Abdominal and Pelvic Imaging of Transgender Patients
Images
The abdominal radiologist plays an important role in providing care for transgender and gender diverse patients, regardless of whether these patients choose to pursue gender affirming hormone therapy or surgery. If a patient does pursue gender affirming surgery, imaging plays an integral role in both preoperative and postoperative management.1
The term “transgender” describes a person whose gender identity is incongruent with their sex assigned at birth.2 In 2016, it was estimated that approximately 1.4 million people in the United States identified as transgender3 – approximately 1 in 250 adults, which has significantly increased in the past decade.4 Not every person with gender incongruence will pursue hormonal therapy or gender-affirming surgery. In 2015, only approximately 25% of national transgender survey respondents had undergone gender-affirming surgery.5 This rate is expected to increase as Medicare and Medicaid in the United States now cover gender-affirming surgical procedures, and many third-party payers are beginning to increase coverage as well.6
Radiologists must be aware of the various treatment options and associated anatomic and pathologic changes in transgender patients to ensure accurate imaging interpretation.6 This article aims to discuss relevant imaging findings of hormonal therapies, nonoperative procedures, and gender-affirming surgeries, with a focus on abdominal and pelvic imaging. Additionally, it is critical for imaging centers to create an inclusive environment for these patients as they expand care; thus, this article will also explore potential pitfalls and strategies to overcome them.
Imaging Findings of Hormonal Therapies
At least 80% of transgender people have taken or want to take gender-affirming hormone therapy, according to the National Transgender Discrimination Survey Report on Health and Health Care.7 Transgender women may take an estrogen with an androgen blocker, with or without a progestogen, to feminize their bodies while suppressing or minimizing male secondary sex characteristics. Transgender men may take testosterone to masculinize their bodies. Additionally, gender nonbinary, gender nonconforming, or gender-diverse individuals may take hormones to develop or minimize masculine or feminine sexual secondary characteristics. The abdominal and pelvic imaging findings in patients undergoing hormone therapy are dependent on the hormone regimen and length of treatment.
Feminizing Hormone Therapy
Imaging findings may be associated with desired effects and deleterious side effects of hormone therapy. For example, feminizing hormones may result in reduced testicular size, subcutaneous fat redistribution, and muscle-mass reduction. Rare harmful side effects that may be evaluated with imaging include venous thromboembolism (exacerbated by smoking and mitigated by transdermal estradiol administration) and liver dysfunction or fulminant hepatitis.8
Studies have found that prostate cancer risk is lower in transgender women receiving androgen-deprivation and estrogen treatment.9 Given that population-based, prostate-specific antigen (PSA) screening is not globally recommended, that there is a low incidence of prostate cancer in transgender women, and that there is a lack of PSA reference values in this population, routine PSA screening is not recommended. However, maintaining an awareness of the presence of the prostate gland in these patients and the possibility of prostate cancer, which may be seen on imaging, is important for radiologists. In patients who have undergone vaginoplasty, transvaginal ultrasound (US) may be the best imaging modality for prostate examination and preferred over digital rectal examination. Similarly, prostatitis and epididymitis may occur and should remain considerations in transgender women with elevated PSA and/or pelvic pain. Routine testicular cancer screening is also not recommended for transgender women who have not undergone orchiectomy, as they are not at increased risk. However, if there is suspicion for a testicular tumor, scrotal US should be performed.10
Masculinizing Hormone Therapy
Masculinizing hormone therapy may result in increased muscle mass, subcutaneous fat redistribution, and clitoral growth. Idiopathic pelvic pain is an uncommon side effect after initiation of testosterone therapy; and, while cessation of menses is expected to occur within 6 months,10 over half of these patients experience persistent uterine bleeding. There is no consensus regarding an increased risk of endometrial hyperplasia and cancer in patients receiving masculinizing hormone therapy; thus, screening for endometrial cancer is not currently recommended.10 Pelvic US may be indicated for evaluation of persistent or abnormal uterine bleeding; however, due to potential significant emotional and physical discomfort, clinicians may elect to pursue computed tomography (CT) or magnetic resonance imaging (MRI) evaluation as a first-line imaging choice.1
Masculinizing hormonal therapy with testosterone in transgender men has been linked to an increase in volume and follicular count of the ovaries; radiologists should be aware that these ovarian changes may resemble polycystic ovarian syndrome on imaging.10 In sexually active transgender men receiving masculinizing hormone therapy and who have not undergone hysterectomy or oophorectomy, differential considerations on imaging for pelvic pain may include pregnancy, tuboovarian abscess, and pelvic inflammatory disease. Pregnancy tests prior to exposure to ionizing radiation and gadolinium administration should be considered in these patients regardless of hormone therapy administration.
Imaging Findings of Nonoperative Procedures
Silicone Injections
Silicone injections are now illegal in the United States; as a consequence, these procedures often are being performed in an unsupervised fashion or outside of the country. These injections are often used for breast augmentation or other soft-tissue contouring procedures.6
Silicone injection results in formation of soft-tissue granulomas within the subcutaneous fat, which can be visualized on imaging, including radiography and CT (Figure 1). On US, this results in the pathognomonic “snowstorm” appearance. Visualized on MRI, silicone demonstrates intermediate T1 signal intensity and increased T2 signal intensity. Silicone-selective suppression sequences can be used which result in the loss of signal in the regions of prior silicone injection. Silicone injections limit mammogram sensitivity; therefore, in this patient population, contrast-enhanced MRI is often the first-line tool for breast cancer screening.6
Gender-affirming Surgery
Gender-affirming surgery, which is performed to alleviate symptoms of gender dysphoria, has beneficial effects across multiple domains. Studies have demonstrated improved quality of life, sexual desire, and overall increased mood.11 The procedure is categorized into “top surgery,” which includes facial feminization or masculinization and chest reconstruction, and “bottom surgery,” which includes vaginoplasty and phalloplasty.12 For the purposes of this discussion, only abdominal imaging findings will be discussed, with a focus on bottom surgery.
