengths.
In addition, UHF scanners have much higher initial and operational expenses than non-UHF scanners. Low manufacturing volumes, as well as the expenditures involved in creating a strong magnetic field, such as magnets and conductors, greatly increase the cost of these scanners. The higher magnetic field contributes significantly to the scanners’ operational costs by increasing shielding and site preparation expenditures.88 Moreover, due to the current lack of efficient body coils for UHF scanners, transmit/receive coils must be utilized, which further increases the overall cost.89
Clinical Applications of UHF MRI
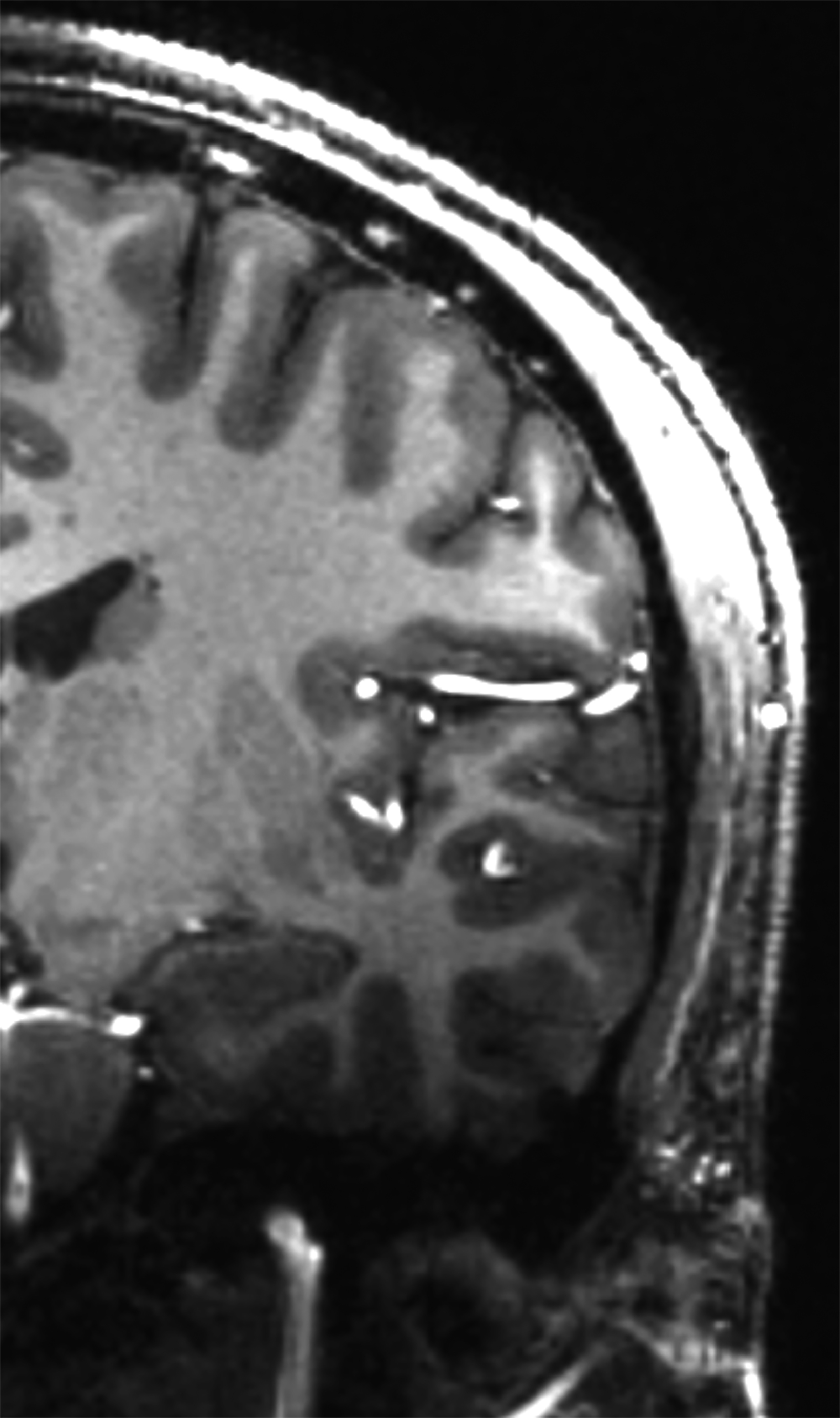
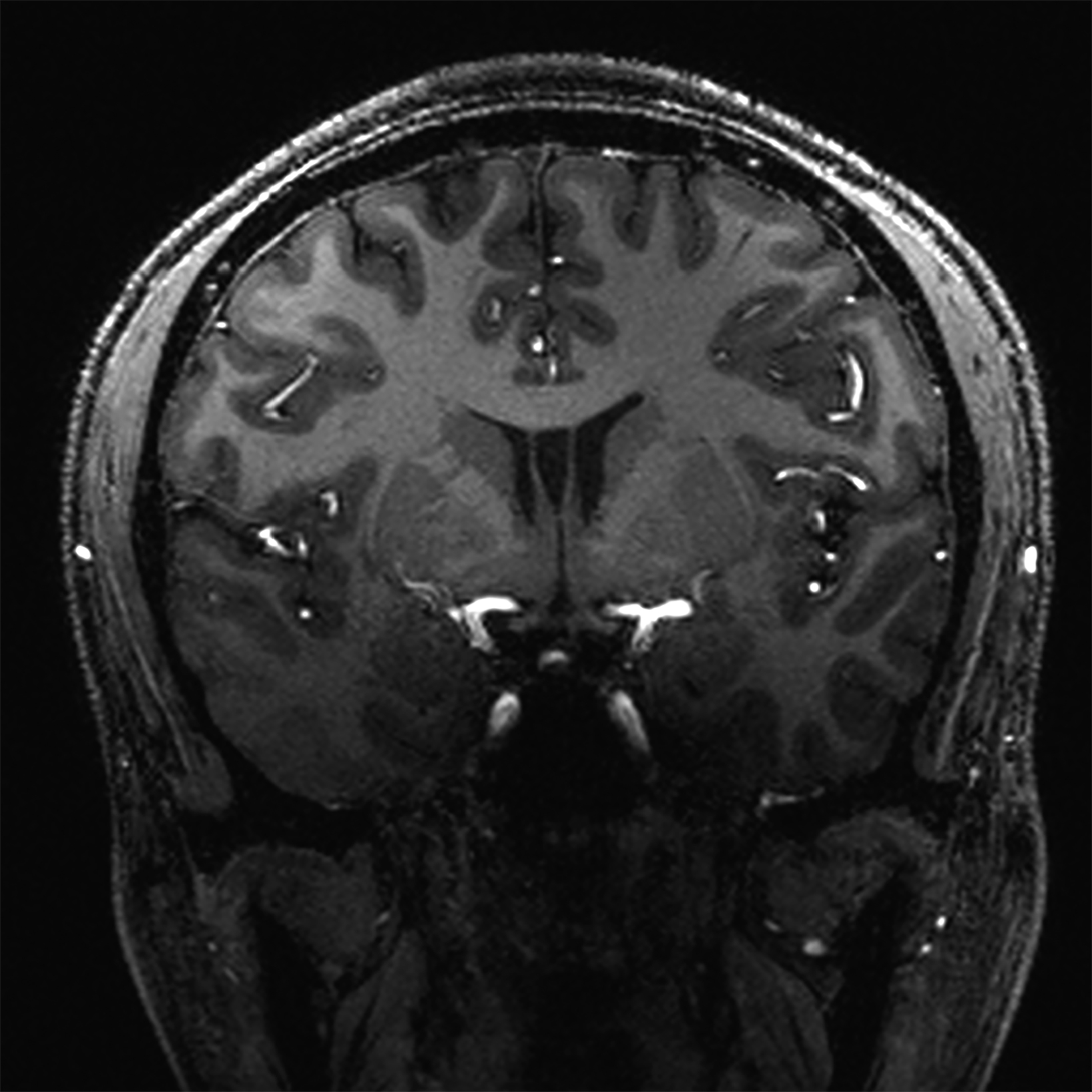
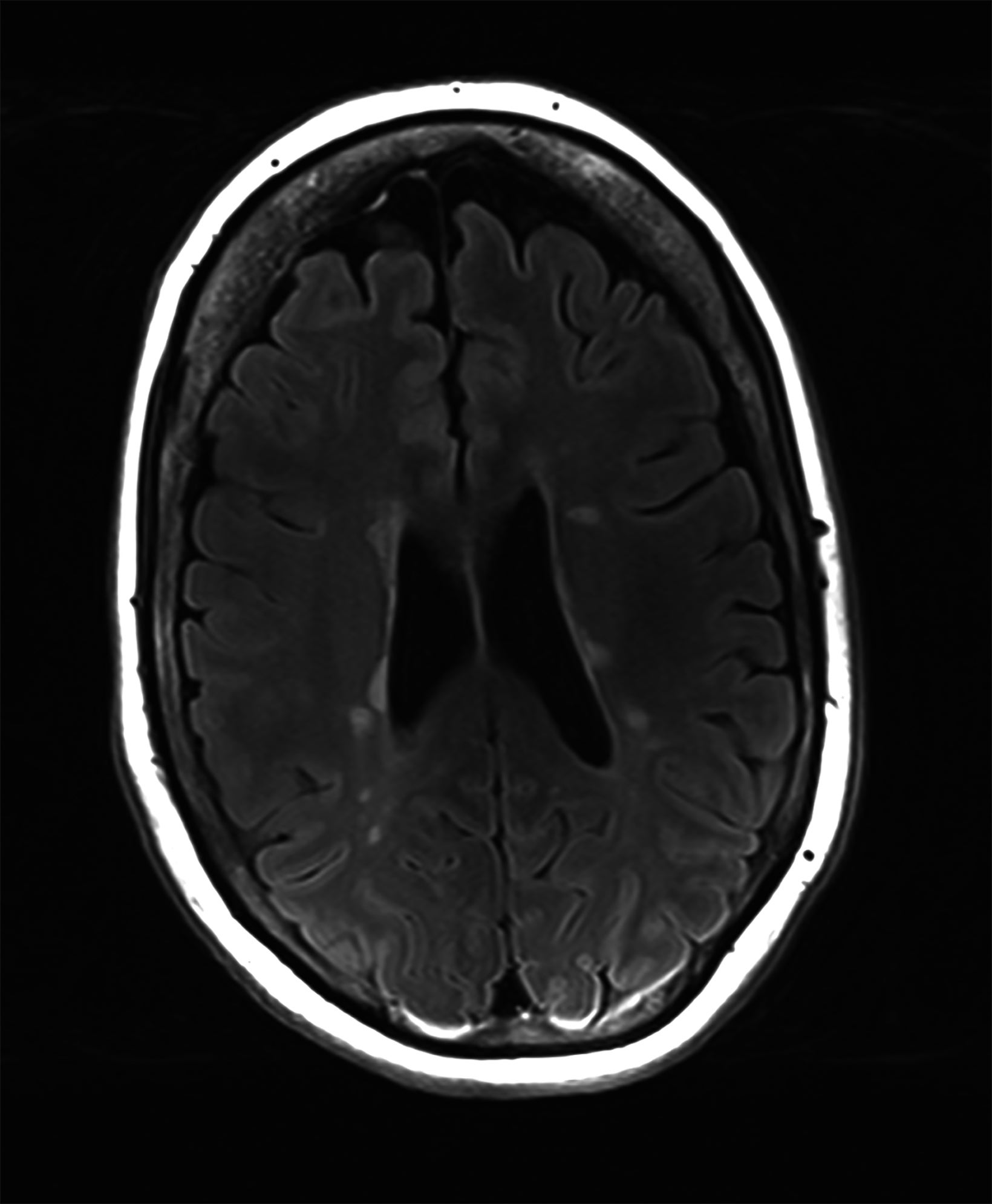
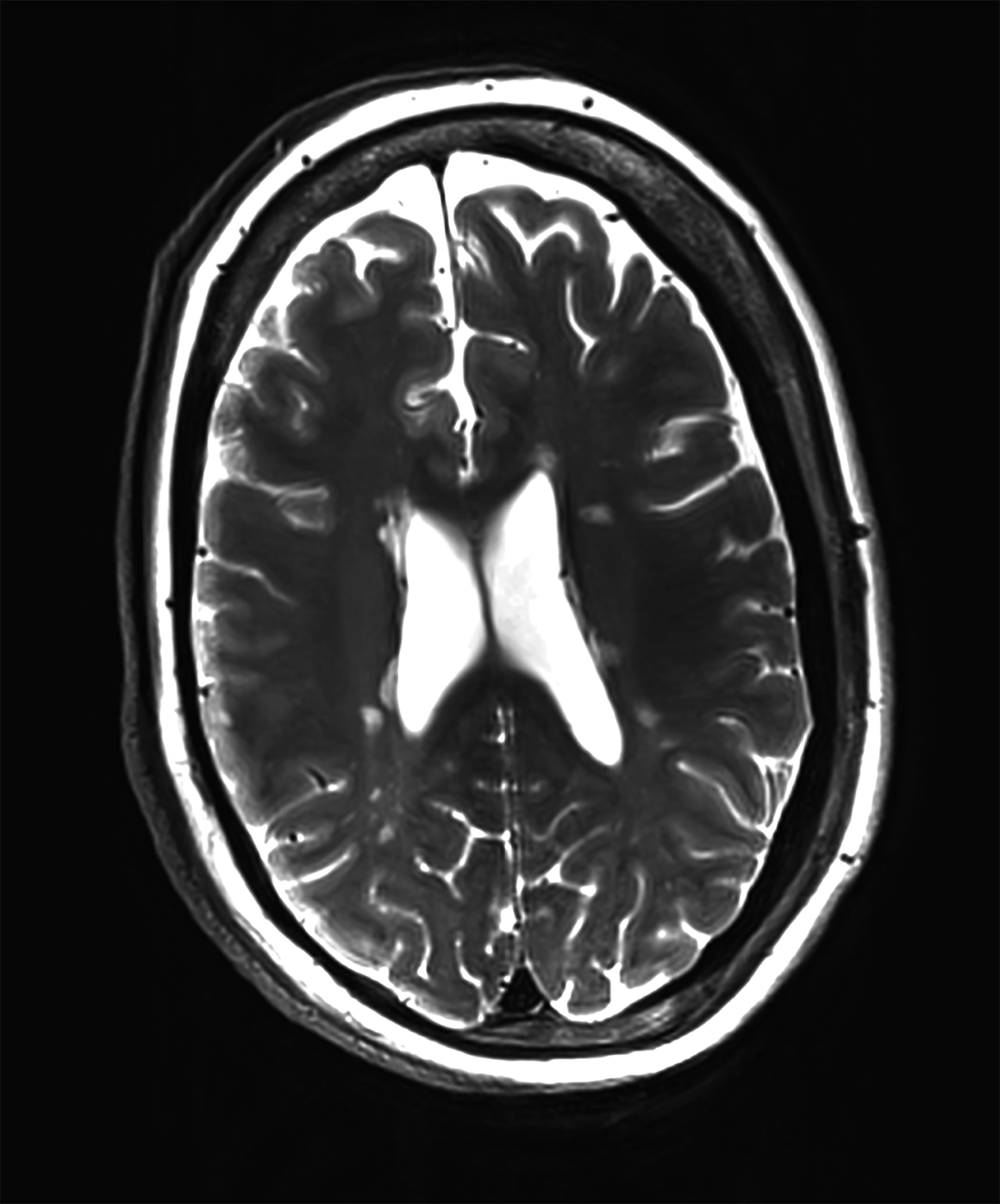
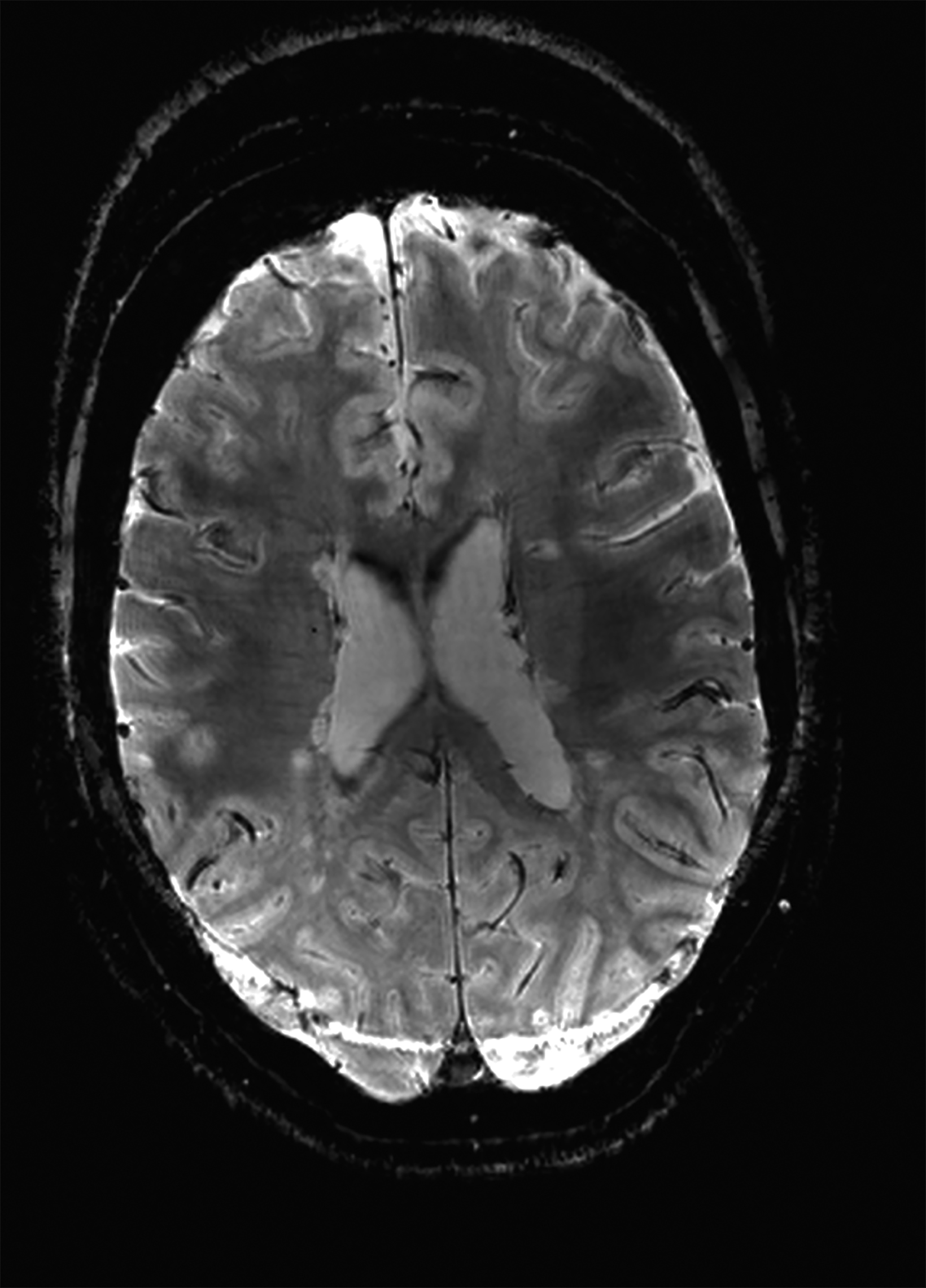
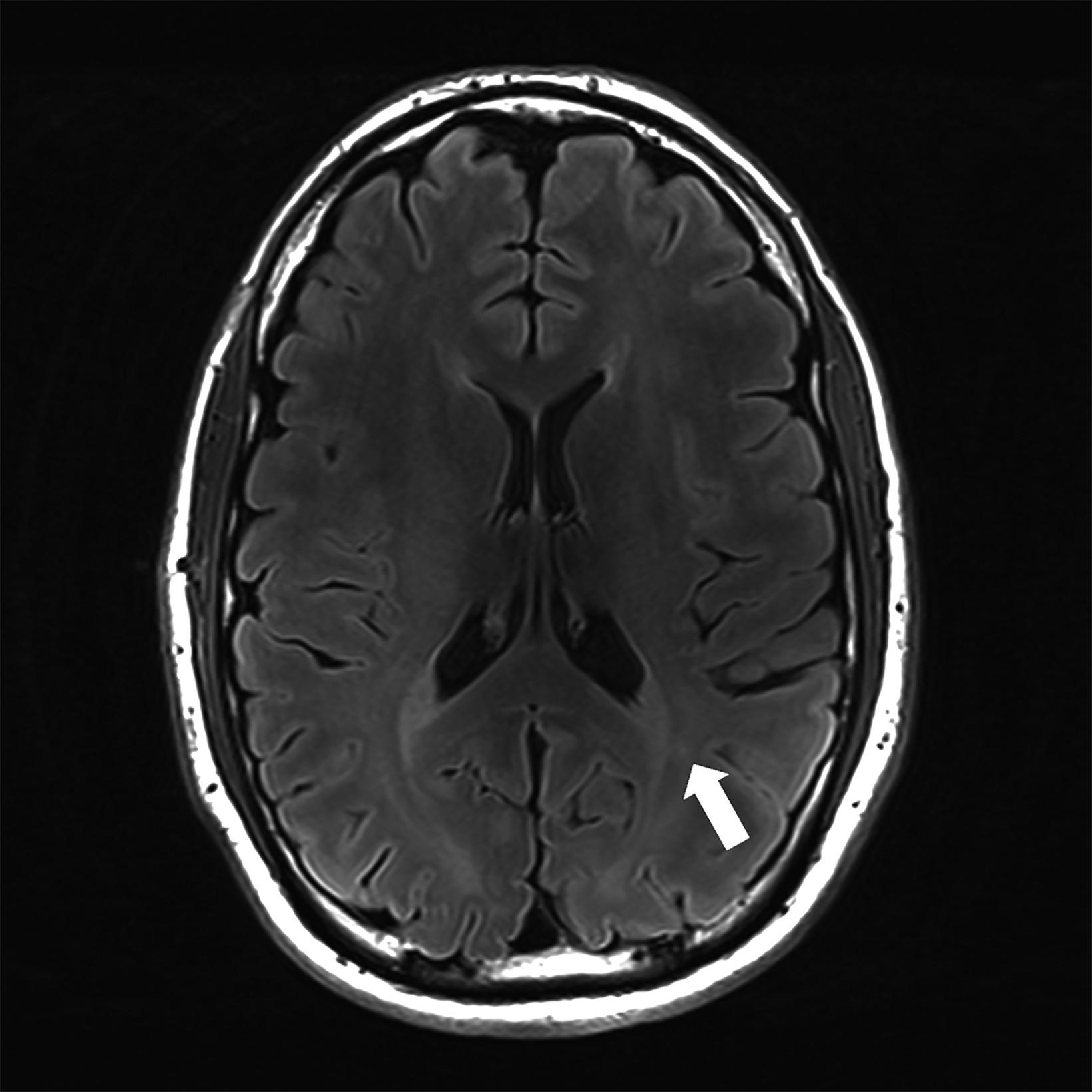
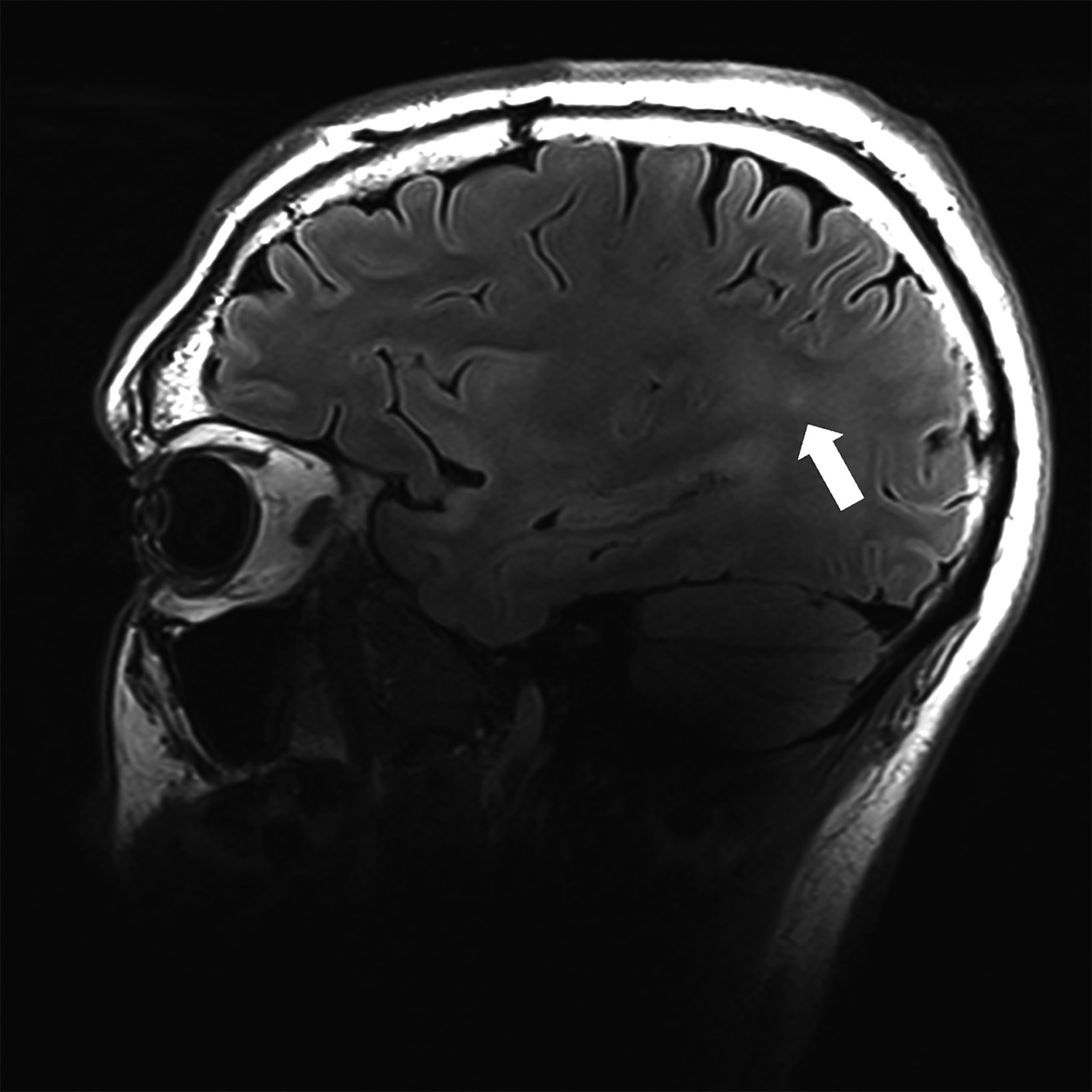
There is growing data on the potential of UHF MRI in improving diagnoses and clinical management of several pathologies. In neuroradiology, UHF MRI’s higher resolution has been shown to enhance the representation of detailed and complex anatomical structures, such as cranial nerves and structures of the brain stem (Figures 4, 5, 6).90,91 