Feminizing Genital Surgery
Transfeminine patients often pursue gender-affirming surgery to align their external genitalia with their gender identity, as well as for receptive intercourse. Multiple components are involved, including penectomy, orchiectomy, and penoscrotal inversion or enteric vaginoplasty.12
Penoscrotal inversion is the most common surgical technique for vaginoplasty. After orchiectomy, penile disassembly is performed, with shortening and repositioning of the urethra. The remaining penile and scrotal skin is inverted to create a neovagina. The neovaginal flap is sometimes sutured to the sacrospinous ligament to prevent prolapse. A neoclitoris that is sensate is created from a portion of the glans penis.6
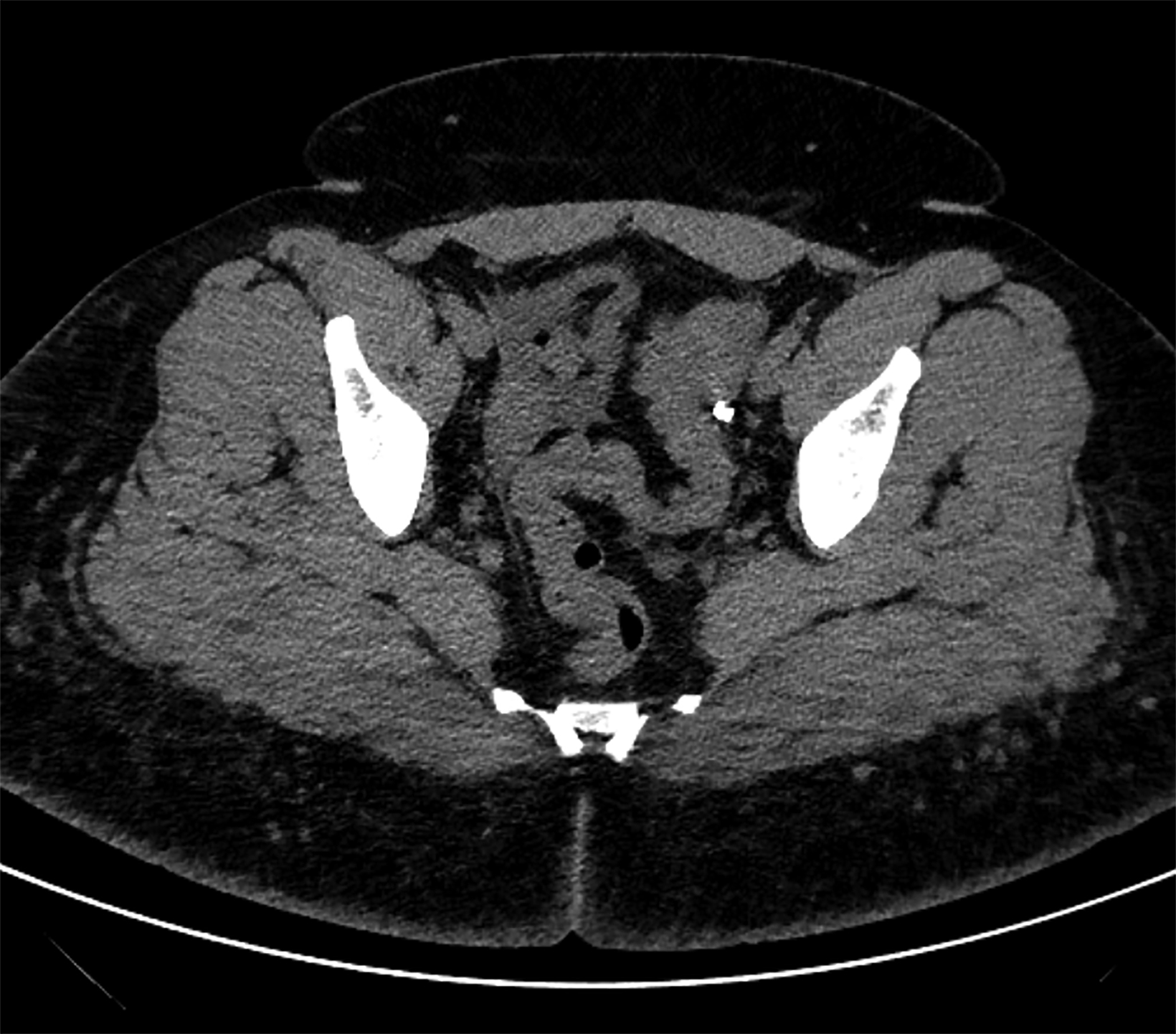
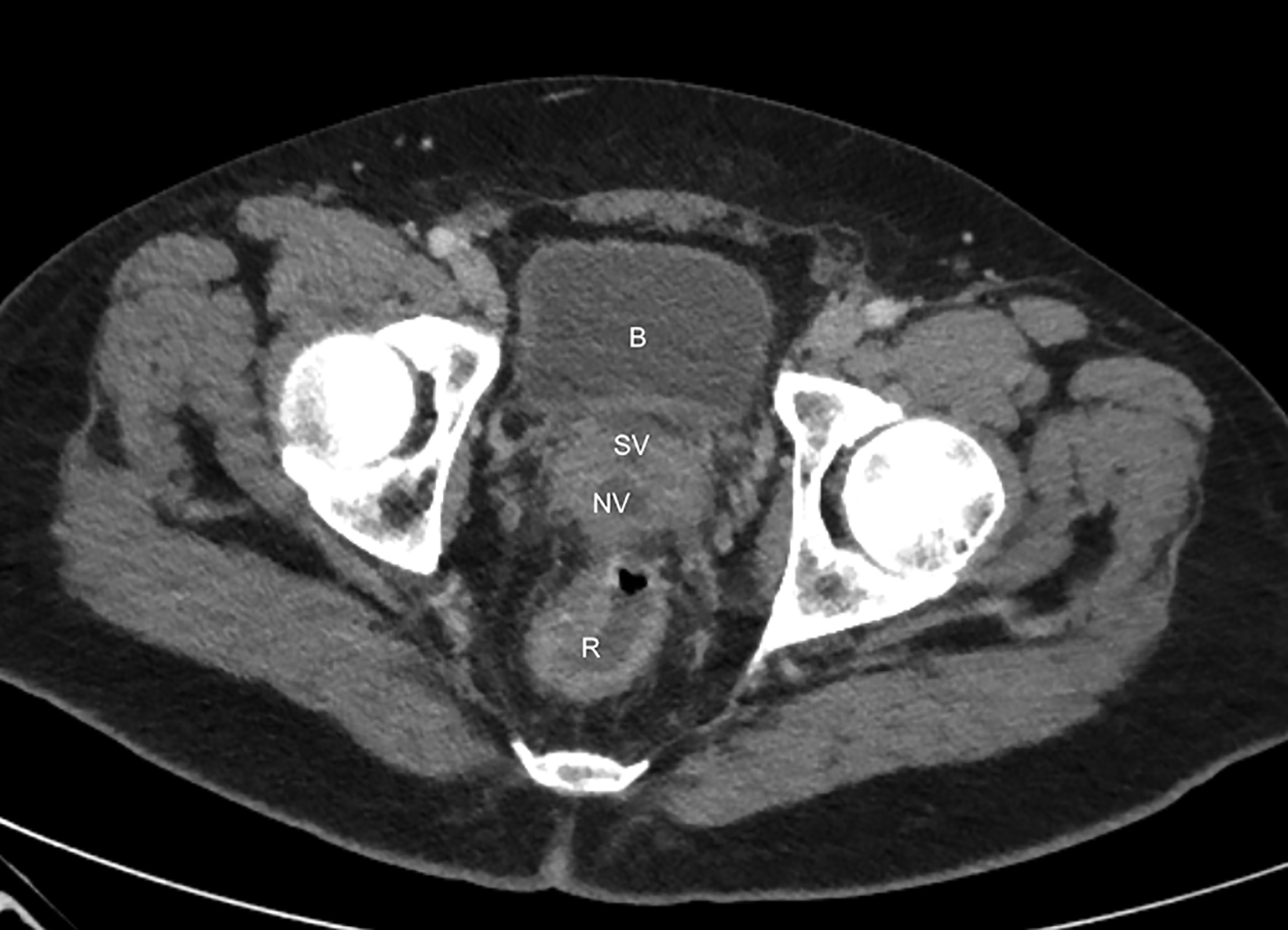
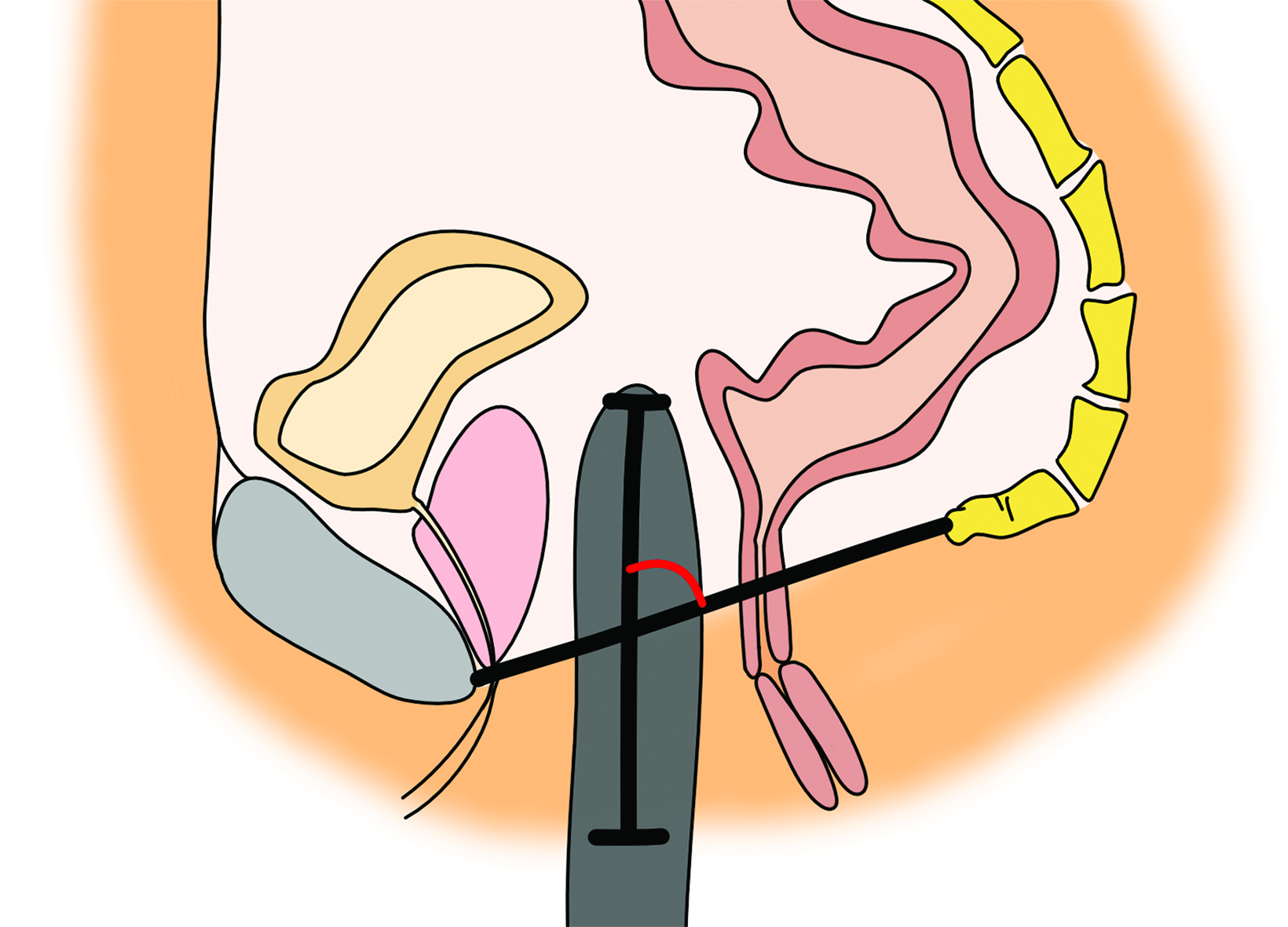
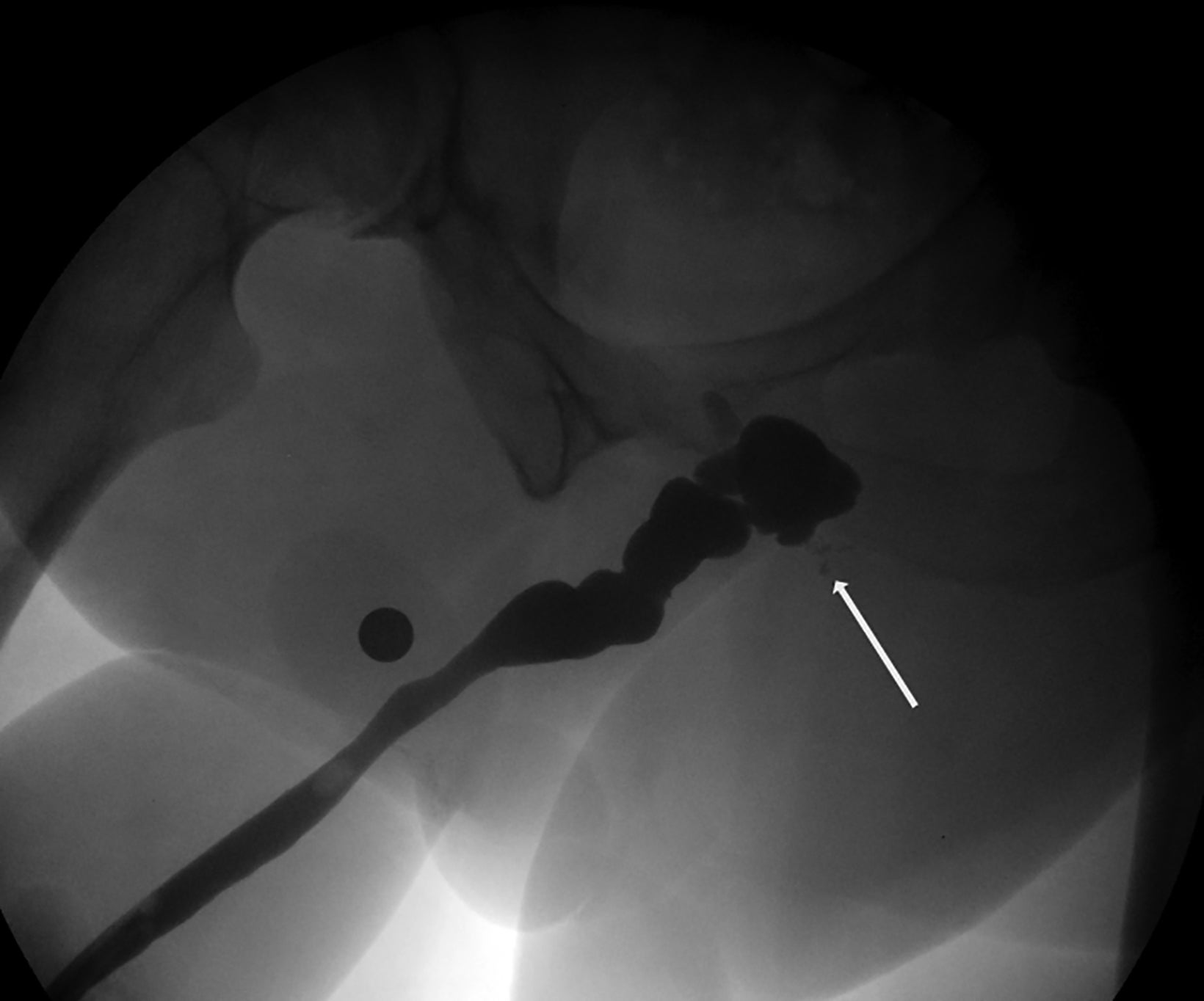
On CT, the neovagina appears as a collapsed tubular structure often surrounded by granulation tissue and associated fat stranding (Figure 2). In the immediate postoperative setting, the neovagina contains surgical packing material; it is essential to not label this packing