UHF MRI enables better detection of arteriovenous malformations (AVMs) and cerebral microaneurysms with diameters of 1 mm or smaller.92,93 In individuals with stroke, small infarctions not identified on 1.5T MRI were detected on 8T MRI images utilizing weighted gradient echo (GRE) and rapid acquisition with relaxation enhancement.94 For patients with multiple sclerosis, recent studies have shown increased diagnostic confidence using UHF MRI compared to 1.5T or 3T (Figures 7 and 8).95 7T MRI has also shown utility in neurodegenerative diseases such as Alzheimer’s disease by providing an accurate volumetric evaluation of hippocampal subfields and the entorhinal cortex.96 Moreover, UHF MRI at 7T could be used to better visualize the substantia nigra and its inner structure in patients with Parkinson’s disease.97 In epileptic patients, UHF MRI systems enable better visualization of epileptic foci by improving SNR and CNR.98,99 The higher spatial resolution of 7T scanners provides the opportunity to better detect and distinguish brain tumors from surrounding tissues.100
In addition to neuroradiology applications, 7T scanners have been approved for a number of musculoskeletal indications. Trabecular bone microarchitecture has been evaluated by using GRE-based pulse sequences and high-resolution spin-echo applied to 7T, leading to enhanced visualization of tissues.101 When the diagnostic performance of 7T was compared to that of 3T in the diagnosis of joint diseases in individuals with knee pain, 7T not only showed considerable improvements in SNR but also enhanced overall diagnostic confidence, particularly for the evaluation of small joint structures.102 T2 and T2* mapping at 7T have demonstrated a positive correlation with water content on the evaluation of cartilage collagen matrix integrity.103 Moreover, T1ρ imaging at 7T MR, which is utilized in the evaluation of proteoglycan content in cartilage, has led to improved sensitivity with the same resolution compared to 3T MRI scanners.104 The feasibility of spine MRI at UHF has been described in prior studies, and recent advances in innovative coil technology enhance 7T spine MR by increasing SNR.105,106
In comparison to neuroimaging and MSK applications, a limited number of studies have focused on the application of UHF MRI in abdominal and thoracic imaging. Ladder et al. reported that the improved SNR and CNR at 7T seen in a variety of abdominal organs allowed the identification of minor pathologies that would otherwise be undetected at lower field strengths.107 The feasibility of good-quality images of the kidneys has been shown at 7T, especially T1 GRE MRI.108 Umutlu, et al, demonstrated that contrast-enhanced MRI cholangiopancreatography (MRCP) is feasible at UHF. The authors reported equivalent results compared to 3T MRCP.109 In prostate MRI, T2 and DWI (± dynamic contrast-enhanced) are the most frequently used sequences. By applying high-resolution T2 TSE MR at 7T, cancerous lesions in both the transition zone and the peripheral zone could be distinguished.110 7T has also shown feasibility in the assessment of breast cancer lesions and in cardiac MRI.111–113
Discussion
Developing a successful MRI service in a healthcare setting is a complicated and multifaceted process that includes financial considerations for equipment purchases, maintenance, and upgrade contracts, as well as operational considerations for safety, quality assurance, and workflow. Additionally, an MRI technologist with expertise in image acquisition and operating logistics specific to the scanner is also required. Therefore, it is crucial to comprehend the opportunities and challenges of each scanner, and the selection of an MRI scanner should consider all of these factors.
Academic Institutions and Research Facilities
Increased SNR and CNR, high spatial resolution, and improved image quality of UHF systems provide the opportunity for better visualization of detailed and complex pathologies. This feature makes UHF MRI ideal for understanding the pathophysiology and investigating the natural history of various disease processes.
On the other hand, low-field MRI systems have great potential for research, particularly related to new applications of these systems and clinical validation of emerging sequences and techniques. Capabilities unique to low-field settings, such as their mobility, increase access and make imaging investigations possible in previously uninvestigated situations, such as the ICU.
Community-based Inpatient Facilities and Hospitals
Mainstream imaging studies are performed on 1.5-3T scanners, as these are the most standardized in terms of acquisition protocols and radiology technologists’ familiarity. Moreover, radiologists are more accustomed to interpreting the images acquired by these scanners.
Low-field scanners can help with intraoperative imaging in community-based inpatient hospitals. The intraoperative application of these scanners in such settings facilitates multidisciplinary communication and provides the opportunity for better patient care in situations requiring urgent and emergent clinical decision-making. Additionally, the portability feature can alleviate many logistical
challenges for patient transport in and out of specific hospital wards. The low cost of low-field scanners might be especially beneficial for inpatient facilities looking to grow their MRI fleet. Many of the routine exams conducted in radiology today do not benefit from the features afforded by higher fields such as 3T. These exams might be offloaded to a less expensive low-field system, freeing up higher field strength scanners for applications that benefit from them.
The presence of UHF scanners in the MRI service of a community-based inpatient facility could potentially improve patient care in clinical scenarios that require higher resolution imaging for enhanced interpretation, accurate diagnosis, and therefore optimal management. However, the relatively limited benefits in this setting need to be balanced with the operational challenges and the cost associated with the maintenance of these scanners.
Private Practice Radiology Settings
Low-field MRI systems are well suited for private practice settings due to their low cost and ease of maintenance. As the cost of an MRI scanner is directly associated with the magnet strength, low-field scanners have lower initial purchase costs. Moreover, owing to decreased energy consumption and safety considerations, these systems require less maintenance cost.
Low-field MRI systems may also broaden the potential patient population for radiology private practice settings; for example, claustrophobic patients and individuals who have difficulty staying in closed MRI systems due to their body habitus may prefer radiology practices that offer more open configuration MRI imaging options. Additionally, the reduced noise associated with low-field systems may result in less anxiety and an improved imaging experience, enhancing value-based care.
Conclusion
Selecting an MRI scanner for a private practice setting, tertiary care institution, or research center is a nuanced task. In order to make an informed decision, it is critical to evaluate the imaging scenarios for which the scanner is intended and in which it excels, as well as a comprehensive understanding of the strengths and limitations of various magnetic fields.
References
- >Barisano G, Sepehrband F, Ma S, et al. Clinical 7 T MRI: Are we there yet? A review about magnetic resonance imaging at ultra-high field. Br J Radiol. 2019;92(1094):20180492.
- Ladd ME, Bachert P, Meyerspeer M, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1-50.
- Alvarez-Linera J. 3T MRI: advances in brain imaging. Eur J Radiol. 2008;67(3):415-426.
- Marques JP, Simonis FFJ, Webb AG. Low-field MRI: An MR physics perspective. J Magn Reson Imaging. 2019;49(6):1528-1542.
- Sarracanie M, Salameh N. Low-Field MRI: How Low Can We Go? A Fresh View on an Old Debate. Frontiers in Physics. 2020;8. doi:10.3389/fphy.2020.00172
- >Przyborowska P, Adamiak Z, Holak P, Zhalniarovich Y. Comparison of Feline Brain Anatomy in 0.25 and 3 Tesla Magnetic Resonance Images. Anat Histol Embryol. 2017;46(2):178-186.
- >Fischer A. Revisiting the Physics behind MRI and bhe Opportunities bhab Lower Field. https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/395bbaf69 3bcd08d/57c490bfbb34/sh-1624_MAGNETOM_Flash_Free-Max_Edition_K4_fischer.pdf
- >Choquet P, Breton E, Goetz C, Marin C, Constantinesco A. Dedicated low-field MRI in mice. Physics in Medicine and Biology. 2009;54(17):5287-5299. doi:10.1088/0031-9155/54/17/014
- Klein HM. Clinical Low Field Strength Magnetic Resonance Imaging: A Practical Guide to Accessible MRI. Springer; 2015.
- >Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019;293(2):384-393.
- >Campbell-Washburn AE, Jiang Y, Körzdörfer G, Nittka M, Griswold MA. Feasibility of MR fingerprinting using a high-performance 0.55 T MRI system. Magnetic Resonance Imaging. 2021;81:88-93. doi:10.1016/j.mri.2021.06.002
- >Hamilton-Basich M. FDA Clears Siemens Healthineers Magnetom Free.Max 80 cm MR Scanner. AXIS Imaging News. Published online July 7, 2021. https:// search.proquest.com/openview/8159ee793b68825befd794cfc51b2de1/1?pq-origsite=gscholar&cbl=2037571
- Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl Radiat Oncol. 2019;18:98-101.
- Cooley CZ, McDaniel PC, Stockmann JP, et al. A portable scanner for magnetic resonance imaging of the brain. Nat Biomed Eng. 2021;5(3):229-239.