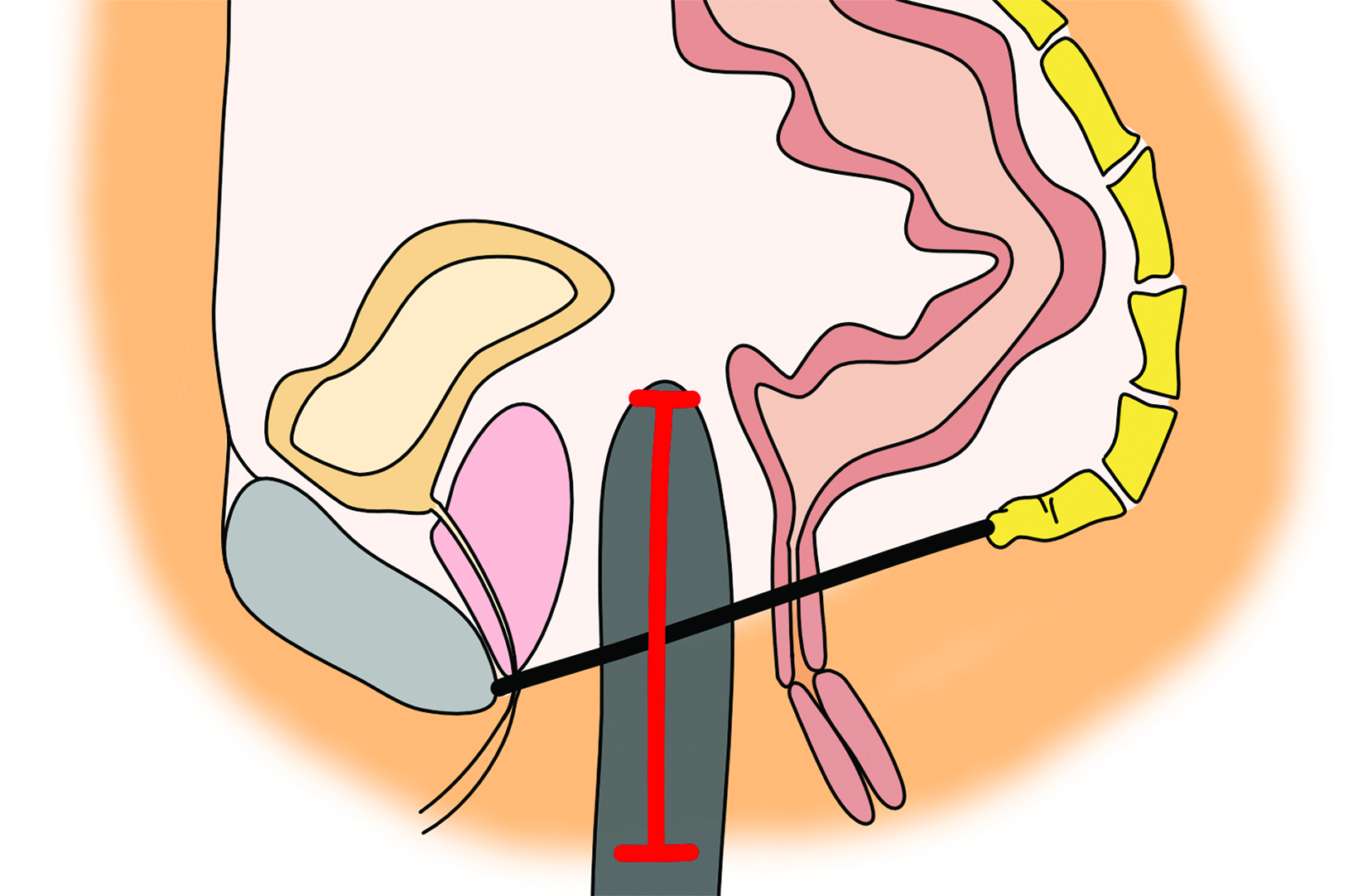
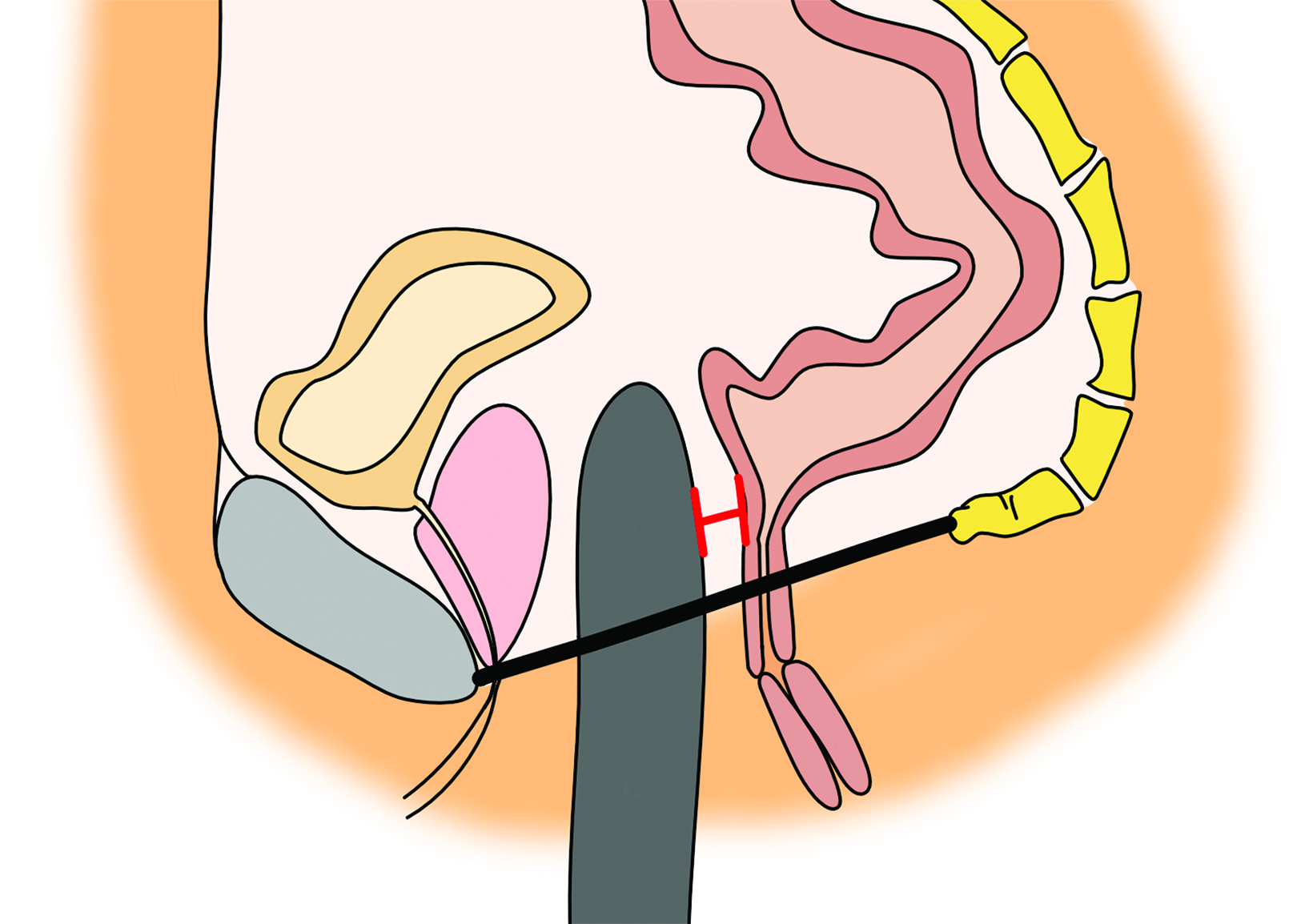
as a postoperative abscess. Small perivaginal hematomas are expected. Residual erectile tissue can be visualized and is hyperenhancing; care should be taken to ensure this is not contrast extravasation in the immediate postoperative setting. In the outpatient setting, vaginal dilators may be in place. In evaluating a neovagina on imaging, including the vaginal depth, angle of inclination, and thickness of the rectovaginal septum is important (Figure 3).6
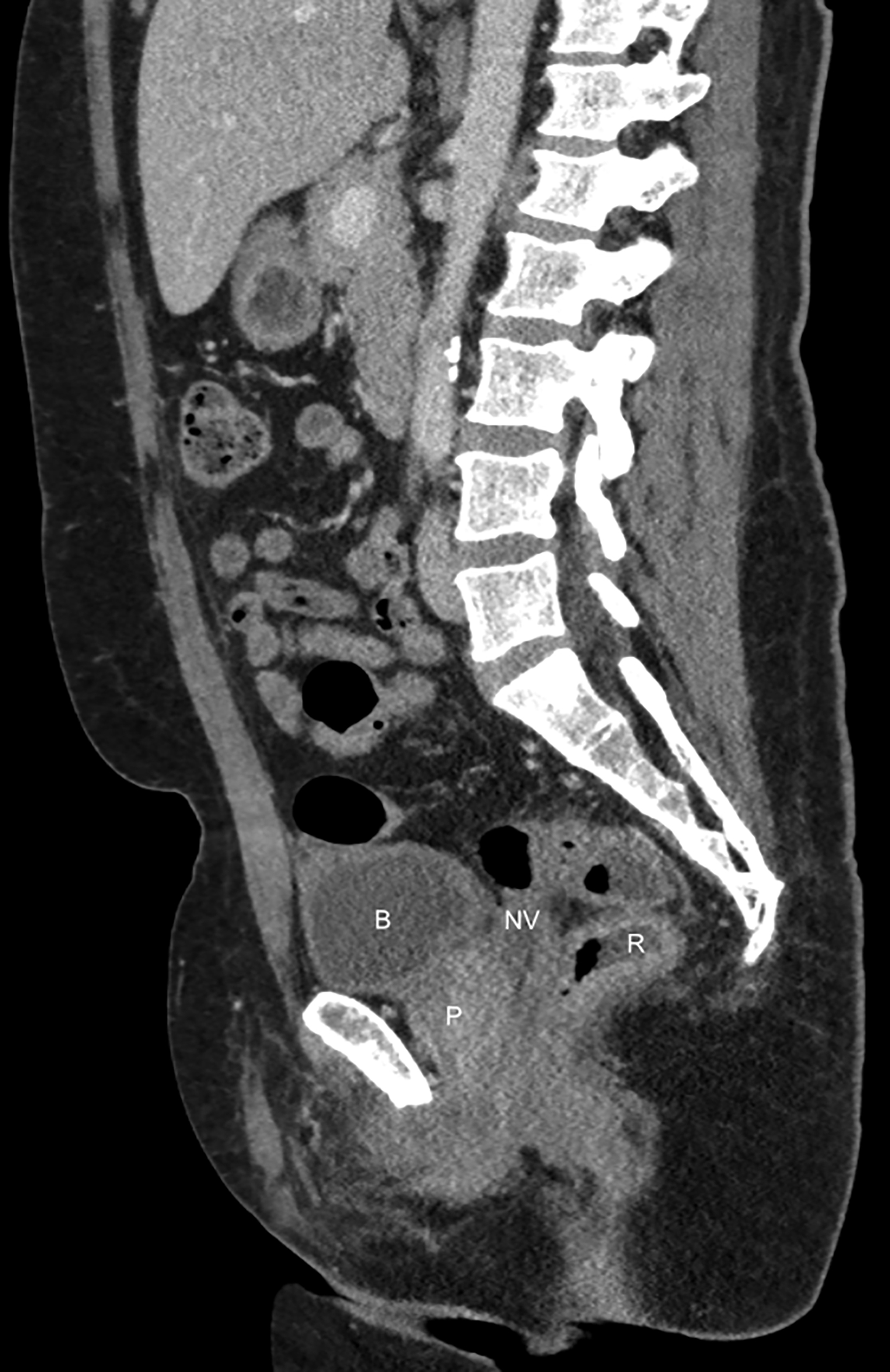