- >O’Reilly T, Teeuwisse WM, de Gans D, Koolstra K, Webb AG. In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array. Magn Reson Med. 2021;85(1):495-505.
- Sarracanie M, LaPierre CD, Salameh N, Waddington DEJ, Witzel T, Rosen MS. Low-Cost High-Performance MRI. Sci Rep. 2015;5:15177.
- >Hamilton-Basich M. Hyperfine Receives FDA Clearance for Portable MRI Technology. AXIS Imaging News; Overland Park. Published online August 16, 2020. https://search.proquest.com/openview/5c609d9987037cd3e2d4e866bfbc9ad0/1?pq-origsite=gscholar&cbl=2037571.
- >Panther A, Thevathasen G, Connell IRO, et al. A dedicated head-only MRI scanner for point-of-care imaging. In: ISMRM 27th Annual Meeting & Exhibition. 2019. https://www.synaptivemedical.com/wp-content/uploads/2020/04/a-dedicated-head-only-mri-scanner-for-point-of-care-imaging.pd
- >Chiragzada S, Hellman E, Michael D, Narayanan R, Nacev A, Kumar D. Initial phantom studies for an office-based low-field MR system for prostate biopsy. Int J Comput Assist Radiol Surg. 2021;16(5):741-748.
- Ghazinoor S, Crues JV 3rd, Crowley C. Low-field musculoskeletal MRI. J Magn Reson Imaging. 2007;25(2):234-244.
- Klein HM. Low-Field Magnetic Resonance Imaging. Rofo. 2020;192(6):537-548.
- Schick F, Pieper CC, Kupczyk P, et al. 1.5 vs 3 Tesla Magnetic Resonance Imaging: A Review of Favorite Clinical Applications for Both Field Strengths-Part 1. Invest Radiol. 2021;56(11):680-691.
- Hori M, Hagiwara A, Goto M, Wada A, Aoki S. Low-field magnetic resonance imaging: Its history and renaissance. Invest Radiol. 2021;56(11):669-679.
- >Heiss R, Nagel AM, Laun FB, Uder M, Bickelhaupt S. Low-Field Magnetic Resonance Imaging: A New Generation of Breakthrough Technology in Clinical Imaging. Invest Radiol. 2021;56(11):726-733.
- >Khodarahmi I, Bonham LW, Weiss CR, Fritz J. Needle Heating During Interventional Magnetic Resonance Imaging at 1.5- and 3.0-T Field Strengths. Invest Radiol. 2020;55(6):396-404.
- >Runge VM, Heverhagen JT. Advocating the Development of Next-Generation, Advanced-Design Low-Field Magnetic Resonance Systems. Invest Radiol. 2020;55(12):747-753.
- >Basar B, Sonmez M, Yildirim DK, et al. Susceptibility artifacts from metallic markers and cardiac catheterization devices on a high-performance 0.55 T MRI system. Magn Reson Imaging. 2021;77:14-20.
- Webb AG. Magnetic Resonance Technology: Hardware and System Component Design. Royal Society of Chemistry; 2016.
- Buckley BW, MacMahon PJ. Radiology and the Climate Crisis: Opportunities and Challenges—Radiology In Training. Radiology. 2021;300(3):E339-E341. doi:10.1148/radiol.2021210851
- Keller PJ, Hunter WW Jr, Schmalbrock P. Multisection fat-water imaging with chemical shift selective presaturation. Radiology. 1987;164(2):539-541.
- Frahm J, Haase A, Hänicke W, Matthaei D, Bomsdorf H, Helzel T. Chemical shift selective MR imaging using a whole-body magnet. Radiology. 1985;156(2):441- 444. doi:10.1148/radiology.156.2.4011907
- Campbell-Washburn AE. 2019 American Thoracic Society BEAR Cage Winning Proposal: Lung Imaging Using High-Performance Low-Field Magnetic Resonance Imaging. Am J Respir Crit Care Med. 2020;201(11):1333-1336.
- Bhattacharya I, Ramasawmy R, Javed A, et al. Assessment of Lung Structure and Regional Function Using 0.55 T MRI in Patients With Lymphangioleiomyomatosis. Invest Radiol. 2022;57(3):178-186.
- Bhattacharya I, Ramasawmy R, Javed A, et al. Oxygen-enhanced functional lung imaging using a contemporary 0.55 T MRI system. NMR in Biomedicine. 2021;34(8). doi:10.1002/nbm.4562
- Javed A, Ramasawmy R, O’Brien K, et al. Self-gated 3D stack-of-spirals UTE pulmonary imaging at 0.55T. Magn Reson Med. 2022;87(4):1784-1798.
- Campbell-Washburn AE, Suffredini AF, Chen MY. High-Performance 0.55-T Lung MRI in Patient with COVID-19 Infection. Radiology. 2021;299(2):E246-E247. doi:10.1148/radiol.2021204155
- Heiss R, Grodzki DM, Horger W, Uder M, Nagel AM, Bickelhaupt S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn Reson Imaging. 2021;76:49-51.
- Campbell-Washburn AE, Malayeri AA. T2-weighted lung imaging using a 0.55-T MRI system. Radiology. Published online 2021. https://pubs.rsna.org/doi/ abs/10.1148/ryct.2021200611
- Bandettini WP, Shanbhag SM, Mancini C, et al. Evaluation of myocardial infarction by cardiovascular magnetic resonance at 0.55-T compared to 1.5-T. JACC Cardiovasc Imaging. 2021;14(9):1866-1868.
- Bandettini WP, Patricia Bandettini W, Shanbhag SM, et al. A comparison of cine CMR imaging at 0.55 T and 1.5 T. Journal of Cardiovascular Magnetic Resonance. 2020;22(1). doi:10.1186/s12968-020-00618-y
- Amin EK, Campbell-Washburn A, Ratnayaka K. MRI-Guided Cardiac Catheterization in Congenital Heart Disease: How to Get Started. Curr Cardiol Rep. Published online February 2, 2022. doi:10.1007/s11886-022-01659-8
- Tavernier T, Cotten A. High- Versus Low-Field MR Imaging. Radiologic Clinics of North America. 2005;43(4):673-681. doi:10.1016/j.rcl.2005.02.001
- Riel KA, Reinisch M, Kersting-Sommerhoff B, Hof N, Merl T. 0.2-Tesla magnetic resonance imaging of internal lesions of the knee joint: a prospective ar- throscopically controlled clinical study. Knee Surgery, Sports Traumatology, Arthroscopy. 1999;7(1):37-41. doi:10.1007/s001670050118
- Van Speybroeck CDE, O’Reilly T, Teeuwisse W, Arnold PM, Webb AG. Characterization of displacement forces and image artifacts in the presence of passive medical implants in low-field (<100 mT) permanent magnet-based MRI systems, and comparisons with clinical MRI systems. Physica Medica. 2021;84:116-124. doi:10.1016/j.ejmp.2021.04.003
- Chandarana H, Bagga B, Huang C, et al. Diagnostic abdominal MR imaging on a prototype low-field 0.55 T scanner operating at two different gradient strengths. Abdom Radiol (NY). 2021;46(12):5772-5780.
- Campbell-Washburn AE, Mancini C, Conrey A, et al. Evaluation of hepatic iron overload using a contemporary 0.55 T MRI system. J Magn Reson Imaging. 2022;55(6):1855-1863.
- Deoni SCL, Medeiros P, Deoni AT, et al. Development of a mobile low-field MRI scanner. Sci Rep. 2022;12(1):5690.
- Sheth KN, Mazurek MH, Yuen MM, et al. Assessment of Brain Injury Using Portable, Low-Field Magnetic Resonance Imaging at the Bedside of Critically Ill Patients. JAMA Neurol. Published online September 8, 2020. doi:10.1001/jamaneurol.2020.3263
- Shah J, Cahn B, By S, et al. Portable, Bedside, Low-field Magnetic Resonance Imaging in an Intensive Care Setting for Intracranial Hemorrhage (270). Neurology. 2020;94(15 Supplement). Accessed March 5, 2022. https://n.neurology.org/content/94/15_Supplement/270.abstract
- Moser E, Laistler E, Schmitt F, Kontaxis G. Ultra-High Field NMR and MRI—The Role of Magnet Technology to Increase Sensitivity and Specificity. Frontiers in Physics. 2017;5. doi:10.3389/fphy.2017.00033
- Robitaille PML, Abduljalil AM, Kangarlu A. Ultra high resolution imaging of the human head at 8 tesla: 2K× 2K for Y2K. J Comput Assist Tomogr. 2000;24(1):2-8.
- Yacoub E, Shmuel A, Pfeuffer J, et al. Imaging brain function in humans at 7 Tesla. Magn Reson Med. 2001;45(4):588-594.
- Robitaille PM, Abduljalil AM, Kangarlu A, et al. Human magnetic resonance imaging at 8 T. NMR Biomed. 1998;11(6):263-265.
- Fda U. Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices—Guidance for Industry and Food and Drug Administration Staff. Published online 2014.
- Office of the Commissioner. FDA clears first 7T magnetic resonance imaging device. U.S. Food and Drug Administration. Published October 12, 2017. Accessed February 19, 2022. https://www.fda.gov/news-events/press-announcements/fda-clears-first-7t-magnetic-resonance-imaging-device
- Ertürk MA, Wu X, Eryaman Y, et al. Toward imaging the body at 10.5 tesla. Magn Reson Med. 2017;77(1):434-443.
- Le Bihan D, Schild T. Human brain MRI at 500 MHz, scientific perspectives and technological challenges. Supercond Sci Technol. 2017;30(3):033003.
- Polimeni JR, Uludağ K. Neuroimaging with ultra-high field MRI: Present and future. Neuroimage. 2018;168:1-6.
- Wu W, Miller KL. Image formation in diffusion MRI: A review of recent technical developments. J Magn Reson Imaging. 2017;46(3):646-662.
- Abduljalil AM, Schmalbrock P, Novak V, Chakeres DW. Enhanced gray and white matter contrast of phase susceptibility-weighted images in ultra-high-field magnetic resonance imaging. J Magn Reson Imaging. 2003;18(3):284-290.
- Polonara G, Scarabino T, Salvolini U. Basics and New Frontiers of MR Spectroscopy with High Tesla. Rivista di Neuroradiologia. 2003;16(2_suppl_part2):144-148.
- de Bazelaire C, Rofsky NM, Duhamel G, et al. Combined T2* and T1 measurements for improved perfusion and permeability studies in high field using dynamic contrast enhancement. Eur Radiol. 2006;16(9):2083-2091.
- Dula AN, Smith SA, Gore JC. Application of Chemical Exchange Saturation Transfer (CEST) MRI for Endogenous Contrast at 7 Tesla. Journal of Neuroimaging. 2013;23(4):526-532. doi:10.1111/j.1552-6569.2012.00751.x
- Öz G, Deelchand DK, Wijnen JP, et al. Advanced single voxel 1 H magnetic resonance spectroscopy techniques in humans: Experts’ consensus recommendations. NMR Biomed. 2020;34(5):e4236.
- Niesporek SC, Nagel AM, Platt T. Multinuclear MRI at Ultrahigh Fields. Top Magn Reson Imaging. 2019;28(3):173-188.
- Platt T, Ladd ME, Paech D. 7 Tesla and Beyond: Advanced Methods and Clinical Applications in Magnetic Resonance Imaging. Invest Radiol. 2021;56(11):705-725.
- Andre JB, Bresnahan BW, Mossa-Basha M, et al. Toward Quantifying the Prevalence, Severity, and Cost Associated With Patient Motion During Clinical MR Examinations. J Am Coll Radiol. 2015;12(7):689-695.
- Federau C, Gallichan D. Motion-Correction Enabled Ultra-High Resolution In-Vivo 7T-MRI of the Brain. PLoS One. 2016;11(5):e0154974.
- Mattern H, Sciarra A, Lüsebrink F, Acosta-Cabronero J, Speck O. Prospective motion correction improves high-resolution quantitative susceptibility mapping at 7T. Magn Reson Med. 2019;81(3):1605-1619.
- Schallmo MP, Weldon KB, Burton PC, Sponheim SR, Olman CA. Assessing methods for geometric distortion compensation in 7T gradient echo fMRI data. bioRxiv. Published online March 10, 2021:2020.07.02.184515. doi:10.1101/2020.07.02.184515
- Yamamoto T, Fukunaga M, Sugawara SK, Hamano YH, Sadato N. Quantitative evaluations of geometrical distortion corrections in cortical surface-based analysis of high-resolution functional MRI data at 7T. J Magn Reson Imaging. 2021;53(4):1220-1234.
- Heilmaier C, Theysohn JM, Maderwald S, Kraff O, Ladd ME, Ladd SC. A large-scale study on subjective perception of discomfort during 7 and 1.5 T MRI examinations. Bioelectromagnetics. 2011;32(8):610-619. doi:10.1002/bem.20680
- Rauschenberg J, Nagel AM, Ladd SC, et al. Multicenter Study of Subjective Acceptance During Magnetic Resonance Imaging at 7 and 9.4 T. Invest Radiol. 2014;49(5):249.
- Krug JW, Rose G, Clifford GD, Oster J. ECG-based gating in ultra high field cardiovascular magnetic resonance using an independent component analysis approach. J Cardiovasc Magn Reson. 2013;15:104.
- Chen I, Saha S. Analysis of an Intensive Magnetic Field on Blood Flow. Electromagnetic Biology and Medicine. 1984;3(1):293-298. doi:10.3109/15368378409035972
- Atkinson IC, Renteria L, Burd H, Pliskin NH, Thulborn KR. Safety of human MRI at static fields above the FDA 8 T guideline: sodium imaging at 9.4 T does not affect vital signs or cognitive ability. J Magn Reson Imaging. 2007;26(5):1222-1227.
- Eryaman Y, Zhang P, Utecht L, et al. Investigating the physiological effects of 10.5 Tesla static field exposure on anesthetized swine. Magn Reson Med. 2018;79(1):511-514.
- Heinrich A, Szostek A, Meyer P, et al. Cognition and sensation in very high static magnetic fields: a randomized case-crossover study with different field strengths. Radiology. 2013;266(1):236-245.