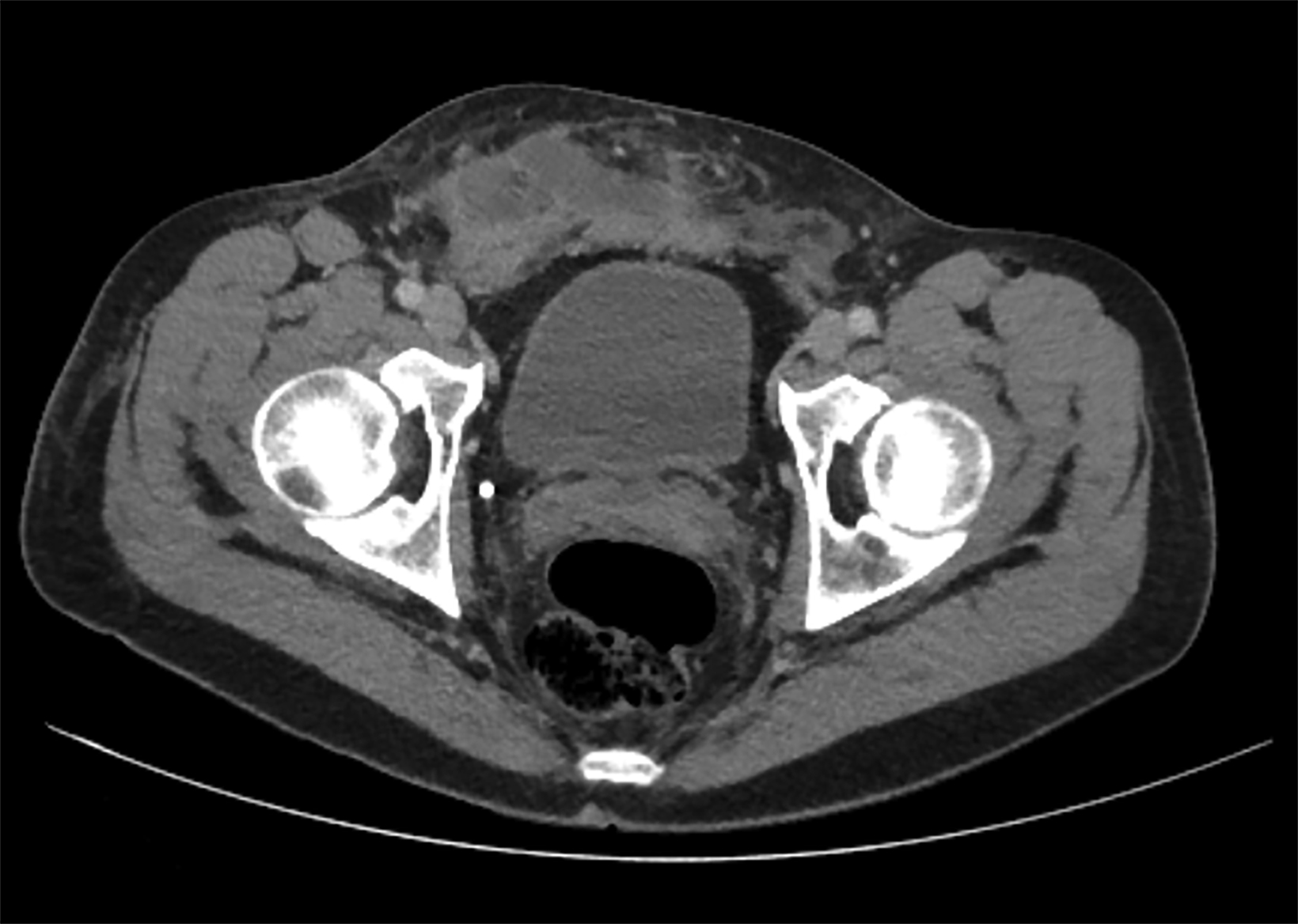
Postoperative complications may be seen in this patient population, with highly variable reported rates, likely owing to slight variances in operative technique. Urinary issues such as strictures requiring surgery have been reported in 13.4% of patients11; these are best evaluated with fluoroscopy. The average intraoperative rectal injury rate after vaginoplasty is approximately 2.4%, with 1.7% of total patients developing rectovaginal fistulization.11 This is best evaluated with small field-of-view, contrast-enhanced pelvic MRI. Wound dehiscence is seen in approximately 12% of patients; however, the incidence of major infection is only 2.1%.11 These complications are well-evaluated utilizing either CT or MRI, depending on the complete clinical scenario (Figure 4). In
the setting of enteric vaginoplasty, which is less commonly performed today, postoperative complications often included acute peritonitis, mucorrhea, neovaginitis, malodor, rectal dysfunction, and anastomotic stricture.11 Vaginal prolapse is also a potential postoperative complication,12 in which case dynamic pelvic MRI is best for evaluation.
Masculinizing Genital Surgery
Masculinizing genital surgeries include hysterectomy, oophorectomy, vaginectomy, phalloplasty, metoidioplasty, and scrotoplasty — most of which are staged to reduce the risk of significant blood loss. Two main surgical options for creating a neophallus include metoidioplasty and phalloplasty, with phalloplasty being the most complex gender-affirming surgery; it often requires a multidisciplinary team approach. The goals of creating a neophallus might include the ability to urinate while standing, maintain sensation, and potentially participate in penetrative sexual intercourse. Numerous surgical techniques exist; the most common and currently preferred technique is the radial forearm free flap. Anterolateral thigh flap, abdominal flap, and latissimus dorsi free flap are additional but less frequently utilized techniques. Metoidioplasty is a separate surgical technique in which the neophallus is created from a hormonally hypertrophied clitoris; however, this can limit neophallus length and prevent the ability to engage in sexual intercourse in some patients.6
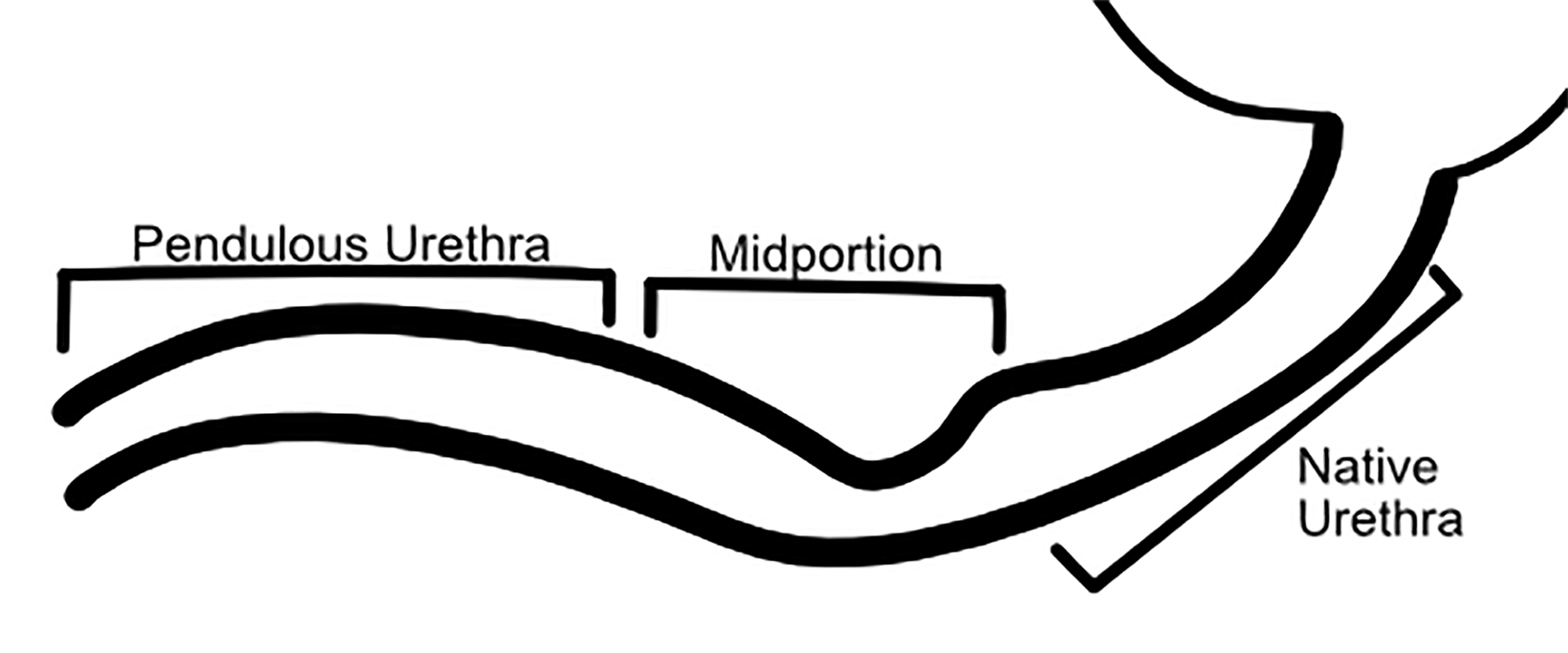
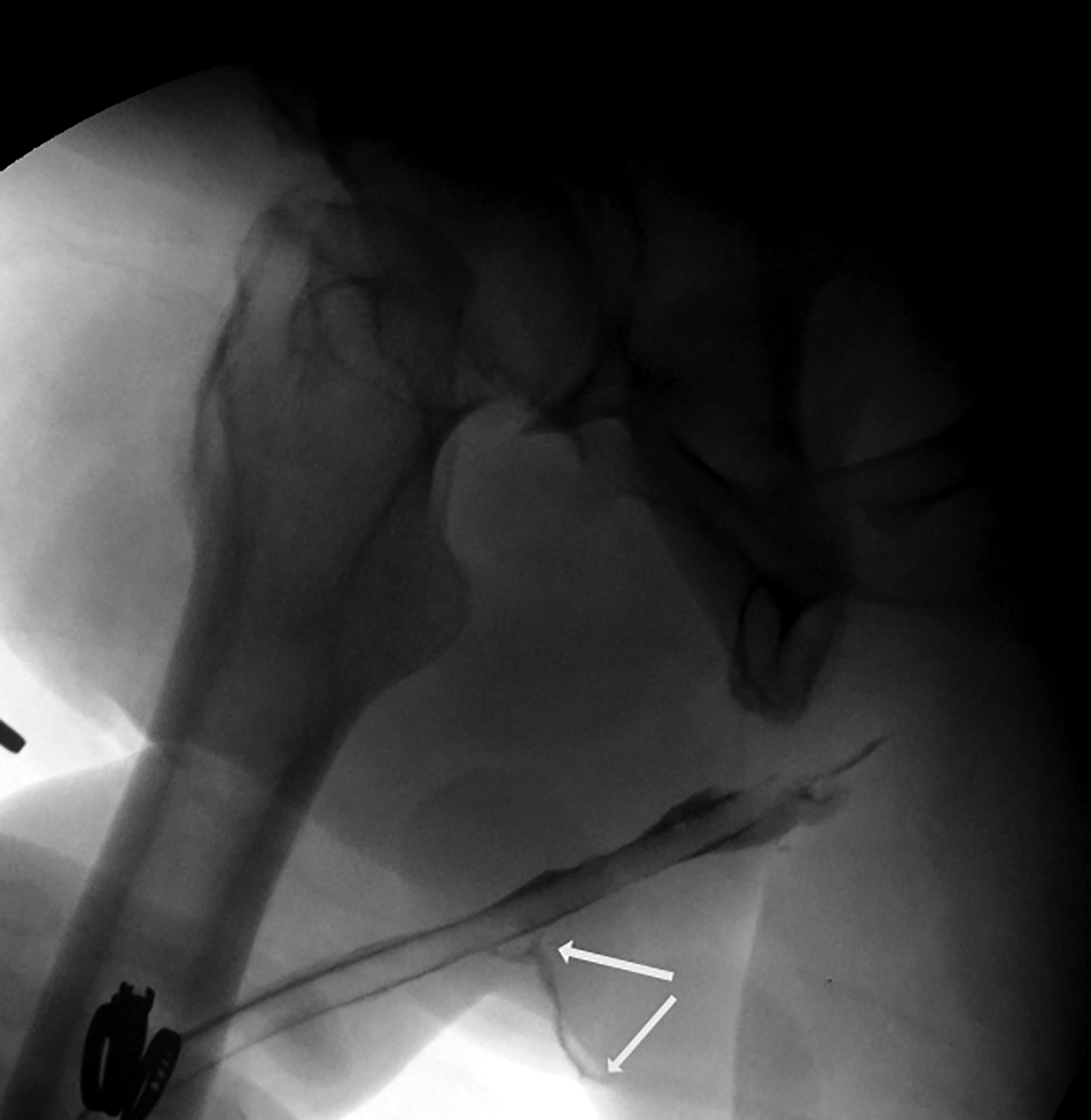
Owing to the number of surgical techniques, data varies in the literature regarding postoperative complications for masculinization surgery. Overall, metoidioplasty is a less-invasive and lower-risk procedure than phalloplasty.12 In patients undergoing phalloplasty, wound dehiscence occurs in 9.8%. This is adequately evaluated with CT to assess for the presence of underlying abscess.11 It is essential to monitor early perfusion of the flap in the immediate postoperative setting using ultrasound with color doppler as well.4 Minor urethral issues (including stenoses, strictures, or fistulas which resolved with treatment) occurred in 24% of patients, with major urethral complications seen in an additional 24.4% of patients.11 These urinary complications are well-evaluated fluoroscopically with retrograde urethrograms (Figures 5 and 6). Reported rates of urethral issues in metoidioplasty are lower, with minor issues in 3.9% of patients, and major urethral issues in 11.4%. However, it was also reported that 15% of patients who underwent metoidioplasty elected to undergo a secondary phalloplasty.11 In phalloplasty patients, donor site complications are also a consideration, and include graft necrosis, excessive scarring, paresthesias, and limitation of wrist motion. However, these are generally beyond the scope of practice for abdominal radiologists.11
Erectile devices can also be considered in patients who intend to engage in penetrative intercourse; they are usually placed months after the neophallus has healed. These devices demonstrate higher rates of complications in postoperative transgender patients compared to cisgender individuals requiring prosthesis.12 This difference is hypothesized to be secondary to earlier age of implantation and lack of supportive cavernosal fascial layers. These potential complications include mechanical device failure, malposition, and infection, and are well evaluated with CT.4 Testicular prostheses, which are usually either silicone or saline filled, may also be placed. Complications of these prostheses include malposition, rupture, and infection, all of which are well evaluated with CT or MRI.4
Creating an Inclusive Environment
As accessibility to gender-affirming care increases, it is essential for radiologists not only to increase their knowledge of the imaging findings but also to ensure that imaging centers improve their inclusivity to ensure positive patient experiences. The 2015 U.S. Transgender Survey found that 33% of responders had at least one negative experience in a healthcare setting related to their gender identity.3 Grimstad, et al, surveyed over 500 transgender and nonbinary patients, and more than 70% reported
having one or more negative experience during an imaging encounter.13 These included not being asked their correct pronouns, incorrect use of pronouns, personnel discomfort, and failure to protect privacy. Additionally, almost 25% were misgendered in the radiology report.13