- Lepsien J, Müller K, von Cramon DY, Möller HE. Investigation of higher-order cognitive functions during exposure to a high static magnetic field. J Magn Reson Imaging. 2012;36(4):835-840.
- Vocht F de, de Vocht F, Stevens T, et al. Cognitive effects of head-movements in stray fields generated by a 7 Tesla whole-body MRI magnet. Bioelectromagnetics. 2007;28(4):247-255. doi:10.1002/bem.20311
- van Nierop LE, Slottje P, van Zandvoort MJE, de Vocht F, Kromhout H. Effects of magnetic stray fields from a 7 Tesla MRI scanner on neurocognition: a double-blind randomised crossover study. Occupational and Environmental Medicine. 2012;69(10):759-766. doi:10.1136/oemed-2011-100468
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). ICNIRP Statement on Diagnostic Devices Using Non-ionizing Radiation: Existing Regulations and Potential Health Risks. Health Phys. 2017;112(3):305.
- Fatahi M, Reddig A, Friebe B, Reinhold D, Speck O. MRI and Genetic Damage: An Update. Current Radiology Reports. 2017;5(6):20.
- Lancellotti P, Nchimi A, Delierneux C, et al. Biological Effects of Cardiac Magnetic Resonance on Human Blood Cells. Circ Cardiovasc Imaging. 2015;8(9):e003697.
- Reddig A, Fatahi M, Roggenbuck D, et al. Impact of in Vivo High-Field-Strength and Ultra-High-Field-Strength MR Imaging on DNA Double-Strand-Break Formation in Human Lymphocytes. Radiology. 2017;282(3):782-789.
- Reddig A, Fatahi M, Friebe B, et al. Analysis of DNA Double-Strand Breaks and Cytotoxicity after 7 Tesla Magnetic Resonance Imaging of Isolated Human Lymphocytes. PLoS One. 2015;10(7):e0132702.
- Foster KR, Moulder JE, Budinger TF. Will an MRI Examination Damage Your Genes? Radiat Res. 2017;187(1):1-6.
- Warner R. Ultra-high field magnets for whole-body MRI. Supercond Sci Technol. 2016;29(9):094006.
- Moser E. Ultra-high-field magnetic resonance: Why and when? World J Radiol. 2010;2(1):37-40.
- Springer E, Dymerska B, Cardoso PL, et al. Comparison of Routine Brain Imaging at 3 T and 7 T. Invest Radiol. 2016;51(8):469-482.
- Straub S, Knowles BR, Flassbeck S, Steiger R, Ladd ME, Gizewski ER. Mapping the human brainstem: Brain nuclei and fiber tracts at 3 T and 7 T. NMR Biomed. 2019;32(9):e4118.
- Wrede KH, Dammann P, Johst S, et al. Non-Enhanced MR Imaging of Cerebral Arteriovenous Malformations at 7 Tesla. Eur Radiol. 2016;26(3):829-839.
- Wrede KH, Matsushige T, Goericke SL, et al. Non-enhanced magnetic resonance imaging of unruptured intracranial aneurysms at 7 Tesla: Comparison with digital subtraction angiography. Eur Radiol. 2017;27(1):354-364.
- Novak V, Abduljalil AM, Novak P, Robitaille PM. High-resolution ultrahigh-field MRI of stroke. Magn Reson Imaging. 2005;23(4):539-548.
- Bruschi N, Boffa G, Inglese M. Ultra-high-field 7-T MRI in multiple sclerosis and other demyelinating diseases: from pathology to clinical practice. Eur Radiol Exp. 2020;4(1):59.
- Wisse LEM, Biessels GJ, Heringa SM, et al. Hippocampal subfield volumes at 7T in early Alzheimer’s disease and normal aging. Neurobiol Aging. 2014;35(9):2039-2045.
- Lehéricy S, Bardinet E, Poupon C, Vidailhet M, François C. 7 Tesla magnetic resonance imaging: a closer look at substantia nigra anatomy in Parkinson’s disease. Mov Disord. 2014;29(13):1574-1581.
- Wang I, Oh S, Blümcke I, et al. Value of 7T MRI and post-processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia. 2020;61(11):2509-2520.
- Opheim G, van der Kolk A, Markenroth Bloch K, et al. 7T Epilepsy Task Force Consensus Recommendations on the Use of 7T MRI in Clinical Practice. Neurology. 2021;96(7):327-341.
- Regnery S, Knowles BR, Paech D, et al. High-resolution FLAIR MRI at 7 Tesla for treatment planning in glioblastoma patients. Radiother Oncol. 2019;130:180-184.
- 1Chang G, Pakin SK, Schweitzer ME, Saha PK, Regatte RR. Adaptations in trabecular bone microarchitecture in Olympic athletes determined by 7T MRI. J Magn Reson Imaging. 2008;27(5):1089-1095.
- Springer E, Bohndorf K, Juras V, et al. Comparison of routine knee magnetic resonance imaging at 3 T and 7 T. Invest Radiol. 2017;52(1):42-54.
- Lazik A, Theysohn JM, Geis C, et al. 7 Tesla quantitative hip MRI: T1, T2 and T2* mapping of hip cartilage in healthy volunteers. Eur Radiol. 2016;26(5):1245-1253.
- Wyatt C, Guha A, Venkatachari A, et al. Improved differentiation between knees with cartilage lesions and controls using 7T relaxation time mapping. J Orthop Translat. 2015;3(4):197-204.
- Wu B, Wang C, Krug R, et al. 7T human spine imaging arrays with adjustable inductive decoupling. IEEE Trans Biomed Eng. 2010;57(2):397-403.
- Rietsch SHG, Brunheim S, Orzada S, et al. Development and evaluation of a 16-channel receive-only RF coil to improve 7T ultra-high field body MRI with focus on the spine. Magn Reson Med. 2019;82(2):796-810.
- Laader A, Beiderwellen K, Kraff O, et al. 1.5 versus 3 versus 7 Tesla in abdominal MRI: A comparative study. PLoS One. 2017;12(11):e0187528.
- Umutlu L, Orzada S, Kinner S, et al. Renal imaging at 7 Tesla: preliminary results. Eur Radiol. 2011;21(4):841-849.
- Fischer A, Kraff O, Orzada S, et al. Ultrahigh-Field Imaging of the Biliary Tract at 7 T: Initial Results of Gadoxetic Acid–Enhanced Magnetic Resonance Cholangiography. Invest Radiol. 2014;49(5):346.
- Vos EK, Lagemaat MW, Barentsz JO, et al. Image quality and cancer visibility of T2-weighted magnetic resonance imaging of the prostate at 7 Tesla. Eur Radiol. 2014;24(8):1950-1958.
- Kraff O, Quick HH. 7T: Physics, safety, and potential clinical applications. J Magn Reson Imaging. 2017;46(6):1573-1589.
- Stehouwer BL, Klomp DWJ, van den Bosch MAAJ, et al. Dynamic contrast-enhanced and ultra-high-resolution breast MRI at 7.0 Tesla. Eur Radiol. 2013;23(11):2961-2968.
- von Knobelsdorff-Brenkenhoff F, Tkachenko V, Winter L, et al. Assessment of the right ventricle with cardiovascular magnetic resonance at 7 Tesla. J Cardiovasc Magn Reson. 2013;15:23.