Initiating staff training covering respectful communication, including correct use of pronouns, and cultural sensitivity and gender-affirming care standards, as well as training in proper imaging techniques for these patients, are essential for medical imaging centers. Radiology residency programs should incorporate LGBTQ culture competency, as well. Gender-neutral restrooms/ changing facilities, as well as LGBTQ-affirming reading materials and signage are also essential to creating a positive and welcoming patient experience. Integrating these issues into organizational accreditation guidelines, such as those provided by the American College of Radiology, would be impactful.13
Conclusion
The rapid evolution of transgender healthcare across the world is allowing for greater access to care and overall improved quality of life for many with gender dysphoria.4 As more transgender patients seek gender-affirming surgery, it is essential for abdominal radiologists to remain current on the latest surgical techniques, their potential complications, and the appropriate imaging modalities to best evaluate them. Additionally, as they seek to provide adequate imaging interpretations, abdominal radiologists should strive for excellence in the transgender patient experience. This will help to maintain their role as integral members of the multidisciplinary transgender care team and to perpetuate a culture of inclusivity within the medical field.
References
- Stowell JT, Zavaletta VA, Carroll EF, Grimstad FW. Multidisciplinary approach to imaging for gender-affirming surgery: engaging surgeons, radiologists, and patients to ensure a positive imaging experience. Ann Transl Med. Apr 2021;9(7):610. doi:10.21037/atm-20-6431.
- National LGBT Health Education Center: Glossary of LGBT Terms for Health Care Teams. Updated June 2017. Accessed March 13, 2022, https://www.lgbtqiahealtheducation.org/wp-content/uploads/2018/03/Glossary-2018-English-update-1.pdf.
- Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? Updated 2016. https://williamsinstitute.law.ucla.edu/ wp-content/uploads/Trans-Adults-US-Aug-2016.pdf. Accessed March 13, 2022,
- Stowell JT, Horowitz JM, Thomas S. Gender-affirming surgical techniques, complications, and imaging considerations for the abdominal radiologist. Abdom Radiol (NY). Jul 2020;45(7):2036-2048. doi:10.1007/s00261-019-02398-1.
- James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality. 2016;
- Stowell JT, Grimstad FW, Kirkpatrick DL, et al. Imaging findings in transgender patients after gender-affirming surgery. Radiographics. Sep-Oct 2019;39(5):1368-1392. doi:10.1148/rg.2019190010
- Nguyen HB, Chavez AM, Lipner E, et al. Gender-affirming hormone use in transgender individuals: impact on behavioral health and cognition. Current Psychiatry Reports. 2018/10/11 2018;20(12):110. doi:10.1007/s11920-018-0973-0.
- Deutsch MB. Overview of feminizing hormone therapy. https://transcare.ucsf.edu/guidelines/feminizing-hormone-therapy. Accessed April 4, 2022.
- de Nie I, de Blok CJM, van der Sluis TM, et al. Prostate cancer incidence under androgen deprivation: nationwide cohort study in trans women receiving hormone treatment. J Clin EndocrinolMetabol. 2020;105(9):e3293-e3299. doi:10.1210/clinem/dgaa412.
- Sowinski JS, Gunderman RB. Transgender patients: what radiologists need to know. AJR Am J Roentgenol. May 2018;210(5):1106-1110. doi:10.2214/AJR.17.18904.
- Oles N, Darrach H, Landford W, et al. Gender affirming surgery: a comprehensive, systematic review of all peer-reviewed literature and methods of assessing patient-centered outcomes (Part 2: Genital Reconstruction). Ann Surg. Jan 1 2022;275(1):e67-e74. doi:10.1097/sla.0000000000004717.
- Hassan O, Sun D, Jha P. Imaging in gender affirmation surgery. Curr Urol Rep. Jan 30 2021;22(2):14. doi:10.1007/s11934-020-01029-3.
- Grimstad FW, Stowell JT, Gaddis M. Survey of experiences of transgender and gender nonbinary patients during imaging encounters and opportunities for improvement. AJR Am J Roentgenol. Nov 2020;215(5):1136-1142. doi:10.2214/ajr.19.22558
References
Citation
EN S, Ghosheh KK P,. Abdominal and Pelvic Imaging of Transgender Patients. Appl Radiol. 2022;(4):7-13.
July 14, 2022